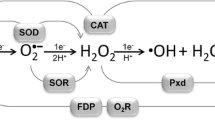
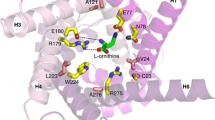
Abstract
The authors wish to dedicate this article to the memory of Professor Eraldo Antonini, who died of an incurable illness on 19 March 1983. It was Professor Antonini who introduced the authors to the study of oxygen-carrying proteins and inspired their scientific activity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ackers, G. K. (1980) Biophys. J. 32, 331.
Adair, G. S. (1925) J. Biol. Chem. 63, 529.
Alberding, N., Lavalette, D. and Austin, R. H. (1981) Proc. Natl Acad. Sci. USA 78, 2307.
Amiconi, G., Antonini, E., Brunori, M., Formaneck, H. and Huber, R. (1972) Eur. J. Biochem. 31, 52.
Amiconi, G., Antonini, E., Brunori, M., Wyman, J. and Zolla, L. (1981) J. Mol. Biol. 152, 111.
Andersen, M. E., Moffat, J. K. and Gibson, Q. H. (1971) J. Biol. Chem. 246, 2796.
Anderson, L. (1975) J. Mol. Biol. 94, 33.
Antonini, E. and Brunori, M. (1969) J. Biol. Chem. 244, 3909.
Antonini, E. and Brunori, M. (1971) Hemoglobin and Myoglobin in their Reaction with Ligands. Elsevier/North-Holland, Amsterdam.
Antonini, E. and Chiancone, E. (1977) Ann. Rev. Biophys. Bioengng. 6, 239.
Antonini, E., Wyman, J., Rossi-Fanelli, A. and Caputo, A. (1962) J. Biol. Chem. 237, 2773.
Antonini, E., Wyman, J., Brunori, M., Bucci, E., Fronticelli, C. and Rossi-Fanelli, A. (1963) J. Biol. Chem. 238, 2950.
Antonini, E., Brunori, M., Colosimo, A., Kuiper, H. A. and Zolla, L. (1983) Biophys. Chem. 18, 117.
Arisaka, F. and van Holde, K. E. (1979) J. Mol. Biol. 134, 41.
Arnone, A. (1972) Nature, Lond. 237, 146.
Asakura, T. and Sono, M. (1974) J. Biol. Chem. 249, 7087.
Bacci, M., Cerdonio, M. and Vitale, S. (1979) Biophys. Chem. 10, 113.
Baldwin, J. M. (1975) Prog. Biophys. Molec. Biol. 29, 225.
Baldwin, J. M. (1980) J. Mol. Biol. 136, 103.
Baldwin, J. M. and Chothia, C. (1979) J. Mol. Biol. 129, 175.
Barlow, C. H., Maxwell, J. C., Wallace, W. J. and Caughey, W. S. (1973) Biochim. Biophys. Res. Commun. 55, 91.
Barra, D., Bossa, F. and Brunori, M. (1981) Nature, Lond. 293, 587.
Bateman, H. (1910) Proc. Cambridge Phil. Soc. 15, 423.
Bates, G., Brunori, M., Amiconi, G., Antonini, E. and Wyman, J. (1968) Biochemistry 7, 3016.
Bauer, C., Forster, M., Gros, G., Mosca, A., Perrella, M., Rollema, H. S. and Vogel, D. (1981) J. Biol. Chem. 256, 8429.
Benesch, R. and Benesch, R. E. (1967) Biochim. Biophys. Res. Commun. 26, 162.
Binotti, I., Giovenco, S., Giardina, B., Antonini, E., Brunori, M. and Wyman, J. (1971) Arch. Biochem. Biophys. 142, 274.
Blake, C. (1983) Trends Biochem. Sci. 8, 11.
Bolton, W. and Perutz, M. F. (1970) Nature, Lond. 228, 551.
Bonaventura, C., Sullivan, B., Bonaventura, J. and Bourne, S. (1974) Biochemistry 13, 4784.
Bonaventura, J., Bonaventura, C., Brunori, M., Giardina, B., Antonini, E., Bossa, F. and Wyman, J. (1974) J. Mol. Biol. 82, 499.
Bonaventura, J., Bonaventura, C., Sullivan, B., Ferruzzi, G., McCurdy, P. R., Fox, J. and Moo-Penn, W. F. (1976) J. Biol. Chem. 251, 7563.
Brix, O., Lykkeboe, G. and Johansen, K. (1979) J. Comp. Physiol. 129, 97.
Brouwer, M. and Kuiper, H. A. (1973) Eur. J. Biochem. 35, 428.
Brouwer, M., Bonaventura, C. and Bonaventura, J. (1978) Biochemistry 11, 2148.
Brouwer, M., Wolters, M. and van Bruggen, E. F. J. (1979) Arch. Biochem. Biophys. 193, 487.
Brown, J. M., Powers, L., Kincaid, B., Larrabee, J. A. and Spiro, T. G. (1980) J. Am. Chem. Soc. 102, 4210.
Brunori, M. (1971) J. Mol. Biol. 55, 39.
Brunori, M. (1975) Curs. Topics Cell. Reg. 9, 1.
Brunori, M. and Schuster, T. M. (1969) J. Biol. Chem. 214, 4046.
Brunori, M., Noble, R. W., Antonini, E. and Wyman, J. (1966) J. Biol. Chem. 241, 5238.
Brunori, M., Antonini, E., Wyman, J. and Anderson, S. R. (1968) J. Mol. Biol. 34, 357.
Brunori, M., Antonini, E., Phelps, C. and Amiconi, G. (1969) J. Mol. Biol. 44, 563.
Brunori, M., Falcioni, G., Fioretti, E., Giardina, B. and Rotilio, G. (1975) Eur. J. Biochem. 53, 99.
Brunori, M., Coletta, M., Giardina, B. and Wyman, J. (1978) Proc. Natl Acad. Sci. USA 75, 4310.
Brunori, M., Kuiper, H. A., Antonini, E., Bonaventura, C. and Bonaventura, J. (1981a) in Invertebrate Oxygen-binding Proteins: Structure, Active Site and Function (Lamy, J. and Lamy, J., eds). Marcel Dekker, New York, p. 693.
Brunori, M., Zolla, L., Kuiper, H. A. and Finazzi-Agrò, A. (1981b) J. Mol. Biol. 153, 1111.
Brunori, M., Giardina, B. and Kuiper, H. A. (1982a) in Inorganic Biochemistry, Vol. III. (H. A. O. Hill, ed.). Royal Society of Chemistry, London, p. 126.
Brunori, M., Kuiper, H. A. and Zolla, L. (1982b) EMBO J. 1, 329.
Bucci, E., Salahuddin, A., Bonaventura, J. and Bonaventura, C. (1978) J. Biol. Chem. 253, 821.
Bunn, H. F., Wohl, R. C., Bradley, T. B., Cooley, M. and Gibson, Q. H. (1974) J. Biol. Chem. 249, 7402.
Case, D. A., Huynh, B. H. and Karplus, M. (1979) J. Am. Chem. Soc. 101, 4433.
Cassoly, R., Gibson, Q. H., Ogawa, S. and Shulman, R. G. (1971) Biochim. Biophys. Res. Commun. 44, 1015.
Cerdonio, M., Congiu-Castellano, A., Calabrese, L., Morante, S., Pispisa, B. and Vitale, S. (1978) Proc. Natl Acad. Sci. USA 75, 4916.
Cerdonio, M., Morante, S., Torrosini, D., Vitale, S., De Young, A. and Noble, R. W. (1984) Proc. Natl Acad. Sci. USA in press.
Chang, C. K. and Traylor, T. G. (1973) J. Am. Chem. Soc. 95, 5810.
Chang, C. K. and Traylor, T. G. (1975) Proc. Natl Acad. Sci. USA 72, 1166.
Chiancone, E., Giardina, B., Brunori, M. and Antonini, E. (1973) in Comparative Physiology (Bolis, L., Schmidt-Nielsen, K. and Maddrell, S. H. P., eds). Elsevier/North-Holland, Amsterdam.
Chiancone, E., Vecchini, P., Verzili, D., Ascoli, F. and Antonini, E. (1981a). J. Mol. Biol. 152, 577.
Chiancone, E., Ferruzzi, G., Bonaventura, C. and Bonaventura, J. (1981b) Biochim. Biophys. Acta 670, 84.
Ching Ming Chung, M. and Ellerton, H. D. (1979) Prog. Biophys. Molec. Biol. 35, 53.
Chothia, C., Wodak, S. and Janin, J. (1976) Proc. Natl Acad. Sci. USA 73, 3793.
Chu, A. H. and Ackers, G. K. (1981) J. Biol. Chem. 256, 1199.
Collman, J. P., Gagné, R. R., Reed, C. A., Halbert, T. R., Lang, G. and Robinson, W. T. (1975) J. Am. Chem. Soc. 97, 1427.
Collman, J. P., Brauman, J. I., Doxsee, K. M., Halbert, T. R. and Suskick, K. S. (1978) Proc. Natl Acad. Sci. USA 75, 564.
Collman, J. P., Brauman, J. I. and Doxsee, K. M. (1979) Proc. Natl Acad. Sci. USA 76, 6035.
Colosimo, A., Brunori, M. and Wyman, J. (1974) Biophys. Chem.2, 338.
Colosimo, A., Brunori, M. and Wyman, J. (1977) in Structure and Function of Haemocyanins (Bannister, J. V., ed.). Springer-Verlag, Berlin, p. 189.
Craik, C. S., Buchanan, S. R. and Beychok, S. (1980) Proc. Natl Acad. Sci. USA 77, 1384.
Craik, C. S., Buchanan, S. R. and Beychok, S. (1981) Nature, Lond. 291, 87.
Dawson, J. W., Gray, H. B., Hoenig, H. E., Rossman, G. R., Schredder, J. M. and Wang, R. H. (1972) Biochemistry 11, 461.
Deatherage, J. F., Loe, R. S., Anderson, C. M. and Moffat, K. (1976) J. Mol. Biol. 104, 687.
De Bruin, S. H. and Janssen, L. H. M. (1973) J. Biol. Chem. 248, 2774.
De Bruin, S. H., Rollema, H. S., Janssen, L. H. M. and van Os, G. A. J. (1974) Biochim. Biophys. Res. Commun. 58, 210.
de Phillips, H. A. (1971) Arch. Biochem. Biophys. 144, 122.
de Waal, D. J. A. and Wilkins, R. G. (1976) J. Biol. Chem. 251, 2339.
Dickinson, J. C. and Chien, J. C. W. (1973) J. Biol. Chem. 248, 5005.
Dunn, J. B. R., Addison, A. W., Bruce, R. E., Loehr, J. S. and Loehr, T. M. (1977) Biochemistry 16, 1743.
Eaton, W. A. (1980) Nature, Lond. 284, 183.
Economides, A. P. and Wells, R. M. G. (1975) Comp. Biochem. Physiol. A51, 219.
Eickman, N. C., Himmelwright, R. S. and Solomon, E. I. (1979) Proc. Natl Acad. Sci. USA 76, 2094.
Eisenberger, P., Shulman, R. G., Kincaid, B. M., Brown, G. S. and Ogawa, S. (1978) Nature, Lond. 274, 30.
Er-el, Z., Shaklai, N. and Daniel, E. (1972) J. Mol. Biol. 64, 341.
Fabry, T. L., Simo, C. and Javaherian, K. (1968) Biochim. Biophys. Acta 160, 118.
Fager, L. Y. and Alben, J. O. (1972) Biochemistry 11, 4786.
Fermi, G. (1975) J. Mol. Biol. 97, 237.
Fermi, G., Perutz, M. F., Dickinson, L. C. and Chien, J. C. W. (1982) J. Mol. Biol. 155, 495.
Flanagan, M. A., Ackers, G. K., Matthew, J. B., Hanania, G. I. H. and Gurd, F. R. N. (1981) Biochemistry 20, 7439.
Freedman, T. B., Loehr, J. S. and Loehr, T. M. (1976) J. Am. Chem. Soc. 98, 2809.
Friedman, J. M., Rousseau, D. L., Ondrias, M. R. and Stepnoski, R. A. (1982) Science 218, 1244.
Fung, L. W.-M. and Ho, C. (1975) Biochemistry 14, 2526.
Garbett, K., Darnall, D. W., Klotz, I. M. and Williams, R. J. P. (1969) Arch. Biochem. Biophys. 135, 419.
Garlick, R. L. and Riggs, A. F. (1982) J. Biol. Chem. 257, 9005.
Garlick, R. L. and Terwilliger, R. C. (1977) Comp. Biochem. Physiol. 57B, 177.
Gay, R. R. and Solomon, E. I. (1978) J. Am. Chem. Soc. 100, 1973.
Gelin, B. R. and Karplus, M. (1977) Proc. Natl Acad. Sci. USA 74, 801.
Giacometti, G. M., Traylor, T. G., Ascenzi, P., Brunori, M. and Antonini, E. (1977) J. Biol. Chem. 252, 7447.
Giardina, B., Brunori, M., Binotti, I., Giovenco, S. and Antonini, E. (1973) Eur. J. Biochem. 39, 571.
Giardina, B., Chiancone, E. and Antonini, E. (1975) J. Mol. Biol. 93, 1.
Gibson, Q. H. (1959) Biochem. J. 71, 293.
Gielens, C., Préaux, G. and Lontie, R. (1973) Arch. Int. Physiol. Biochim. 81, 182.
Gielens, C., Préaux, G. and Lontie, R. (1975) Eur. J. Biochem. 60, 271.
Gilbert, W. (1979) in Eukaryotic Gene Regulation ICN-UCLA Symp. Molecular and Cell Biology 14 (1). Academic Press, New York.
Gill, S. J., Gaud, H. T. and Barisas, B. G. (1980) J. Biol. Chem. 255, 7855.
Gò, M. (1981) Nature, Lond. 291, 90.
Griffith, J. S. (1956) Proc. R. Soc. Lond. Ser. A 235, 23.
Gros, G., Rollema, H. S. and Forster, R. E. (1981) J. Biol. Chem. 256, 5471.
Guidotti, G. (1967) J. Biol. Chem. 242, 3685.
Gurd, F. R. N., Matthew, J. B., Wittebort, R. J., Morrow, J. S. and Friend, S. H. (1980) in Biophysics and Physiology of CO2 (Bauer, C., Gros, G. and Bartels, H., eds). Springer-Verlag, Berlin, p. 89.
Hendrickson, W. A. (1978) Naval Res. Reviews 31, 1.
Herman, Z. S. and Loew, G. H. (1980) J. Am. Chem. Soc. 102, 1815.
Hewitt, J. A., Kilmartin, J. V., Ten Eyck, L. F. and Perutz, M. F. (1972) Proc. Natl Acad. Sci. USA 69, 203.
Hill, A. V. (1910) J. Physiol. 40,
Hoffman, R., Chen, M. M.-L. and Thorn, D. L. (1977) Inorg. Chem. 16, 503.
Hopfield, J. J., Shulman, R. G. and Ogawa, S. (1971) J. Mol. Biol. 61, 425.
Hoylaert, M., Préaux, G., Witters, R. and Lontie, R. (1979) Arch. Int. Physiol. Biochim. 87, 417.
Imai, K. (1979) J. Mol. Biol. 133, 233.
Imai, K. and Yonetani, T. (1975) J. Biol. Chem. 250, 2227.
Imai, K., Ikeda-Saito, M., Yamamoto, H. and Yonetani, T. (1980) J. Mol. Biol. 138, 635.
Imaizumi, K., Imai, K., and Tyuma, I. (1982) J. Mol. Biol. 159, 703.
Ip, S. H. C. and Ackers, G. K. (1977) J. Biol. Chem. 252, 82.
Jameson, G. B., Molinaro, F. S., Ibers, J. A., Collman, J. P., Brauman, J. I., Rose, E. and Suslick, K. S. (1978) J. Am. Chem. Soc. 100, 6769.
Jameson, G. B., Molinaro, F. S., Ibers, J. A., Collman, J. P., Brauman, J. I., Rose, E. and Suslick, K. S. (1980) J. Am. Chem. Soc. 102, 3224.
Janin, J. and Wodak, S. J. (1984) EMBO J. in press.
Kellett, G. L. (1971) J. Mol. Biol. 59, 401.
Kilmartin, J. V. (1974) FEBS Lett. 38, 147.
Kilmartin, J. V., Breen, J. J., Roberts, G. C. K. and Ho, C. (1973a) Proc. Natl Acad. Sci. USA 70, 1246.
Kilmartin, J. V., Fogg, J., Luzzana, M. and Rossi-Bernardi, L. (1973b) J. Biol. Chem. 248, 7039.
Kilmartin, J. V., Hewitt, J. A. and Wootton, J. F. (1975) J. Mol. Biol. 93, 203.
Kilmartin, J. V., Anderson, N. L. and Ogawa, S. (1978) J. Mol. Biol. 123, 71.
Kilmartin, J. V., Fogg, J. H. and Perutz, M. F. (1980) Biochemistry 19, 3189.
Klarman, A. and Daniel, E. (1980) Biochemistry 19, 5176.
Konkel, D. A., Tilghmann, S. M. and Leder, P. (1978) Cell 15, 1125.
Koshland, D. E., Nemethy, G. and Filmer, D. (1966) Biochemistry 5, 365.
Koster, A. S. (1975) J. Chem. Phys. 63, 3284.
Kuiper, H. A., Torensma, R. and van Bruggen, E. F. J. (1976) Eur. J. Biochem. 68, 425.
Kuiper, H. A., Antonini, E. and Brunori, M. (1977) J. Mol. Biol. 116, 569.
Kuiper, H. A., Brunori, M. and Antonini, E. (1978) Biochim. Biophys. Res. Commun. 82, 1062.
Kuiper, H. A., Forlani, L., Chiancone, E., Antonini, E., Brunori, M. and Wyman, J. (1979) Biochemistry 18, 5849.
Kuiper, H. A., Coletta, M., Zolla, L., Chiancone, E. and Brunori, M. (1980a) Biochim. Biophys. Acta 626, 412.
Kuiper, H. A., Finazzi-Agrò, A., Antonini, E. and Brunori, M. (1980b) Proc. Natl Acad. Sci. USA 77, 2387.
Kurtz, A., Rollema, H. S. and Bauer, C. (1981) Arch. Biochem. Biophys. 210, 200.
Kurtz, D. M., Shriver, D. F. and Klotz, I. M. (1977) Coord. Chem. Revs 24, 145.
Kwiatkowski, L. D. and Noble, R. W. (1982) J. Biol. Chem. 257, 8891.
La Mar, G. N. and de Ropp, J. S. (1982) J. Am. Chem. Soc. 104, 5203.
Lamy, J., Lamy, J. and Weill, J. (1979a) Arch. Biochem. Biophys. 193, 140.
Lamy, J., Lamy, J. and Weill, J. (1979b) Arch. Biochem. Biophys. 196. 324.
Lang, G. and Marshall, W. (1966) Proc. Phys. Soc. London 87, 3.
Langerman, N. and Sturtevant, J. M. (1971) Biochemistry 10, 2809.
Lerch, K. (1981) in Metal Ions in Biological Systems (Sigel, H., ed.). Marcel Dekker, New York, p. 143.
Loehr, J. S. and Loehr, T. M. (1979) in Advances in Inorganic Biochemistry Vol. I (Eichhorn, G. L. and Marzilli, L. O. eds). Elsevier/North Holland, Amsterdam, pp. 235–52.
Loehr, J. S., Freedman, T. B. and Loehr, T. M. (1974) Biochim. Biophys. Res. Commun. 56, 510.
Loehr, J. S., Lammers, P. J., Brimhall, B. and Hermandson, M. A. (1978a) Biochemistry 7, 3868.
Loehr, J. S., Lammers, P. J., Brimhall, B. and Hermandson, M. A. (1978b) J. Biol. Chem. 253, 5726.
Lontie, R. and Witters, R. (1981) in Metal Ions in Biological Systems (Sigel, H., ed.). Marcel Dekker, New York, p. 229.
McConnell, H., Deal, W. J. and Ogata, R. G. (1969) Biochemistry 8, 2580.
Mangum, C. P. and Kondon, M. (1975) Comp. Biochem. Physiol. 50A, 777.
Manwell, C. (1960) Science 132, 550.
Matthew, J. B., Hanania, G. I. H. and Gurd, F. R. N. (1979) Biochemistry 18, 1928.
Mellema, J. E. and Klug, A. (1972) Nature, Lond. 239, 145.
Michalowicz, A., Pin, S. and Alpert, B. (1983) Biophys. J. 41, 413a.
Miller, K. and van Holde, K. E. (1974) Biochemistry 13, 1668.
Mills, F. C. and Ackers, G. K. (1979) Proc. Natl Acad. Sci. USA 76, 273.
Misra, H. P. and Fridovich, I. (1972) J. Biol. Chem. 247, 6960.
Moffat, J. K. (1971) J. Mol. Biol. 55, 135.
Moffat, K., Deatherage, J. F. and Seybert, D. W. (1979) Science 206, 1035.
Monod, J., Wyman, J. and Changeux, J.-P. (1965) J. Mol. Biol. 12, 88.
Moss, T. H., Maleski, C. and York, J. L. (1971) Biochemistry 10, 840.
Nagai, K. and Kitagawa, T. (1980) Proc. Natl Acad. Sci. USA 77, 2033.
Nagai, K., La Mar, G. N., Jue, T. and Bunn, H. F. (1982) Biochemistry 21, 842.
Nickerson, K. W. and van Holde, K. E. (1971) Comp. Biochem. Physiol. 39, 855.
Nigen, A. M. and Manning, J. M. (1975) J. Biol. Chem. 250, 8248.
Nigen, A. M., Manning, J. M. and Alben, J. O. (1980) J. Biol. Chem. 255, 5525.
Nishikura, K. (1978) Biochem. J. 173, 671.
Noble, R. W., Gibson, Q. H., Brunori, M., Antonini, E. and Wyman, J. (1969) J. Biol. Chem. 241, 3905.
Noble, R. W., Parkhurst, L. J. and Gibson, Q. H. (1970) J. Biol. Chem. 245, 6628.
Norne, J.-E., Chiancone, E., Forsén, S., Antonini, E. and Wyman, J. (1976) FEBS Lett. 94, 410.
O’Donnell, S., Mandaro, R., Schuster, T. M. and Arnone, A. (1979) J. Biol. Chem. 254, 12204.
Ogawa, S. and Shulman, R. G. (1971) Biochim. Biophys. Res. Commun. 42, 9.
O’Hagen, J. E. (1961) in Haematin Enzymes. Pergamon Press, London, p. 173.
Ohnoki, S., Mitomi, T., Hata, R. and Satake, K. (1973) J. Biochem. 73, 717.
Okamura, M. Y., Klotz, J. M., Johnson, C. E., Winter, M. R. C. and Williams, R. J. P. (1969) Biochemistry 8, 1951.
Okamura, M. Y. and Klotz, I. M. (1973) in Inorganic Biochemistry (Eichhorn, G. L., ed.). Elsevier, New York.
Olafson, B. D. and Goddard III, W. A. (1977) Proc. Natl Acad. Sci. USA 74, 1315.
Parkhurst, L. J. (1979) Ann. Rev. Phys. Chem. 30, 503.
Pauling, L. and Coryell, C. D. (1936) Proc. Natl Acad. Sci. USA 22, 210.
Perrella, M., Kilmartin, J. V., Fogg, J. and Rossi-Bernardi, L. (1975) Nature, Lond. 256, 759.
Perutz, M. F. (1970) Nature, Lond. 228, 726.
Perutz, M. F. (1972) Nature, Lond. 237, 495.
Perutz, M. F. (1978) Science 201, 1187.
Perutz, M. F. (1979) Ann. Rev. Biochem. 48, 327.
Perutz, M. F. (1980) Proc. R. Soc. Lond. Series B 208, 135.
Perutz, M. F. (1984) Adv. Prot. Chem. in press.
Perutz, M. F. and Brunori, M. (1982) Nature, Lond. 299, 421.
Perutz, M. F. and Ten Eyck, L. F. (1971) Symp. Quant. Biol. XXXVI, 295.
Perutz, M. F., del Pulsinelli, P., Ten Eyck, L., Kihnartin, J. V., Shibata, S., Iucki, I., Miyaji, T. and Hamilton, H. B. (1971) Nature New Biol. 232, 147.
Perutz, M. F., Ladner, J. E., Simon, S. R. and Ho, C. (1974a) Biochemistry 13, 2163.
Perutz, M. F., Heidner, E. J., Ladner, J. E., Beetlestone, J. G., Ho, C. and Slade, E. F. (1974b) Biochemistry 13, 2187.
Perutz, M. F., Kilmartin, J. V., Nishikura, K., Fogg, J. H., Butler, P. J. G. and Rollema, H. S. (1980) J. Mol. Biol. 138, 649.
Perutz, M. F., Bauer, C., Gros, G., Leclercq, F., Vandecasserie, C., Schnek, A. G., Braunitzer, G., Friday, A. E. and Joysey, K. A. (1981) Nature, Lond. 291, 682.
Pettigrew, D. W., Romeo, P. H., Tsapis, A., Thillet, J., Smith, M. L., Turner, B. W. and Ackers, G. K. (1982) Proc. Natl Acad. Sci. USA 79, 1849.
Phillips, S. E. V. (1980) J. Mol. Biol. 142, 531.
Philo, J. S., Dreyer, U. and Schuster, T. M. (1984) Biochemistry 23, 865.
Poyart, C., Bursaux, E., Bohn, B. and Guesnon, P. (1980) Biochim. Biophys. Acta 626, 417.
Reed, C. A. and Cheung, S. K. (1977) Proc. Natl Acad. Sci. USA 74, 1780.
Reed, C. A., Mashiko, T., Scheidt, W. R., Spartalian, K. and Lang, G. (1980) J. Am. Chem. Soc. 102, 2302.
Roche, J., Bessis, M. and Thiery, J. P. (1960) Biochim. Biophys. Acta 41, 182.
Rollema, H. S., De Bruin, S. H., Janssen, L. H. M. and van Os, G. A. J. (1975) J. Biol. Chem. 250, 1333.
Rollema, H. S., Gros, G. and Bauer, C. (1980) J. Biol. Chem. 255, 2756.
Root, R. W. (1931) Biol. Bull. (Woods Hole, Mass.) 61, 427.
Rossi-Fanelli, A. and Antonini, E. (1959) Arch. Biochem. Biophys. 80, 269.
Rossi-Fanelli, A., Antonini, E. and Caputo, A. (1961) J. Biol. Chem. 236, 391.
Roughton, F. J. W. (1949) in Hemoglobin. Butterworth, London, p. 83.
Roughton, F. J. W. and Lyster, R. L. J. (1965) Hvalradets Skr. 48, 185.
Rubin, M. M. and Changeux, J.-P. (1966) J. Mol. Biol. 21, 265.
Russu, I. M., Tseng Ho, N. and Ho, C. (1980) Biochemistry 19, 1043.
Salhany, J. M., Castillo, G. L. and Ogawa, S. (1976) Biochemistry 15, 5344.
Sawicki, C. A. and Gibson, Q. H. (1977) J. Biol. Chem. 252, 5783.
Sawicki, J. and Lang, G. (1984) Proc. Natl Acad. Sci. USA in press.
Scholander, P. F. and van Dam, L. (1954) Biol. Bull. (Woods Hole, Mass.) 107, 247.
Schoot-Uiterkamp, A. J. M. and Mason, H. S. (1973) Proc. Natl Acad. Sci. USA 70, 993.
Shaanan, B. (1982) Nature, Lond. 296, 683.
Shelnutt, J. A., Rousseau, D. L., Friedman, J. M. and Simon, S. R. (1979) Proc. Natl Acad. Sci. USA 76, 4409.
Shulman, R. G., Ogawa, S. and Hopfield, J. J. (1975) Quart. Rev. Biophys. 8, 325.
Siezen, R. J. and van Bruggen, E. F. J. (1974) J. Mol. Biol. 90, 91.
Stanford, M. A., Swartz, J. C., Phillips, T. E. and Hoffman, B. M. (1980) J. Am. Chem. Soc. 102, 4492.
Steigemann, W. and Weber, E. (1979) J. Mol. Biol. 127, 309.
Stein, P., Mitchell, M. and Spiro, T. G. (1980) J. Am. Chem. Soc. 102, 7795.
Stenkamp, R. E., Siecker, L. C. and Jensen, L. H. (1976) Proc. Natl Acad. Sci. USA 73, 349.
Stenkamp, R. E., Siecker, L. C., Jensen, L. H. and McQueen, Jr, J. E. (1978) Biochemistry 17, 2499.
Stenkamp, R. E., Siecker, L. C., Jensen, L. H. and Sanders-Loehr, J. (1981) Nature, Lond. 291, 263.
Swaney, J. B. and Klotz, J. M. (1971) Arch. Biochem. Biophys. 147, 475.
Takano, T. (1977) J. Mol. Biol. 110, 569.
Tan, A. L., Noble, R. W. and Gibson, Q. H. (1973) J. Biol. Chem. 248, 2880.
Tan, K. H., Keresztes-Nagy, S. and Frankfater, A. (1975) Biochemistry 14, 4280.
Terwilliger, R. C. (1974) Comp. Biochem. Physiol. 48A, 745.
Terwilliger, R. C., TerwiIliger, N. B., Bonaventura, C. and Bonaventura, J. (1977) Biochim. Biophys. Acta 494, 416.
Thamann, T. J., Loehr, J. S. and Loehr, T. M. (1977) J. Am. Chem. Soc. 99, 4187.
Traylor, T. G. (1981) Acc. Chem. Res. 14, 102.
Traylor, T. G., Deardurff, L. A., Coletta, M., Ascenzi, P., Antonini, E. and Brunori, M. (1983) J. Biol. Chem. 258, 12147.
Tsai, T. E., Groves, J. L. and Wu, C. S. (1981) J. Chem. Phys. 74, 4306.
Tyuma, I. and Ueda, Y. (1975) Biochim. Biophys. Res. Commun. 65, 1278.
van Breemen, J. F. L., Wichterjes, T., Muller, M. F. J., van Driel, R. and van Bruggen, E. F. J. (1975) Eur. J. Biochem. 60, 129.
van Breemen, J. F. L., Ploegman, J. H. and van Bruggen, E. F. J. (1979) Eur. J. Biochem. 100, 61.
van Bruggen, E. F. J. (1978) Ninth Intern. Congr. Electron Microscopy, Toronto, 13, p. 450.
vanden Berg (1978) Nature, Lond. 276, 37.
van der Deen, H. and Hoving, H. (1979) Biophys. Chem. 9, 169.
van Driel, R. (1973) Biochemistry 12, 2696.
van Driel, R., Brunori, M. and Antonini, E. (1974) J. Mol. Biol. 89, 103.
van Driel, R., Kuiper, H. A., Antonini, E. and Brunori, M. (1978) J. Mol. Biol. 121, 431.
van Heel, M. and Frank, J. (1980) in Pattern Recognition in Pratica (Gelsema, E. S. and Kanal, L. N., eds). Elsevier/North-Holland, Amsterdam.
van Holde, K. E. and Miller, K. I. (1982) Quart. Rev. Biophys. 15, 1.
van Holde, K. E. and van Bruggen, E. F. J. (1971) in Biological Macromolecules vol. V (Timasheff, S. N. and Fasman, G. A., eds). Marcel Dekker, New York, p. 1.
van Schaick, E. J. M., Schutter, W. G., Gaykema, W. P. J., van Bruggen, E. F. J. and Hal, W. G. J. (1981) in Invertebrate Oxygen-binding Proteins: Structure, Active Site and Function (Lamy, J. and Lamy, J., eds). Marcel Dekker, New York, p. 353.
Viggiano, G. and Ho, C. (1979) Proc. Natl Acad. Sd. USA 76, 3673.
Vinogradov, S. N., Shlom, J. M., Hall, B. C., Kapp, O. S. and Mizukami, H. (1977) Biochim. Biophys. Acta 492, 136.
Warshel, A. (1977) Proc. Natl Acad. Sci. USA 74, 1789.
Warshel, A. and Weiss, R. M. (1981) J. Am. Chem. Soc. 103, 446.
Waxman, L. (1971) J. Biol. Chem. 246, 7318.
Weiss, J. J. (1964) Nature, Lond. 202, 83.
Wittenberg, J. B. and Wittenberg, B. A. (1962) Nature, Lond. 194, 106.
Wittenberg, J. B., Wittenberg, B. A., Peisach, J. and Blumberg, W. E. (1970) Proc. Natl Acad. Sci. USA 67, 1846.
Wood, E. J. (1980) Essays in Biochem. 16, 1.
Wood, E. J., Cayley, G. R. and Pearson, J. S. (1977) J. Mol. Biol. 109, 1.
Wyman, J. (1948) Adv. Prot. Chem. 4, 407.
Wyman, J. (1964) Adv. Prot. Chem. 19, 224.
Wyman, J. (1969) Nobel Symposia 11, 266.
Wyman, J., Gill, S. T., Gaud, H. T., Colosimo, A., Giardina, B., Kuiper, H. A. and Brunori, M. (1978) J. Mol. Biol. 124, 161.
Yamamoto, H., Kayne, F. J. and Yonetani, T. (1974) J. Biol. Chem. 249, 691.
Yonetani, T., Drott, H. R., Leigh, J. S., Reed, G. H., Waterman, M. R. and Asakura, T. (1970) J. Biol. Chem. 245, 2998.
Yonetani, T., Yamamoto, H. and Woodrow III, G. V. (1974) J. Biol. Chem. 249, 682.
York, J. L. and Bearden, A. J. (1970) Biochemistry 9, 4549.
Zerner, M., Gouterman, M. and Kobayashi, H. (1966) Theoret. Chim. Acta 6, 363.
Zolla, L., Kuiper, H. A., Vecchini, P., Antonini, E. and Brunori, M. (1978) Eur. J. Biochem. 87, 467.
Zolla, L., Kuiper, H. A., Brunori, M. and Antonini, E. (1981) in Invertebrate Oxygen-binding Proteins: Structure, Active Site and Function (Lamy, J. and Lamy, J., eds). Marcel Dekker, New York, p. 719.
Editor information
Editors and Affiliations
Copyright information
© 1985 The contributors
About this chapter
Cite this chapter
Brunori, M., Coletta, M., Giardina, B. (1985). Oxygen carrier proteins. In: Harrison, P.M. (eds) Metalloproteins. Topics in Molecular and Structural Biology. Palgrave Macmillan, London. https://doi.org/10.1007/978-1-349-06375-8_6
Download citation
DOI: https://doi.org/10.1007/978-1-349-06375-8_6
Publisher Name: Palgrave Macmillan, London
Print ISBN: 978-1-349-06377-2
Online ISBN: 978-1-349-06375-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)