Abstract
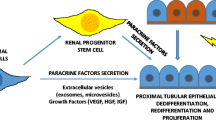
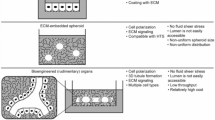
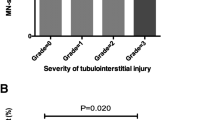
Stem/progenitor cells are in the focus of regenerative medicine for a future therapy of acute and chronic renal failure. However, broad knowledge about parenchymal regeneration in kidney is lacking. For that reason developmental pathways leading from stem/progenitor cells to newly formed tubules have to be investigated. A new technique promotes renal stem/progenitor cells to form numerous tubules between layers of polyester fleeces. This artificial interstitium replaces coating by extracellular matrix proteins, supports spatial extension of renal tubules, and can be used with chemically defined Iscove’s modified Dulbecco’s medium (IMDM) during a long-term culture period of 13 days. The development of tubules is stimulated by aldosterone and depends on the applied hormone concentration. The tubulogenic effect cannot be mimicked by precursors of the aldosterone synthesis pathway or by other steroid hormones. Antagonists such as spironolactone or canrenoate prevent the development of tubules, which indicates that the mineralocorticoid receptor (MR) is involved. Administration of geldanamycin, radicicol, quercetin, or KNK 437 in combination with aldosterone blocks development of tubules by disturbing the contact between MR and heat-shock proteins. Transmission electron microscopy (TEM) further demonstrates that generated tubules exhibit a junctional complex between the apical and the lateral plasma membrane. At the basal aspect a continuously developed basal lamina is present. Immuno-label for Troma I (cytokeratin Endo-A) shows isoprismatic cells, while label for laminin γ1, occludin, and Na/K-ATPase α5 confirms typical features of a polarized epithelium. Finally, the introduced system makes it possible to pile and pave renal stem/progenitor cells, so that the spatial development of tubules can be systematically investigated.
Similar content being viewed by others
References
Mansilla E, Drago H, Sturla F, Bossi S, Salas E, Marin GH, Ibar R, Soratti C. Matrix superhighways configurations: new concepts for complex organ regeneration. Transplant Proc. 2007; 39(7):2431–2433.
Humphreys BD, Bonventre JV. The contribution of adult stem cells to renal repair. Nephrol Ther. 2007; 3:3–10.
Hammerman MR. Cellular therapies for kidney failure. Expert Opin Biol Ther. 2006; 6:87–97.
Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006; 17(1):188–198.
Hogan BL, Koledziej M. Molecular mechanisms of tubulogenesis. Nat Rev. 2002; 3:513–523.
Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell 2003; 112:19–28.
Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol. 2005; 100:40–45.
Walid S, Eisen R, Ratcliffe DR, Dai K, Hussain M, Ojakian GK, The PI. 3-kinase and mTOR signaling pathways are important modulators of epithelial tubule formation. J Cell Physiol. 2008; 216:469–479.
Minuth WW. Neonatal rabbit kidney cortex in culture as tool for the study of collecting duct formation and nephron differentiation. Differentiation 1987; 36:12–22.
Minuth WW. Patent DE 199 52 847. Vorrichtung zum Kultivieren und/oder Differenzieren und/oder Halten von Zellen und/oder Geweben; 2006.
Heber S, Denk L, Minuth WW. Modulating the development of renal tubules growing in serum-free culture medium at an artificial interstitium. Tissue Eng. 2007; 13:281–291.
Hu K, Denk L, de Vries U, Minuth WW. Chemically defined medium environment for the development of renal stem cells into tubules. Biotechnol J. 2007; 2:992–995.
Blattmann A, Denk L, Strehl R, Castrop H, Minuth WW. The formation of pores in the basal lamina of regenerated tubules. Biomaterials 2008; 29(18):2749–2756.
Minuth WW, Denk L, Hu K. The role of polyester interstitium and aldosterone during structural development of renal tubules in serum-free medium. Biomaterials 2007; 28:8–28.
Minuth WW, Denk L, Hu K, Castrop H, Gomez-Sanchez C. Tubulogenic effect of aldosterone is attributed to intact binding and intracellular response of the mineralocorticoid receptor. CEJB 2007; 3:307–325.
Minuth WW, Blattmann A, Denk L, Castrop H. Mineralocorticoid receptor, heat shock proteins and immunophilins participate in the transmission of the tubulogenic signal of aldosterone. J Epithel Biol Pharmacol. 2008; 1:24–34.
Minuth WW, Denk L, Castrop H. Generation of tubular superstructures by piling of renal stem/progenitor cells. Tissue Eng C Methods 2008; 14(1):3–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Minuth, W., Denk, L., Roessger, A. (2011). Regenerating Tubules for Kidney Repair. In: Artmann, G., Minger, S., Hescheler, J. (eds) Stem Cell Engineering. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-11865-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-11865-4_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-11864-7
Online ISBN: 978-3-642-11865-4
eBook Packages: EngineeringEngineering (R0)