Abstract
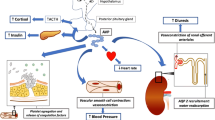
Despite the introduction of multiple new pharmacological agents over the past three decades in the field of heart failure (HF), overall prognosis remains poor. Hyponatremia is prevalent in HF patients and has been suggested as a contributor to poor response to standard therapy. Elevated levels of arginine vasopressin (AVP), a peptide hormone produced in the hypothalamus, play a role in development of hyponatremia, and AVP and its surrogate, copeptin, are related to changes in osmolality, hemodynamics, neuro-hormones as well as in overall outcome in HF patients. Of current pharmacological interest are the selective and non-selective vasopressin receptor antagonists (VRAs), which inhibit vasoconstriction and cardiac remodeling mediated by the V1a receptors in smooth blood vessels, and water retention (increased urine osmolality and decreased water excretion) by increasing aquaporin-2 water channels mediated by the V2 receptors in the renal collecting tubules. The optimal use of VRAs is yet to be determined, especially in patients with congestive HF. Although long-term effects on improvement in mortality have not been shown in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, the only long-term outcome trial to date, many short-term studies indicate beneficial aquaretic- and hemodynamic-effects of the VRAs. In contrast to loop diuretics, these new agents tend to increase urine flow and the excretion of electrolyte-free water (so-called aquaresis) in patients with HF, without substantial changes in sodium or potassium excretion. This chapter reviews the role of AVP and copeptin in HF, and the treatment potential of VRAs in HF.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ACTIV:
-
Acute and chronic therapeutic impact of a vasopressin 2 antagonist
- ADHF:
-
Acute decompensated heart failure
- AE:
-
Adverse event
- AVP:
-
Arginine vasopressin
- BNP:
-
B-type natriuretic peptide
- BP:
-
Blood pressure
- BUN:
-
Blood urea nitrogen
- BW:
-
Body weight
- CHF:
-
Congestive heart failure
- CI:
-
Cardiac index
- CIBIS-II:
-
Cardiac insufficiency bisoprolol study II
- CO:
-
Cardiac output
- CV:
-
Cardiovascular
- CVP:
-
Central venous pressure
- DILIPO:
-
DILutIonal hyponatremia
- ECLIPSE:
-
EffeCt of toLvaptan on hemodynamIc Parameters in Subjects with hEart failure
- EF:
-
Ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- EVEREST:
-
Efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan
- HF:
-
Heart failure
- HR:
-
Heart rate
- iv:
-
Intravenous
- KCCQ:
-
Kansas City Cardiomyopathy Questionnaire
- LV:
-
Left ventricular
- MAP:
-
Mean arterial pressure
- METEOR:
-
Multicenter evaluation of tolvaptan effect on remodeling
- NE:
-
Norepinephrine
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- NYHA:
-
New York Heart Association
- OD:
-
Once daily
- PARADIGM-HF:
-
Prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure
- PCWP:
-
Pulmonary capillary wedge pressure
- p-Na+ :
-
Plasma sodium
- PVR:
-
Pulmonary vascular resistance
- QOL:
-
Quality of life
- RA:
-
Renin activity
- RALES:
-
Randomized aldactone evaluation study
- RAP:
-
Right atrial pressure
- RAS:
-
Renin angiotensin system
- SD:
-
Standard deviation
- SIADH:
-
Syndrome of inappropriate antidiuretic hormone
- s-K+ :
-
Serum potassium
- s-Na+ :
-
Serum sodium
- SOLVD:
-
Studies of left ventricular dysfunction
- SV:
-
Stroke volume
- SVR:
-
Systemic vascular resistance
- VRA:
-
Vasopressin receptor antagonist
- WRF:
-
Worsening renal function
References
Aronson D, Verbalis JG, Mueller M et al (2011) Short- and long-term treatment of dilutional hyponatraemia with satavaptan, a selective arginine vasopressin V2-receptor antagonist: the DILIPO study. Eur J Heart Fail 13:327–336
Balling L, Gustafsson F (2016) Copeptin in heart failure. Adv Clin Chem 73:29–64
Balling L, Schou M, Videbaek L et al (2011) Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail 13:968–973
Balling L, Kistorp C, Schou M et al (2012) Plasma copeptin levels and prediction of outcome in heart failure outpatients: relation to hyponatremia and loop diuretic doses. J Card Fail 18(5):351–358
Bettari L, Fiuzat M, Felker GM et al (2012) Significance of hyponatremia in heart failure. Heart Fail Rev 17:17–26
CIBIS-II (1999) The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. CIBIS-II Investigators and Committees. Lancet 353(9146):9–13
Cooper HA, Dries DL, Davis CE et al (1999) Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 100:1311–1315
Creager MA, Faxon DP, Cutler SS et al (1986) Contribution of vasopressin to vasoconstriction in patients with congestive heart failure: comparison with the renin-angiotensin system and the sympathetic nervous system. J Am Coll Cardiol 7:758–765
Decaux G, Soupart A, Vassart G (2008) Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 371:1624–1632
Domanski M, Norman J, Pitt B et al (2003) Diuretic use, progressive heart failure and death in patients in the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol 42:705–708
Ghali JK, Tam SW (2010) The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists. J Card Fail 16:419–431
Ghali JK, Orlandi C, Abraham WT (2012) The efficacy and safety of lixivaptan in outpatients with heart failure and volume overload: results of a multi-centre, randomized, double-blind, placebo-controlled, parallel-group study. Eur J Heart Fail 14:642–651
Gheorghiade M, Gattis WA, O’Conner CM et al (2004) Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 291:1963–1971
Gheorghiade M, Konstam MA, Burnett JC Jr et al (2007) Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA 297:1332–1343
Gines P, Wong F, Watson H et al (2008) Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia. A randomized trial. Hepatology 48:204–213
Goldsmith SR (1988) Baroreflex control of vasopressin secretion in normal humans. In: Cowley AW, Liard J-F, Ausiello DA (eds) Vasopressin: cellular and integrative functions. Raven Press, New York, pp 389–397
Goldsmith SR (1992) Baroreflex loading maneuvers do not suppress increased plasma arginine vasopressin in patients with congestive heart failure. J Am Coll Cardiol 19:1180–1184
Goldsmith SR (2013) Hyponatremia in heart failure: time for a trial. J Card Fail 19(6):398–400
Goldsmith SR, Gheorghiade M (2005) Vasopressin antagonism in heart failure. J Am Coll Cardiol 46:1785–1791
Goldsmith SR, Francis GS, Cowley AW et al (1983) Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol 1:1385–1390
Goldsmith SR, Francis GS, Cowley AW et al (1986) Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol 8:779–783
Goldsmith SR, Dodge D, Cowley AW (1989) The effect of moderate hypotension on arginine vasopressin levels in normal humans. Am J Med Sci 298:295–298
Goldsmith SR, Elkayam U, Haught H et al (2008) Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: a dose-ranging pilot study. J Card Fail 14:641–647
Goldsmith SR, Gilbertson DT, Mackedanz SA et al (2011) Renal effects of conivaptan, furosemide, and the combination in patients with chronic heart failure. J Card Fail 17:982–989
Hauptman PJ, Burnett J, Gheorghiade M et al (2013) Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 19:390–397
Hirano T, Yamamura Y, Nakamura S et al (2000) Effects of the V2-receptor antagonist OPC-41061 and the loop diuretic furosemide alone and in combination in rats. J Pharmacol Exp Ther 292:288–294
Holmes CL, Landry DW, Granton JT (2003) Science review: vasopressin and the cardiovascular system part 1 – receptor physiology. Crit Care 7(6):427–434
Jackson EK (2006) Vasopressin and other agents affecting the renal conservation of water. In: Brunton L, Lazo JS, Parker KL (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 11th edn. McGraw-Hill, New York, pp 771–778
Jovanovich AJ, Berl T (2013) Whereas vaptans do and do not fit in the treatment of hyponatremia. Kidney Int 83:563–567
Konstam MA, Gheorghiade M, Burnett JC Jr et al (2007) Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 297:1319–1331
Lanfeur D, Sabbah HN, Goldsmith SR et al (2013) Association of arginine vasopressin levels with outcomes and the effect of V2 blockage in patients hospitalized for heart failure with reduced ejection fraction. Insights from the EVEREST trial. Circ Heart Fail 6:47–52
Lilly LS, Dzau VJ, Williams GH et al (1984) Hyponatremia in congestive heart failure: implications for neurohumoral activation and responses to orthostasis. J Clin Endocrinol Metab 59:924–930
Maggioni AP, Dahlström U, Filippatos G et al (2013) EUR observational research programme: regional differences and 1-year follow-up results of the heart failure pilot survey (ESC-HF Pilot). Eur J Heart Fail 15:808–817
Mao ZL, Stalker D, Keirns J (2009) Pharmacokinetics of conivaptan hydrochloride, a vasopressin V1A/V2-receptor antagonist, in patients with euvolemic or hypervolemic hyponatremia and with or without congestive heart failure from a prospective, 4-day open-label study. Clin Ther 31:1542–1550
McMurray JJ, Packer M, Desai AS et al (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371(11):993–1004
Mondritzki T, Kolkhof P, Sabbah HN et al (2011) Differentiation of arginine vasopressin antagonistic effects by selective V2 versus dual V2/V1a receptor blockage in a preclinical heart failure model. Am J Ther 18:31–37
Morgenthaler NG, Struck J, Alonso C et al (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52(1):112–119
Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93:1137–1146
Neuhold S, Huelsmann M, Strunk G et al (2008) Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol 52:266–272
Ohnishi A, Orita Y, Okahara R et al (1993) Potent aquaretic agent: a novel nonpeptide selective vasopressin 2 antagonist (OPC-31260) in men. J Clin Invest 92:2653–2659
Packer M, Lee WH, Kessler PD et al (1987) Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 75:IV80–IV92
Peri A (2013) Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab 98:1321–1332
Pitt B, Zannad F, Remme WJ et al (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717
Robinson FH, Farr LE (1940) The relation between clinical edema and the excretion of an antidiuretic substance in the urine. Ann Intern Med 14:42
Schrier RW, Berl T, Anderson RJ (1979) Osmotic and nonosmotic control of vasopressin release. Am J Physiol 236:F321–F332
Schrier RW, Gross P, Gheorghiade M et al (2006) Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355:2099–2112
Serradeil-Le Gal C, Lacour C, Valette G et al (1996) Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest 98:2729–2738
SOLVD (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325(5):293–302
Stoiser B, Mörtl D, Hülsmann M et al (2006) Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest 36:771–778
Szatalowicz VL, Arnold PE, Chaimovitz C et al (1981) Radio-immunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med 305:263–266
Tahara A, Tomura Y, Wada K-I et al (1997) Pharmacological profile of YM087, a novel potent nonpeptide vasopressin V1A and V2 receptor antagonist, in vitro and in vivo. J Pharmacol Exp Ther 282:301–308
Udelson JE, Smith WB, Hendrix GH et al (2001) Acute hemodynamic effects of conivaptan, a dual V1A and V2 vasopressin receptor antagonist, in patients with advanced heart failure. Circulation 104:2417–2423
Udelson JE, McGrew FA, Flores E et al (2007) Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 49:2151–2159
Udelson JE, Orlandi C, Ouyang J et al (2008) Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction. J Am Coll Cardiol 52:1540–1545
Udelson JE, Bilsker M, Hauptman PJ et al (2011) A multi-center, randomized, double-blind, placebo-controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail 17:973–981
Verbalis JG (2006) Whole-body volume regulation and escape from antidiuretics. Am J Med 119:S21–S29
Yamamura Y, Nakamura S, Itoh S et al (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287:860–867
Yamane Y (1968) Plasma ADH level in patients with chronic congestive heart failure. Jpn Circ J 32:745–759
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix: Main Study Characteristics and Results of the Vasopressin Receptor Antagonist Clinical Trials
Appendix: Main Study Characteristics and Results of the Vasopressin Receptor Antagonist Clinical Trials
Study | Design | Treatment | Target receptor | Patient characteristics | Effect on primary (important secondary) endpoints |
|---|---|---|---|---|---|
Creager et al. (1986), n = 10 | Controlled | iv V1a RA of 0.5 mg administered over 5 min % diuretics 24 h prior to experiment | V1A | Chronic NYHA III–IV average EF = 24 ± 10% only men | ↓ SVR and ↑ CI when baseline AVP was above normal |
Udelson et al. (2001), n = 142 | Multicenter trial with a baseline inpatient phase, and a randomized, double-blind treatment phase | Single dose iv conivaptan (10, 20, or 40 mg) vs. placebo in a 1:1:1:1 ratio + standard therapy | V1A/V2 | Chronic NYHA III–IV EF ≤ 40% | ↓ PCWP, ↓ RAP and dose-dependent ↑ in urine output in the treatment group vs. placebo no difference in CI, SVR, PVR, BP, HR between the groups |
The ACTIV in CHF study (Gheorghiade et al. 2004), n = 319 | Multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging, phase 2 feasibility trial | Oral tolvaptan OD (30, 60, or 90 mg) vs. placebo in a 1:1:1:1 ratio up to 60 days + standard therapy | V2 | Acute/chronic NYHA III–IV EF ≤ 40% | Day 1+ at discharge: non-dose-dependent ↓ BW and ↑ urine volume in the treatment group vs. placebo60-day mortality: trend toward ↓ mortality in the treatment group |
The EVEREST clinical status trials (Gheorghiade et al. 2007) trial A: n = 2,048, trial B: n = 2,085 | Multicenter, randomized, double-blind, placebo-controlled, phase 3 | Oral tolvaptan 30 mg OD vs. placebo in a 1:1 ratio for minimum 60 days + standard therapy | V2 | Acute NYHA III–IV EF ≤ 40% | At day 7/discharge: ↓ BW, ↑ Na+, ↓ dyspnea, and ↓ edema in the treatment group vs. placebo general clinical status did not differ between the groups |
The EVEREST outcome trial (Konstam et al. 2007), trials A and B: n = 4,133 | Multicenter, randomized, double-blind, placebo-controlled, phase 3 | Oral tolvaptan 30 mg OD vs. placebo in a 1:1 ratio for minimum 60 days + standard therapy | V2 | Acute NYHA III–IV EF ≤ 40% | No difference in mortality or re-hospitalization between groups KCCQ summary score not improved at outpatient week 1 sustained ↓ BW and ↑ Na+ in the treatment group |
The METEOR trial (Udelson et al. 2007), n = 240 | Multicenter, randomized, double-blind, placebo-controlled | Oral tolvaptan 30 mg OD vs. placebo in a 1:1 ratio for 1 year + standard therapy | V2 | Chronic NYHA II–III EF ≤ 30% | No effect on LV remodeling, although small ↓ LVEDV index in the treatment group vs. placebo (no significant between group difference) favorable effect of treatment on mortality/HF hospitalization |
The ECLIPSE trial (Udelson et al. 2008), n = 181 | International, multicenter, randomized, placebo-controlled | Oral tolvaptan 15, 30, or 60 mg OD vs. placebo in a 1:1 ratio | V2 | Acute NYHA II–III EF ≤ 30% | ↓ PCWP, ↓ PAP (all treatment groups), ↓ RAP (tolvaptan 15 and 30 mg) vs. placebo dose-dependent ↑ in urine output no difference in CI, PVR, SVR between groups |
Goldsmith et al. (2008), n = 170 | Multicenter, double-blind, dose-ranging pilot study | iv loading dose of conivaptan 20 mg, followed by two successive 24 h continuous infusions of 40, 80, or 120 mg/day vs. placebo in a 1:1:1:1 ratio+ standard therapy | V1A/V2 | Acute pulmonary congestion, respiratory symptoms NYHA III–IV mean (SD) EF = 29.5% (15.6) | ↑ urine output, ↓ BW in the treatment group vs. placebo no difference in worsening HF, respiratory status at 48 hours, or electrolyte disturbances between the groups |
Mao et al. (2009), n = 203 | Multicenter, 4-day open-label | iv loading dose over 30 min of conivaptan 20 mg, followed by a continuous 4-day infusion of 20 or 40 mg/day | V1A/V2 | CHF (n = 90) s-Na+ ≤ 130 mmol/L euvolemic- or hypervolemic-hyponatremia | Conivaptan concentrations: highest after 30 min loading dose, declined during day 1, and were maintained by the continuous infusion no difference with regard to status of volume or CHF |
Udelson et al. (2011), n = 83 | Multicenter, randomized, double-blind, placebo-controlled, parallel group | Oral tolvaptan 30 mg, furosemide 80 mg, or tolvaptan 30 mg + furosemide 80 mg vs. placebo in a 1:1:1:1 ratio + standard therapy | V2 | Acute: signs of congestion (edema, rales) NYHA II–III EF ≤ 40% | Day 8: ↓ BW in all treatment groups, and ↑ urine volume in the two treatment groups with tolvaptan vs. placebo ↑ Na+ within the normal range, no change in K+ or BP in the group with tolvaptan |
Goldsmith et al. (2011), n = 8 | Randomized, cross-over study | Three separate study days separated with a 7-day washout periods: iv furosemide (<80 mg), iv loading dose of conivaptan 20 mg, followed by a continuous infusion at 1.2 mg/h for 4 h, or the combination | V1A/V2 | Chronic NYHA II–III EF ≤ 23 ± 7% only men | ↑ urine volume in all treatment groups and ↑ urinary Na+ excretion with furosemide therapy (combination > monotherapy) no difference in hemodynamics, neurohormonal levels, renal blood flow, or GFR |
The DILIPO study (Aronson et al. 2011), n = 118 | Multicenter, randomized, double-blind, placebo-controlled phase; followed by a 1-year open-label non-comparative phase | Oral satavaptan OD (25 or 50 mg) vs. placebo in a 1:1:1 ratio up to 4 days, followed by satavaptan 12.5–25 mg + fluid restriction 1–1.5 L | V2 | Chronic (n = 90) NYHA III–IV s-Na+ = 115–132 mmol/L | Higher ↑ in Na+, ↓ time to response, and ↑ BW reduction in the treatment group vs. placebo similar results in the CHF subgroup the increase in Na+ was maintained long-term |
Mondritzki et al. (2011), n = 6 | Randomized, controlled | After iv infusion of AVP to keep its levels controlled, randomization to either conivaptan or tolvaptan, both iv 0.1 mg/kg bolus | V2 vs. V1A/V2 | Mongrel dogs were paced continuously at 220 beats/min after 14 days they underwent acute testing | ↑ CO and ↓ SVR with conivaptan ↓ CO and ↑ SVR with tolvaptan no difference in effect on MAP, dP/dtmax, CVP, and urine output between the groups |
Ghali et al. (2012), n = 170 | Multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 2 | Oral lixivaptan 100 mg OD vs. placebo in a 2:1 ratio for 8 weeks + standard therapy | V2 | Chronic NYHA II–III EF < 40% (n = 57%) EF ≥ 40% (n = 43%) | Day 1 + weeks 1, 2, 4: ↓ BW in the treatment group vs. placebo improvement in orthopnea and dyspnea in the treatment group |
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Vishram-Nielsen, J.K., Gustafsson, F. (2017). Vasopressin and Vasopressin Antagonists in Heart Failure. In: Bauersachs, J., Butler, J., Sandner, P. (eds) Heart Failure. Handbook of Experimental Pharmacology, vol 243. Springer, Cham. https://doi.org/10.1007/164_2017_28
Download citation
DOI: https://doi.org/10.1007/164_2017_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59658-7
Online ISBN: 978-3-319-59659-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)