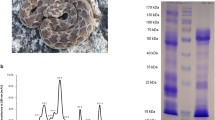
Abstract
India is the home to several venomous snake species which are responsible for the highest number of snake envenomings and deaths in the world. Nevertheless, the ‘Big Four’ species of snakes namely Russell’s viper (Daboia russelii), spectacled cobra (Naja naja), common krait (Bungarus caeruleus), and saw-scaled viper (Echis carinatus) account for most cases of envenoming, morbidity, and mortality primarily in the rural parts of the country. The composition of these snake venoms have been determined using conventional biochemical approaches. However, these studies faced the inevitable limitation of identification of non-enzymatic and low abundance venom toxins. In the last 5 years, mass spectrometry investigations of Indian snake venoms have provided comprehensive information on their venom proteomes. In addition, proteomic analysis has also been employed to identify and characterize snake venom protein complexes and serves as a tool for screening of candidate pharmacologically active drug prototypes from snake venoms. Further, mass spectrometry analysis has also found its way for the identification and quantification of poorly immunogenic venom toxins against commercial antivenoms and this information is of paramount importance for improved design and production of snake antivenoms for better hospital management of snakebite victims. In this review article, we have focused the recent advances in snake venom proteomics research in India that has facilitated the understanding of the pathophysiology of snake envenomation, provided insights for improvement for antivenom production and also discover drug prototypes from snake venom.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- 1D SDS-PAGE:
-
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- 2D SDS-PAGE:
-
Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- 3FTx:
-
Three-finger toxin
- AChE:
-
Acetylcholine esterase
- AChR:
-
Acetylcholine receptor
- AKI:
-
Acute kidney injury
- APase:
-
Aminopeptidase
- ARF:
-
Acute renal failure
- ASPro:
-
Aspartic protease
- ChE:
-
Choline esterase
- CRISP:
-
Cysteine-rich secretory protein
- CTX:
-
Cytotoxin
- CVF:
-
Cobra venom factor
- Cys:
-
Cystatin
- Dis:
-
Disintegrin
- ECV:
-
Echis carinatus venom
- EI:
-
Eastern India
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESI-LC-MS/MS:
-
Electrospray ionization liquid chromatography tandem mass spectrometry
- GC:
-
Glutaminyl cyclase
- GF:
-
Gel filtration
- HhV:
-
Hypnale hypnale venom
- Hya:
-
Hyaluronidase
- kDa:
-
Kilo Dalton
- KSPI:
-
Kunitz-type serine protease inhibitor
- KTX:
-
Kaouthiotoxin
- KV:
-
Krait venom
- LAAO:
-
l-amino acid oxidase
- LNTX:
-
Long neurotoxin
- MALDI:
-
Matrix-assisted laser desorption/ionization
- NCBI:
-
National Center for Biotechnology Information
- NGF:
-
Nerve growth factor
- NI:
-
Northern India
- NkV:
-
Naja kaouthia venom
- NnV:
-
Naja naja venom
- NP:
-
Natriuretic peptide
- NT:
-
5′-Nucleotidase
- OLP:
-
Ohanin-like protein
- PAV:
-
Polyvalent antivenom
- PDE:
-
Phosphodiesterase
- PLA2 :
-
Phospholipase A2
- PLB:
-
Phospholipase B
- RP-HPLC:
-
Reversed phased high-performance liquid chromatography
- RVV:
-
Russell’s viper venom
- SI:
-
Southern India
- SVMP:
-
Snake venom metalloprotease
- SVSP:
-
Snake venom serine protease
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
- WI:
-
Western India
References
Bawaskar H, Bawaskar P (2015) Snake bite poisoning. J Mahatma Gandhi Inst Med Sci 20:5
Bawaskar HS, Bawaskar PH, Punde DP, Inamdar MK, Dongare RB, Bhoite RR (2008) Profile of snakebite envenoming in rural Maharashtra, India. J Assoc Phys India 56:88–95
Burbaum J, Tobal GM (2002) Proteomics in drug discovery. Curr Opin Chem Biol 6:427–433
Casewell NR, Wuster W, Vonk FJ, Harrison RA, Fry BG (2013) Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol 28:219–229
Chanda A, Kalita B, Patra A, Senevirathne W, Mukherjee AK (2018a) Proteomic analysis and antivenomics study of Western India Naja naja venom: correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Rev Proteomics 16(2):171–184
Chanda A, Patra A, Kalita B, Mukherjee AK (2018b) Proteomics analysis to compare the venom composition between Naja naja and Naja kaouthia from the same geographical location of eastern India: correlation with pathophysiology of envenomation and immunological cross-reactivity towards commercial polyantivenom. Expert Rev Proteomics 15:949–961
Chiappinelli V, Hue B, Mony L, Sattelle D (1989) Kappa-bungarotoxin blocks nicotinic transmission at an identified invertebrate central synapse. J Exp Biol 141:61–71
Chippaux JP (2017) Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins Incl Trop Dis 23:38
Choudhury M, McCleary RJR, Kesherwani M, Kini RM, Velmurugan D (2017) Comparison of proteomic profiles of the venoms of two of the ‘Big Four’ snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon 135:33–42
Daltry JC, Wuster W, Thorpe RS (1996) Diet and snake venom evolution. Nature 379:537–540
De Toni LG, Menaldo DL, Cintra AC, Figueiredo MJ, de Souza AR, Maximiano WM, Jamur MC, Souza GE, Sampaio SV (2015) Inflammatory mediators involved in the paw edema and hyperalgesia induced by Batroxase, a metalloproteinase isolated from Bothrops atrox snake venom. Int Immunopharmacol 28:199–207
Deka A, Sharma M, Sharma M, Mukhopadhyay R, Doley R (2017) Purification and partial characterization of an anticoagulant PLA2 from the venom of Indian Daboia russelii that induces inflammation through upregulation of proinflammatory mediators. J Biochem Mol Toxicol 31:e21945
Dutta S, Gogoi D, Mukherjee AK (2015) Anticoagulant mechanism and platelet deaggregation property of a non-cytotoxic, acidic phospholipase A2 purified from Indian cobra (Naja naja) venom: inhibition of anticoagulant activity by low molecular weight heparin. Biochimie 110:93–106
Dutta S, Chanda A, Kalita B, Islam T, Patra A, Mukherjee AK (2017) Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: correlation of venom composition with its biochemical and pharmacological properties. J Proteomics 156:29–39
Dutta S, Sinha A, Dasgupta S, Mukherjee AK (2019) Binding of a Naja naja venom acidic phospholipase A2 cognate complex to membrane-bound vimentin of rat L6 cells: implications in cobra venom-induced cytotoxicity. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamem.2019.02.002
Faisal T, Tan KY, Sim SM, Quraishi N, Tan NH, Tan CH (2018) Proteomics, functional characterization and antivenom neutralization of the venom of Pakistani Russell’s viper (Daboia russelii) from the wild. J Proteomics 183:1–13
Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246:64–71
Fry BG (2005) From genome to “venome”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res 15:403–420
Fry BG, Wickramaratna JC, Hodgson WC, Alewood PF, Kini RM, Ho H, Wuster W (2002) Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: taxonomic and toxinological implications. Rapid Commun Mass Spectrom 16:600–608
Gutierrez JM, Theakston RD, Warrell DA (2006) Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med 3:e150
Jagadeesha DK, Shashidhara Murthy R, Girish KS, Kemparaju K (2002) A non-toxic anticoagulant metalloprotease: purification and characterization from Indian cobra (Naja naja naja) venom. Toxicon 40:667–675
Jayanthi GP, Gowda TV (1988) Geographical variation in India in the composition and lethal potency of Russell’s viper (Vipera russelli) venom. Toxicon 26:257–264
Kalita B, Patra A, Mukherjee AK (2017) Unraveling the proteome composition and immuno-profiling of western India Russell’s Viper venom for in-depth understanding of its pharmacological properties, clinical manifestations, and effective antivenom treatment. J Proteome Res 16:583–598
Kalita B, Mackessy SP, Mukherjee AK (2018a) Proteomic analysis reveals geographic variation in venom composition of Russell’s Viper in the Indian subcontinent: implications for clinical manifestations post-envenomation and antivenom treatment. Expert Rev Proteomics 15:837–849
Kalita B, Patra A, Das A, Mukherjee AK (2018b) Proteomic analysis and immuno-profiling of eastern India Russell’s Viper (Daboia russelii) venom: correlation between RVV composition and clinical manifestations post RV bite. J Proteome Res 17:2819–2833
Kalita B, Singh S, Patra A, Mukherjee AK (2018c) Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. Int J Biol Macromol 118:375–385
Karas M, Hillenkamp F (1988) Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem 60:2299–2301
Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ (2008) The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 5:e218
Kemparaju K, Prasad BN, Gowda VT (1994) Purification of a basic phospholipase A2 from Indian saw-scaled viper (Echis carinatus) venom: characterization of antigenic, catalytic and pharmacological properties. Toxicon 32:1187–1196
Kemparaju K, Krishnakanth TP, Veerabasappa Gowda T (1999) Purification and characterization of a platelet aggregation inhibitor acidic phospholipase A2 from Indian saw-scaled viper (Echis carinatus) venom. Toxicon 37:1659–1671
Kini RM, Doley R (2010) Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56:855–867
Koh CY, Kini RM (2012) From snake venom toxins to therapeutics-cardiovascular examples. Toxicon 59:497–506
Kumar JR, Basavarajappa BS, Arancio O, Aranha I, Gangadhara NS, Yajurvedi HN, Gowda TV (2008) Isolation and characterization of “Reprotoxin”, a novel protein complex from Daboia russelii snake venom. Biochimie 90:1545–1559
Kumar JR, Basavarajappa BS, Vishwanath BS, Gowda TV (2015) Biochemical and pharmacological characterization of three toxic phospholipase A2s from Daboia russelii snake venom. Comp Biochem Physiol C: Toxicol Pharmacol 168:28–38
Lomonte B, Calvete JJ (2017) Strategies in ‘snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J Venom Anim Toxins Incl Trop Dis 23:26
Mandal S, Bhattacharyya D (2008) Two l-amino acid oxidase isoenzymes from Russell’s viper (Daboia russelli russelli) venom with different mechanisms of inhibition by substrate analogs. FEBS J 275:2078–2095
Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL (1978) Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Investig 61:661–670
McNamee D (2001) Tackling venomous snake bites worldwide. Lancet 357:1680
Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, Rodriguez PS, Mishra K, Whitaker R, Jha P, Million Death Study Collaborators (2011) Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis 5:e1018
Montecucco C, Gutierrez JM, Lomonte B (2008) Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci 65:2897–2912
Morais IC, Pereira GJ, Orzáez M, Jorge RJ, Bincoletto C, Toyama MH, Monteiro HS, Smaili SS, Pérez-Payá E, Martins AM (2015) l-amino acid oxidase from Bothrops leucurus venom induces nephrotoxicity via apoptosis and necrosis. PLoS ONE 10:e0132569
Mukherjee AK (2008a) Characterization of a novel pro-coagulant metalloprotease (RVBCMP) possessing alpha-fibrinogenase and tissue haemorrhagic activity from venom of Daboia russelli russelli (Russell’s viper): evidence of distinct coagulant and haemorrhagic sites in RVBCMP. Toxicon 51:923–933
Mukherjee AK (2008b) Phospholipase A2-interacting weak neurotoxins from venom of monocled cobra Naja kaouthia display cell-specific cytotoxicity. Toxicon 51:1538–1543
Mukherjee AK (2010) Non-covalent interaction of phospholipase A2 (PLA2) and kaouthiotoxin (KTX) from venom of Naja kaouthia exhibits marked synergism to potentiate their cytotoxicity on target cells. J Venom Res 1:37–42
Mukherjee AK (2014a) A major phospholipase A2 from Daboia russelii russelii venom shows potent anticoagulant action via thrombin inhibition and binding with plasma phospholipids. Biochimie 99:153–161
Mukherjee AK (2014b) The pro-coagulant fibrinogenolytic serine protease isoenzymes purified from Daboia russelii russelii venom coagulate the blood through factor V activation: role of glycosylation on enzymatic activity. PLoS ONE 9:e86823
Mukherjee AK, Mackessy SP (2013) Biochemical and pharmacological properties of a new thrombin-like serine protease (Russelobin) from the venom of Russell’s Viper (Daboia russelii russelii) and assessment of its therapeutic potential. Biochem Biophys Acta 1830:3476–3488
Mukherjee AK, Mackessy SP (2014) Pharmacological properties and pathophysiological significance of a Kunitz-type protease inhibitor (Rusvikunin-II) and its protein complex (Rusvikunin complex) purified from Daboia russelii russelii venom. Toxicon 89:55–66
Mukherjee AK, Maity CR (1998) The composition of Naja naja venom samples from three districts of West Bengal, India. Comp Biochem Physiol A: Mol Integr Physiol 119:621–627
Mukherjee AK, Maity CR (2002) Biochemical composition, lethality and pathophysiology of venom from two cobras—Naja naja and N. kaouthia. Comp Biochem Physiol B: Biochem Mol Biol 131:125–132
Mukherjee A, Ghosal S, Maity C (2000) Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon 38:163–175
Mukherjee AK, Chatterjee S, Majumder S, Saikia D, Thakur R, Chatterjee A (2014a) Characterization of a pro-angiogenic, novel peptide from Russell’s viper (Daboia russelii russelii) venom. Toxicon 77:26–31
Mukherjee AK, Dutta S, Mackessy SP (2014b) A new C-type lectin (RVsnaclec) purified from venom of Daboia russelii russelii shows anticoagulant activity via inhibition of FXa and concentration-dependent differential response to platelets in a Ca2+-independent manner. Thromb Res 134:1150–1156
Mukherjee AK, Kalita B, Thakur R (2014c) Two acidic, anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor Xa via phospholipids independent, non-enzymatic mechanism. PLoS ONE 9:e101334
Mukherjee AK, Mackessy SP, Dutta S (2014d) Characterization of a Kunitz-type protease inhibitor peptide (Rusvikunin) purified from Daboia russelii russelii venom. Int J Biol Macromol 67:154–162
Mukherjee AK, Saviola AJ, Burns PD, Mackessy SP (2015) Apoptosis induction in human breast cancer (MCF-7) cells by a novel venom l-amino acid oxidase (Rusvinoxidase) is independent of its enzymatic activity and is accompanied by caspase-7 activation and reactive oxygen species production. Apoptosis 20:1358–1372
Mukherjee AK, Dutta S, Kalita B, Jha DK, Deb P, Mackessy SP (2016a) Structural and functional characterization of complex formation between two Kunitz-type serine protease inhibitors from Russell’s Viper venom. Biochimie 128–129:138–147
Mukherjee AK, Kalita B, Mackessy SP (2016b) A proteomic analysis of Pakistan Daboia russelii russelii venom and assessment of potency of Indian polyvalent and monovalent antivenom. J Proteomics 144:73–86
Mukherjee AK, Saviola AJ, Mackessy SP (2018) Cellular mechanism of resistance of human colorectal adenocarcinoma cells against apoptosis-induction by Russell’s Viper venom l-amino acid oxidase (Rusvinoxidase). Biochimie 150:8–15
Oh AMF, Tan CH, Ariaranee GC, Quraishi N, Tan NH (2017) Venomics of Bungarus caeruleus (Indian krait): comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J Proteomics 164:1–18
Ohno M, Menez R, Ogawa T, Danse JM, Shimohigashi Y, Fromen C, Ducancel F, Zinn-Justin S, Boulain JC, Tamiya T, Ménez A (1998) Molecular evolution of snake toxins: is the functional diversity of snake toxins associated with a mechanism of accelerated evolution? Prog Nucleic Acid Res Mol Biol 59:307–364
Pathan J, Mondal S, Sarkar A, Chakrabarty D (2017) Daboialectin, a C-type lectin from Russell’s viper venom induces cytoskeletal damage and apoptosis in human lung cancer cells in vitro. Toxicon 127:11–21
Patra A, Kalita B, Chanda A, Mukherjee AK (2017) Proteomics and antivenomics of Echis carinatus carinatus venom: correlation with pharmacological properties and pathophysiology of envenomation. Sci Rep 7:17119
Patra A, Kalita B, Mukherjee AK (2018) Assessment of quality, safety, and pre-clinical toxicity of an equine polyvalent anti-snake venom (Pan Africa): determination of immunological cross-reactivity of antivenom against venom samples of Elapidae and Viperidae snakes of Africa. Toxicon 153:120–127
Patra A, Chanda A, Mukherjee AK (2019) Quantitative proteomic analysis of venom from Southern India common krait (Bungarus caeruleus) and identification of poorly immunogenic toxins by immune-profiling against commercial antivenom. Expert Rev Proteomics. https://doi.org/10.1080/14789450.2019.1609945 (in press)
Prasad NB, Uma B, Bhatt SK, Gowda VT (1999) Comparative characterisation of Russell’s viper (Daboia/Vipera russelli) venoms from different regions of the Indian peninsula. Biochem Biophys Acta 1428:121–136
Punde DP (2005) Management of snake-bite in rural Maharashtra: a 10-year experience. Natl Med J India 18:71–75
Pung YF, Wong PT, Kumar PP, Hodgson WC, Kini RM (2005) Ohanin, a novel protein from king cobra venom, induces hypolocomotion and hyperalgesia in mice. J Biol Chem 280:13137–13147
Raut S, Raut P (2015) Snake Bite management experience in western Mah (India). Toxicon S 103:89–90
Saikia D, Thakur R, Mukherjee AK (2011) An acidic phospholipase A2 (RVVA-PLA2-I) purified from Daboia russelli venom exerts its anticoagulant activity by enzymatic hydrolysis of plasma phospholipids and by non-enzymatic inhibition of factor Xa in a phospholipids/Ca2+ independent manner. Toxicon 57:841–850
Saikia D, Bordoloi NK, Chattopadhyay P, Choklingam S, Ghosh SS, Mukherjee AK (2012) Differential mode of attack on membrane phospholipids by an acidic phospholipase A2 (RVVA-PLA2-I) from Daboia russelli venom. Biochem Biophys Acta 1818:3149–3157
Saikia D, Majumdar S, Mukherjee AK (2013) Mechanism of in vivo anticoagulant and haemolytic activity by a neutral phospholipase A2 purified from Daboia russelii russelii venom: correlation with clinical manifestations in Russell’s Viper envenomed patients. Toxicon 76:291–300
Schetinger M, Rocha J, Ahmed M, Morsch V (2009) Snake venom acetylcholinesterase. In: Mackessy SP, Mackessy SP (eds) Handbook of venoms and toxins of reptiles. CRC Press, Boca Raton, pp 207–219
Sharma M, Das D, Iyer JK, Kini RM, Doley R (2015) Unveiling the complexities of Daboia russelii venom, a medically important snake of India, by tandem mass spectrometry. Toxicon 107:266–281
Sharma M, Iyer JK, Shih N, Majumder M, Mattaparthi VS, Mukhopadhyay R, Doley R (2016) Daboxin P, a major Phospholipase A2 enzyme from the Indian Daboia russelii russelii venom targets Factor X and Factor Xa for its anticoagulant activity. PLoS ONE 11:e0153770
Shashidharamurthy R, Jagadeesha DK, Girish KS, Kemparaju K (2002) Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol Cell Biochem 229:93–101
Sintiprungrat K, Watcharatanyatip K, Senevirathne WD, Chaisuriya P, Chokchaichamnankit D, Srisomsap C, Ratanabanangkoon K (2016) A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J Proteomics 132:131–143
Suchithra N, Pappachan JM, Sujathan P (2008) Snakebite envenoming in Kerala, South India: clinical profile and factors involved in adverse outcomes. Emerg Med J 25:200–204
Suntravat M, Yusuksawad M, Sereemaspun A, Perez JC, Nuchprayoon I (2011) Effect of purified Russell’s viper venom-factor X activator (RVV-X) on renal hemodynamics, renal functions, and coagulopathy in rats. Toxicon 58:230–238
Tan NH, Fung SY, Tan KY, Yap MK, Gnanathasan CA, Tan CH (2015) Functional venomics of the Sri Lankan Russell’s viper (Daboia russelii) and its toxinological correlations. J Proteomics 128:403–423
Teixeira C, Cury Y, Moreira V, Picolo G, Chaves F (2009) Inflammation induced by Bothrops asper venom. Toxicon 54:67–76
Thakur R, Mukherjee AK (2015) A brief appraisal on Russell’s Viper venom (Daboia russelii russelii) proteinases. In: Gopalakrishnakone P, Inagaki H, Mukherjee AK, Rahmy T, Vogel CW (eds) Snake venoms, toxinology. Springer, Dordrecht, pp 1–18
Thakur R, Mukherjee AK (2017) Pathophysiological significance and therapeutic applications of snake venom protease inhibitors. Toxicon 131:37–47
Thakur R, Kumar A, Bose B, Panda D, Saikia D, Chattopadhyay P, Mukherjee AK (2014) A new peptide (Ruviprase) purified from the venom of Daboia russelii russelii shows potent anticoagulant activity via non-enzymatic inhibition of thrombin and factor Xa. Biochimie 105:149–158
Thakur R, Chattopadhyay P, Ghosh SS, Mukherjee AK (2015) Elucidation of procoagulant mechanism and pathophysiological significance of a new prothrombin activating metalloprotease purified from Daboia russelii russelii venom. Toxicon 100:1–12
Thakur R, Kini S, Kurkalang S, Banerjee A, Chatterjee P, Chanda A, Chatterjee A, Panda D, Mukherjee AK (2016) Mechanism of apoptosis induction in human breast cancer MCF-7 cell by Ruviprase, a small peptide from Daboia russelii russelii venom. Chem Biol Interact 258:297–304
Theakston RD, Phillips RE, Warrell DA, Galagedera Y, Abeysekera DT, Dissanayaka P, de Silva A, Aloysius DJ (1990) Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkine antivenom. Trans R Soc Trop Med Hyg 84:301–308
Utkin YN (2019) Last decade update for three-finger toxins: newly emerging structures and biological activities. World J Biol Chem 10:17–27
Vanuopadath M, Sajeev N, Murali AR, Sudish N, Kangosseri N, Sebastian IR, Jain ND, Pal A, Raveendran D, Nair BG, Nair SS (2018) Mass spectrometry-assisted venom profiling of Hypnale hypnale found in the Western Ghats of India incorporating de novo sequencing approaches. Int J Biol Macromol 118:1736–1746
Warrell DA, Gutierrez JM, Calvete JJ, Williams D (2013) New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J Med Res 138:38–59
Weldon CL, Mackessy SP (2010) Biological and proteomic analysis of venom from the Puerto Rican Racer (Alsophis portoricensis: Dipsadidae). Toxicon 55:558–569
Whitaker R (2006) Common Indian snakes: a field guide. Macmillan, London
White J (2005) Snake venoms and coagulopathy. Toxicon 45:951–967
Wong KY, Tan CH, Tan KY, Quraishi NH, Tan NH (2018) Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J Proteomics 175:156–173
Yamazaki Y, Matsunaga Y, Tokunaga Y, Obayashi S, Saito M, Morita T (2009) Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. J Biol Chem 284:9885–9891
Acknowledgements
This study was supported by a financial grant from Department of Biotechnology, New Delhi, sponsored research project Unit of Excellence in Biotechnology in NER of India (BT/412/NE/UExcel/2013) to AKM. BK received Senior Research Fellowships from DBT U-Excel project grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalita, B., Mukherjee, A.K. Recent advances in snake venom proteomics research in India: a new horizon to decipher the geographical variation in venom proteome composition and exploration of candidate drug prototypes. J Proteins Proteom 10, 149–164 (2019). https://doi.org/10.1007/s42485-019-00014-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42485-019-00014-w
Keywords
Profiles
- Bhargab Kalita View author profile