Abstract
Circadian rhythms are fundamental for regulating physiological processes in organisms, with disruptions often linked to metabolic disorders. This study investigated the role of the roraa gene in zebrafish, particularly its influence on circadian rhythms and metabolic regulation. Using quantitative PCR and in situ hybridization, we confirmed the rhythmic expression of roraa and explored its oscillatory mechanisms. The construction of roraa knockout mutants revealed that the absence of roraa disrupts circadian clock function, as evidenced by the reduced expression of core clock genes and altered behavioral rhythms, while the transgenic zebrafish lines which overexpress roraa just have opposite results. Additionally, we demonstrated that Roraa directly regulates per2 expression through the RORE element in its promoter. Furthermore, the transcriptome analysis and quantitative PCR indicated that the metabolism related genes, especially lipid metabolism related genes were obviously changed in roraa−/− mutants compare with WT. Our findings underscore the critical role of Roraa in coordinating circadian and metabolic processes, providing insights into potential therapeutic targets for addressing metabolic disorders related to circadian disruption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The circadian clock is a core system that regulates the 24-h day‒night rhythm in organisms, and it is widely present in plants, animals, and microorganisms [1]. The circadian clock maintains rhythmicity through feedback loops composed of the transcription and translation of core clock genes [2]. Proteins of core clock genes such as Bmal1 and Clock form transcriptional activation complexes that initiate the expression of downstream genes, including Per and Cry [1, 3]. As Per and Cry proteins accumulate within the cell, they inhibit the activity of Bmal1 and Clock through negative feedback mechanisms, thus sustaining a 24-h oscillatory cycle [4]. Recent studies have identified RER-ERB and RORA as important regulatory factors in additional loops that interact with Bmal1 via this feedback mechanism. RORA enhances the stability of circadian rhythms by regulating the expression of Bmal1, whereas REV-ERB participates in this regulatory process by inhibiting Bmal1 expression [5]. The coordinated functioning of these loops is crucial for maintaining normal physiological functions, impacting processes such as sleep, metabolism, and hormone secretion [6]. Therefore, a deeper understanding of the molecular mechanisms underlying the circadian clock is not only important for fundamental biological research but also provides new perspectives for studying diseases related to circadian disruption.
RORΑ has garnered increasing attention for its critical role in regulating circadian rhythms and metabolic processes [7]. Previous studies have indicated that Rora plays a key role in regulating the expression of core circadian clock genes, particularly by influencing the expression of Bmal1 and Clock to maintain the normal oscillation of circadian rhythms [5]. Additionally, Rora is also involved in lipid metabolism [8], glucose metabolism [9], and inflammatory responses [10]. Studies have shown that the absence of Rora is closely associated with metabolic diseases, such as obesity and diabetes, highlighting its pivotal role in metabolic homeostasis [7]. Research by Han et al. shows that Rora regulates lipid metabolism and influences inflammatory responses in macrophages, providing new insights into its role in the immune system [11]. While the functions of Rora in mice and other model organisms (Such as Caenorhabditis elegans [12] and drosophila [13]) have been somewhat studied, its specific mechanisms in zebrafish remain to be thoroughly investigated, particularly concerning its interactions with circadian and metabolic pathways.
Zebrafish (Danio rerio) have gained prominence as excellent model organisms because of their extensive application in genetics, developmental biology, and circadian research [14]. The transparency of zebrafish embryos allows for live observations, and their short life cycle and high reproductive capacity make them invaluable tools for studying human diseases and gene functions [15]. Furthermore, zebrafish share a high degree of genomic similarity with mammals, making them a valuable model system in circadian research. Previous studies have demonstrated that circadian clock genes in zebrafish (such as Bmal1, Clock, and Per) are highly conserved with those in mammals, underscoring the importance of zebrafish in investigating the mechanisms of circadian rhythms [16]. However, the specific functions of Rora in zebrafish have yet to be fully explored. Previous research has revealed two rora genes in zebrafish, namely, roraa and rorab [17]; thus, understanding the rora genes in zebrafish is important for elucidating the mechanisms of the circadian clock.
In this study, we successfully generated roraa-deficient mutants via the CRISPR/Cas9 system. Our findings reveal the potential mechanism by which Roraa regulates per2 expression through the RORE in the per2 promoter, as well as the effects of roraa deletion on the expression of circadian clock genes and PPAR signaling pathway metabolism-related genes. Through this research, we hope to fill existing gaps in the literature, unveil the mechanistic role of Roraa in zebrafish, and provide new insights into the relationship between metabolic diseases and circadian rhythm disorders.
Methods and materials
Experimental animals and rearing conditions
In this study, AB strain zebrafish embryos were used for experiments. Both larvae and adult zebrafish were reared in a standard environment at a temperature of 28.5 °C, adhering strictly to a 14-h light and 10-h dark cycle (light:dark = 14:10), with lights on at 9 AM and off at 11 PM each day. All zebrafish rearing and experimental procedures complied with the relevant regulations outlined in the"Administrative Regulations on Laboratory Animals of the People's Republic of China"and the"Measures for the Administration of Laboratory Animals,"and approval was obtained from the Animal Ethics Committee.
Gene editing and mutation screening of zebrafish roraa
The roraa gene sequence of zebrafish was obtained from the Ensembl database (www.ensembl.org). The cDNA sequence of the target gene was entered into the CRISPRscan.org website to predict gRNA target sites, selecting those with high scores and activity. The specificity of these target sites was subsequently confirmed by comparison with sequences in the NCBI database. The synthesized active gRNA was coinjected with Cas9 mRNA into single-cell-stage zebrafish embryos to create F0 mutants. After maturation, F0 adults were crossed with wild-type zebrafish to screen for F1 mutants with frameshift mutations, which were then reared separately. F1 mutants were subsequently crossed to select stable F2 homozygous mutant lines for further experiments and all the experiments was performed with F3 generation zebrafish.
Construction of transgenic zebrafish via the Tol2 kit system
This study utilized the Tol2kit transgenic system and Gateway vectors to construct a zebrafish model for overexpressing Roraa. First, the CDS of the roraa gene was cloned and inserted into the pME-MCS plasmid. Then, through a Gateway™ LR reaction (using Gateway™ LR Clonase™ II Enzyme Mix, Invitrogen, 11,791,020), the pME-roraa, p5E-hsp70 l, and p3E-polyA sequences were integrated into the pDestTol2 CG2 plasmid. Successfully constructed recombinant plasmids were coinjected with Tol2 mRNA into single-cell-stage zebrafish embryos, and F0 fish with cardiac fluorescent markers were screened and cultivated. After maturation, F0 adults were crossed with wild-type zebrafish to select transgenic lines that could stably inherit the trait for further experimental studies.
RNA extraction and qRT‒PCR analysis
Under LD or DD conditions, juvenile zebrafish (120 to 144 h postfertilization) of the wild-type and roraa−/− mutants were collected every 4 h, with 30 juveniles sampled at each time point. Total RNA from the zebrafish was extracted via TRIzol reagent (Vazyme, Code No:R701). The extracted RNA was reverse transcribed into cDNA via a qRT‒PCR reverse transcription kit (Vazyme, Code No:R423-01). A qRT‒PCR system was subsequently prepared via a fluorescence quantitative reagent kit, and the reactions were detected via a Hangzhou Longy 2000 fluorescent quantitative PCR instrument. In a qRT-PCR experiment, the total reaction volume is 20 µL, which includes 20 ng of template RNA, 0.5 µL of primers, and 10 µL of qPCR Master Mix. The temperature cycling starts with an initial denaturation at 95 °C for 3 min, followed by 40 cycles, each consisting of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s. The data were analyzed through relative quantification via the ΔΔCT method to calculate the relative expression levels of the genes, with β-actin as the internal control. The relative expression levels of the target genes in each sample were determined via the 2−ΔΔCT method. All primers used for qRT‒PCR are listed in the supplementary Table 1.
Behavior monitoring of zebrafish larvae
Behavioral analyses of zebrafish larvae were conducted under both DD and LD conditions. A 48-well plate was used for the experiments, with one 96 hpf zebrafish placed in each well. The plate contained 24 wild-type (WT) larvae and 24 roraa−/− mutants or overexpressing transgenic larvae. For overexpressing transgenic larvae, a 1-h heat shock at 37 °C was applied prior to the experiment. The 48-well plate was then placed in the Zebrabox behavior monitoring system (Videotrack, ViewPoint Life Sciences, Montreal, Canada). The mutant larvae were placed directly into the wells without any special treatment. The movement of the larvae was continuously monitored over 5 days via an automated video tracking system (ViewPoint Life Sciences), and the movement data of each zebrafish were recorded with Zebralab 3.10 software (ViewPoint Life Sciences) for subsequent analysis.
In situ hybridization of zebrafish
Zebrafish embryos were initially treated with 0.3% PTU to inhibit pigmentation. Wild-type (WT) embryos at 80 hpf and 88 hpf were fixed in 4% PFA. After fixation, the embryos were allowed to settle, dehydrated with methanol and stored at −20 °C for later experiments. For hybridization, the samples underwent gradient rehydration treatment in the sequence of 75%–50%–25%-PBST. Following rehydration, the Dig-labeled probes were added to the samples in 50% formamide hybridization buffer and incubated overnight at 70 °C. The next day, the samples were washed with a gradient of 2 × SSC/0.2% Tween and 0.2 × SSC/0.2% Tween. After washing, the samples were blocked and incubated overnight at 4 °C with anti-Digoxin-AP blocking solution. On the third day, the antibodies were retrieved, and color development solution was added for detection. The results were observed and photographed via a stereomicroscope.
Promoter cloning and plasmid construction
The promoter or CDSs of the target genes were first obtained from the Ensembl database (www.ensembl.org). Specific primers for homologous recombination were designed via the CE design program to ensure the accuracy of the subsequent amplification and cloning of both the promoter and CDSs. The promoter sequence was amplified via the use of genomic DNA as a template, and the amplified product was subsequently cloned and inserted into the pGL4.17 plasmid via homologous recombination to construct the promoter reporter vector. Simultaneously, the CDS was amplified from zebrafish cDNA and cloned and inserted into the pcDNA3.1 plasmid to create the CDS expression vector. To predict potential regulatory elements in the promoter region, the JASPAR database (jaspar.genereg.net) was used. Regulatory elements with high predicted scores were selected, and their positions were annotated via SnapGene software for subsequent analysis and experimental design.
On the basis of the predicted promoter elements, primers were designed to delete specific element regions. The promoter-pGL4.17 plasmid (10 ng) was used as a template for PCR amplification with KOD polymerase. The primer concentration in the reaction system was 0.2 μM, and the total volume was 50 μL. The amplification conditions were as follows: 98 °C for 2 min (predenaturation), 98 °C for 30 s (denaturation), an annealing temperature (based on the primer Tm value) for 30 s, and 72 °C for 1 min, for a total of 35 cycles, followed by a final extension at 72 °C for 10 min. The PCR products were analyzed via 1% agarose gel electrophoresis. After the gel was cut and recovered, seamless cloning was performed on the pGL4.17 plasmid. The recombinant plasmids were subsequently transformed into competent E. coli, and individual colonies were selected and cultured overnight in LB medium. The following day, the plasmids were extracted via a plasmid mini-prep kit, and the extracted plasmids were sent to a sequencing company for confirmation against the reference sequence to verify the successful deletion of the target elements. In this study, the per2-luc and pcDNA3.1-Tef/Bmal1b/Clock1a/Roraa plasmids were constructed on the basis of established methods from previous literature. For the me1, cpt2, fads2, and fabp2 promoter–luc reporter plasmids, seamless cloning kits were used. The specific steps included connecting the PCR-amplified promoter sequences with pGL4.17 vector fragments via the seamless connection reaction system according to the kit's instructions. The connection reaction mixture was incubated at 16 °C for 1 h, followed by direct transformation into competent cells for plasmid extraction.
Dual luciferase reporter assay
Human embryonic kidney (HEK) 293 T cells were used for the experiments. HEK293 T cells were cultured in high-glucose DMEM containing 10% fetal bovine serum (FBS) and penicillin‒streptomycin in a cell incubator at 37 °C with 5% CO₂. When the cells reached an appropriate density (approximately 70–80% confluence), they were seeded into 24-well plates, with an appropriate amount of cell suspension added to achieve the required cell density for the experiments. When the cells in the 24-well plates reached 70–80% confluence, transfection experiments were conducted. In accordance with the experimental design, 200 ng of the promoter-luc reporter plasmid was transfected into each well, along with 200 ng each of the Bmal1b and Clock1a plasmids or other plasmids as needed. Transfection was performed via Lipofectamine™ 3000 according to the manufacturer's instructions. Twenty-four hours posttransfection, the cells were collected for luciferase activity detection. The Dual-Luciferase® Reporter Assay System (Promega, Code No:E1910) was used according to the manufacturer's protocol, and the relative light units (RLUs) of firefly luciferase and Renilla luciferase were measured. The data were used to calculate relative luciferase activity, with the activity of Renilla luciferase used as an internal control.
Sample acquisition for sequencing and transcriptome analysis
Sample acquisition for sequencing
The samples sequenced included zebrafish larvae collected at seven time points under DD conditions, as well as larval samples collected at ZT14 under LD conditions, encompassing both the wild-type (WT) and the roraa−/− mutant groups. After sample collection, the samples were preserved on dry ice and immediately shipped to a sequencing company. The sequencing company conducted quality assessments to ensure that the samples met the standards for transcriptome sequencing and performed high-throughput sequencing.
Transcriptome gene rhythm analysis
For the transcriptome data collected at seven time points under DD conditions, the MetaCycle analysis package was used to analyze the gene expression levels in WT and roraa−/− mutants, identifying a set of genes with significant rhythmicity (excluding novel genes). Comparative analysis was subsequently performed on the genes whose rhythmic expression was lost in the roraa−/− mutants, and their expression changes in the mutants were assessed. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted for both the rhythmic loss genes and those that maintained rhythmic expression in roraa−/− larvae. Further analysis was performed on the changes in rhythmic parameters, such as cycle, phase, and amplitude.
Differential gene expression analysis of the transcriptome
For the transcriptome data at each time point, visualization of differentially expressed genes was conducted via the R package ggplot2. Venn diagrams were used to analyze the intersection of differentially expressed genes at each time point, identifying gene sets that exhibited differential expression across various time points. Finally, GO and KEGG pathway enrichment analyses were conducted for the DEGs at each time point to further elucidate the potential biological functional changes in the roraa−/− mutants.
Data analysis
Most of the experimental data in this study were statistically analyzed via GraphPad Prism 8.4.3 and the Shapiro–Wilk test was used for the normality test. For deep sequencing data, R Studio and relevant cloud platforms (BMKCloud Bioinformatics Analysis Platform, https://www.biocloud.net/) were utilized for data processing and analysis. Intergroup differences were calculated via Student's t test and two-way ANOVA via GraphPad Prism 8.0.2. When p < 0.05, the differences between groups were marked as “*”; p < 0.01 as “**” and p < 0.001 as “****” “”; when p > 0.05, it was marked as “ns,” indicating that there was no statistically significant difference between groups. The rhythmicity analysis of the qRT‒PCR data was performed via the JTK cycle algorithm, whereas the rhythmicity analyses of the behavioral and transcriptome data were conducted via the MetaCycle R language program. When p < 0.05, the results indicated significant rhythmicity; when p > 0.05, the results indicated the absence of rhythmicity.
Results
Rhythmic expression of the roraa gene in zebrafish is regulated by the circadian clock
To clarify the role of the roraa gene in circadian regulation, we analyzed its expression rhythm in wild-type zebrafish under DD (dark/dark) and LD (light/dark) conditions via quantitative PCR. We first collected zebrafish larval samples under both conditions and measured the expression levels of roraa mRNA via qPCR. JTK cycle analysis revealed that the expression of roraa exhibited significant circadian rhythmicity under both DD and LD conditions (p < 0.05) (Fig. 1A, Supplementary Fig. 1 A), indicating that the expression of the roraa gene oscillates cyclically with light/dark alternation.
Circadian expression of the roraa gene in zebrafish is regulated by the circadian clock. A Under LD (light/dark) conditions, qRT‒PCR was used to measure the mRNA expression levels of the roraa gene in wild-type (WT) zebrafish. JTK cycle analysis revealed that roraa expression exhibited significant circadian oscillation (p < 0.05). B In situ hybridization was performed to detect roraa gene expression under LD conditions, revealing that roraa expression during the day was significantly greater than that at night, demonstrating circadian rhythmic differences. C Statistical results from in situ hybridization under LD conditions revealed that roraa expression was significantly higher during the day than at night. D Potential cis-regulatory elements on the roraa promoter fragment were predicted via the JASPAR database; two D-box elements, two E-box elements, and one RORE element, which may play key regulatory roles in the circadian expression of roraa, were identified. E Dual-luciferase reporter assays revealed that the heterodimers formed by the Bmal1b and Clocka proteins, as well as the Tefa, Roraa, Rorb, and Rorc proteins, significantly activated the expression of the roraa gene. F Diagram showing the deletion of E-box elements in the roraa-luc plasmid. G The results from dual-luciferase reporter assays demonstrated that the activation of the roraa gene by Bmal1b and Clocka was significantly reduced when E-box1 and E-box2 elements were deleted from the roraa-luc plasmid, indicating the important role of E-box elements in the regulation of roraa expression. All the data are presented as the means ± standard errors of the means (SEMs) (n = 3); ** indicates p < 0.01, **** indicates p < 0.0001 (Student's t test)
To further verify the rhythmic expression of roraa, we conducted in situ hybridization experiments. The qRT‒PCR results revealed that the expression of roraa during the day (CT8/ZT8) was significantly greater than that at night (CT16/ZT16). Therefore, we selected these two time points for sample collection under both LD and DD conditions. The results revealed that roraa expression was significantly greater during the day and lower at night under both LD and DD conditions (Fig. 1B and 1 C, Supplementary Fig. 1B and 1 C).
To explore potential regulatory elements in the promoter region of the roraa gene, we used the JASPAR database to predict the cloned roraa promoter segment. The results indicated the presence of two D-box elements, two E-box elements, and one RORE in this promoter segment (Fig. 1D). These findings suggest that these elements may participate in the regulation of rhythmic expression in roraa.
Further functional validation experiments demonstrated that the heterodimers formed by Bmal1b and Clock1a significantly activated roraa expression, increasing roraa mRNA levels by approximately two-fold compared with those in the control group. Additionally, Tefa significantly increased roraa expression, whereas the activation effects of Roraa, Rorb, and Rorc were relatively weak (Fig. 1E). To confirm the critical role of the E-box elements in this regulatory process, we constructed roraa promoter mutants lacking E-box elements (Fig. 1F). The results revealed that deletion of the E-box elements significantly weakened the activation of roraa by the Bmal1b and Clock1a heterodimers (Fig. 1G), indicating the importance of E-box elements in the rhythmic expression of the roraa gene.
In summary, these results indicate that the rhythmic expression of the roraa gene is precisely regulated by the core circadian clock, particularly through the direct action of Bmal1b and Clock1a heterodimers on the E-box elements in the promoter.
Construction of Zebrafish roraa−/− Mutants via the CRISPR/Cas9 System
To investigate the specific functions of RORα in this organism, we constructed zebrafish roraa−/− mutants via the CRISPR/Cas9 system. First, we obtained the exon and intron sequences of the roraa gene from the Ensembl database and found that the gene comprises ten exons (Fig. 2A). We then predicted target activity via online tools and designed suitable primers P3, P1, and P2 for PCR amplification of the target sequence. P3 and P4 were used to synthesize gRNA and to test its activity in experiments. The synthesized gRNA was subsequently coinjected with Cas9 mRNA into single-cell-stage zebrafish embryos. After 24 h, we collected the injected embryos and uninjected controls for target activity detection.
Construction of zebrafish roraa−/− mutant lines via the CRISPR/Cas9 technique. A Schematic diagram of the roraa exons and target site design. The black boxes represent exons, and the gray boxes represent UTR sequences. The gRNA was designed for the third exon, with the red area indicating the PAM site. B Evaluation of mutation efficiency in embryos after gRNA injection. Twenty-four hours postinjection, the injected embryos were compared with noninjected WT embryos for activity assessment, followed by TA cloning and random selection of colonies for PCR. The results from the 4% agarose gel electrophoresis clearly differed between the experimental group colonies and the WT group (with the corresponding colony numbers marked in red). C Comparison of DNA sequencing results between the WT and mutant strains. The mutation types in the mutants included − 28 bp and − 5 bp. D Comparison of amino acid sequences between the WT and mutant strains. The base deletion led to a frameshift mutation and an early stop codon, resulting in a truncated protein, with the mutant losing part of the DNA-binding domain and the entire ligand-binding domain
In this study, we used TA cloning technology to randomly select 15 colonies from the experimental group for PCR amplification and electrophoresis on a 4% agarose gel (conditions: 150 V, 25 min). The electrophoresis results revealed that the PCR bands from the experimental group differed significantly from those from the WT group, indicating high activity of the target on the third exon (Fig. 2B). On the basis of these results, we performed large-scale microinjections to establish the F0 mutant line. After the F0 generation matured, it was crossed with wild-type zebrafish to produce F1 offspring, which were subjected to TA cloning and sequencing analysis. Ultimately, we identified mutants carrying −28 bp and −5 bp frameshift mutations (Fig. 2C).
Analysis via SnapGene revealed that the frameshift mutation resulted in a deletion of 28 bases in the CDS of the roraa gene, leading to the early formation of a termination codon and resulting in a truncated protein (Fig. 2D). This mutation caused the complete loss of the ligand-binding domain and partial loss of the DNA-binding domain. We further screened for heterozygous fish lines in the F1 generation and obtained F2 homozygous mutants through crosses. Following PCR screening of F2, the mutation type was confirmed to ensure the stability and heritability of the experimental animal model. All the experiments were performed with F3 generation zebrafish.
Impact of roraa gene knockout on zebrafish behavioral rhythm and core clock gene expression
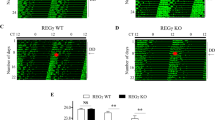
To verify the impact of roraa gene knockout on the circadian clock and to explore whether this gene mutation affects circadian phenotypes, we first conducted a behavioral analysis of the zebrafish mutants. The behavioral rhythm of zebrafish is typically regulated by a 24-h light/dark cycle (light:dark = 14 h:10 h). Therefore, we placed the wild-type (WT) and roraa−/− mutant zebrafish, born on the same day, in an artificial climate chamber to simulate a light/dark cycle (14 h/10 h), with light controlled from 9 AM to 11 PM daily. At 4 dpf, 24 WT and 24 mutant larvae were placed in 48-well plates and cultured under normal light/dark conditions.
Over the next three days, we measured and analyzed the average activity levels of the zebrafish larvae under light and dark conditions. The analysis results revealed that under LD conditions, the average daily swimming distance of the roraa−/− mutants was significantly lower than that of the WT zebrafish; this difference was primarily observed in terms of daytime activity, where the average activity of the WT zebrafish was significantly greater than that of the mutants (Fig. 3A‒D). Further analysis revealed that under DD conditions, the activity cycles of the roraa−/− mutants did not differ significantly from those of the WT, but the mutants' activity phase was delayed by approximately 2 h compared with that of the WT, with the activity amplitude being markedly lower, approximately one-third of that of the WT (Supplementary Fig. 2 A–D). These results indicate that the knockout of the roraa gene significantly altered the activity patterns of zebrafish, leading to notable changes in their circadian behavioral phenotypes.
Behavioral rhythms and gene expression changes in roraa−/− and WT zebrafish under LD conditions. A Activity curves for WT and roraa−/− plants under LD (14 h/300 lx: 10 h/30 lx) conditions. B Total activity of zebrafish, showing that compared with WT zebrafish, roraa−/− zebrafish had significantly lower activity during the day. C Average activity of zebrafish under light conditions, where roraa−/− zebrafish presented significantly lower activity during the day than did WT zebrafish. D Average activity of zebrafish under dark conditions, with no significant difference between roraa−/− and WT zebrafish. E–H Expression levels of the clock genes bmal1b, clock1a, cry1aa, and per2. The black curve represents activity changes in WT, whereas the red curve represents activity changes in roraa−/−; the method for difference analysis was Student's t test, where ns indicates p > 0.05 and *** indicates p < 0.001
To explore the potential molecular mechanisms underlying these behavioral changes, we analyzed the changes in the expression of core clock genes in zebrafish larvae via qRT‒PCR. Under both LD and DD conditions, samples were collected every four hours starting from 120 hpf, totaling seven time points. Under LD conditions, the expression levels of the bmal1b, clock1a, cry1aa, and per2 genes all tended to decrease (Fig. 3E). Similarly, under DD conditions, these genes also showed comparable decreases (Supplementary Fig. 2E–H). Additionally, we examined the changes in the expression of other clock genes and detected significant changes in expression under both DD and LD conditions (Supplementary Figs. 3–4).
In summary, the knockout of the roraa gene resulted in significant changes in the behavioral rhythm of zebrafish, which may be attributed to notable alterations in the expression levels of core clock genes within the zebrafish.
Roraa regulates the expression of circadian genes per2 through the RORE element
In the roraa−/− mutants, we observed a significant reduction in the expression of several core clock genes, suggesting that Roraa may play an important role in the transcriptional regulation of these genes. To test this hypothesis, we first conducted a predictive analysis of the RORE in the promoter region of the per2 gene. The results indicated that the per2 promoter contains a conserved RORE (Fig. 4A). To further investigate the regulatory effect of Roraa on per2 expression, we cotransfected cells with the per2-luc reporter plasmid and the Roraa expression plasmid. The results revealed that Roraa significantly activated per2 transcription in a dose-dependent manner, with increasing Roraa expression leading to progressively increased per2-luc reporter gene expression (Fig. 4B).
Transcriptional regulation of the clock gene per2 by Roraa in zebrafish. A Analysis of the cloned promoter sequence elements on the per2-luc plasmid, with a sequence length of 595 bp that includes one RORE element. A schematic diagram showing the deletion of the RORE in the per2 promoter sequence. B The gradient activation effect of Roraa on per2 expression, with activation increasing with increasing concentrations of Roraa at 100 ng, 200 ng, and 400 ng. C The activation effect of Roraa on per2 disappeared after the deletion of the RORE element, indicating the key role of the RORE element in Roraa's regulation of per2 expression. D Comparison of the relative expression levels of per2 in zebrafish treated with SR3335 and SR1078. “ + ” indicates cells transfected with the specific plasmid, whereas “-” indicates the control group without plasmid transfection. The method for difference analysis was Student's t test; ns indicates p > 0.05, * indicates p < 0.05, and *** indicates p < 0.001
To clarify the role of the RORE element in Roraa's regulation of per2, we constructed a per2 promoter mutant lacking the RORE element. The experimental results revealed that deletion of the RORE element significantly abolished the activation of per2-luc by Roraa (Fig. 4C), indicating the crucial role of the RORE in Roraa's transcriptional regulation of per2. To further validate this regulatory mechanism, we treated zebrafish larvae with the RORα inhibitor SR3335 and the activator SR1078. SR3335 treatment significantly inhibited per2 expression, whereas SR1078 treatment significantly increased per2 expression (Fig. 4D, Supplementary Fig. 5), which is consistent with the findings from the in vitro experiments.
In summary, these results indicate that Roraa can directly regulate the transcription of the circadian gene per2 through the RORE in its promoter, thereby affecting its expression.
Effects of Roraa overexpression on zebrafish behavioral rhythms and clock gene expression
To further validate the regulatory role of Roraa in zebrafish behavioral rhythms and clock gene expression, we constructed heat shock-inducible Roraa-overexpressing transgenic zebrafish and conducted relevant experiments (Supplementary Fig. 6). The results of the behavioral tests revealed that Roraa overexpression significantly increased the activity of the transgenic larvae and advanced their activity phase (Fig. 5A–D). This behavioral phenotype contrasts with that of the roraa−/− mutants, further confirming the importance of Roraa in regulating behavioral rhythms.
Whole-body overexpression of roraa disrupts the behavioral rhythms of zebrafish. A Activity change curves for WT and Tg(hsp70 l;CG2) after heat shock at 37 °C. B–D Analysis results of the period, phase, and amplitude. The R package MetaCycle was used for rhythmic analysis of the behavior data, and there were no significant differences in the activity period between WT and roraa−/−; however, the activity phase of roraa−/− zebrafish was advanced by approximately 5 h, and the activity amplitude was significantly lower than that of WT zebrafish. E Changes in the expression of the core clock genes bmal1b, clock1a, cry1aa, and per2 in WT and Tg(hsp70 l;CG2) after heat shock at 37 °C. The black curve represents activity changes in WT, whereas the green curve represents activity changes in Tg(hsp70 l;CG2). The method for difference analysis was Student's t test; ns indicates p > 0.05, * indicates p < 0.05
Additionally, we analyzed the expression levels of clock genes in the transgenic larvae. The results indicated that, compared with the WT, Roraa overexpression significantly upregulated the expression of most clock genes, including per2, with expression trends opposite those observed in the roraa−/− mutants (Fig. 5E). These results suggest that Roraa overexpression not only regulates zebrafish behavioral rhythms but also affects clock gene expression, further supporting its critical role in behavioral rhythm regulation.
Simultaneously, we analyzed clock gene expression in the transgenic larvae. The results revealed that the expression of most clock genes was significantly greater than that in the WT, and the expression patterns of these genes were opposite to those observed in the roraa−/− mutants. These findings indicate that Roraa overexpression can significantly regulate the expression of zebrafish clock genes, further supporting the key role of Roraa in regulating zebrafish behavioral rhythms (Supplementary Fig. 7).
Transcripome sequence in WT and roraa−/− mutant
To further investigate the impact of Roraa expression abnormalities on circadian rhythm, we conducted transcriptome analysis to compared the rhythmic gene from the WT and roraa−/− mutants (Supplementary Fig. 8a. Supplementary Tables 2, 3). The results showed that 2535 genes exhibit rhythm only in WT, 1748 genes exhibit rhythm only in roraa−/−, and 617 genes exhibit rhythm in both WT and roraa−/− (Supplementary Fig. 8b). So not only the core circadian genes were altered in roraa−/− mutants, but other functional genes were changed as well. Then we focused on those lose-rhythmic genes between WT and roraa−/− mutants, since some important pathway related to these genes may be influenced. The results showed that the pentose phosphate pathway, peroxisomes, arginine and proline metabolism, amino acid biosynthesis, and more importantly, the PPAR signaling pathway and fatty acid metabolism was altered in roraa−/− mutants (Fig. 6. Supplementary Tables 4, 5).
Changes in rhythmic genes and potentially affected signaling pathways after roraa gene mutation, as revealed by transcriptome sequencing. A Clustering analysis of genes whose rhythmic expression was lost in the roraa−/− mutants compared with the WT. B KEGG pathway enrichment analysis of genes whose rhythmic expression was lost in roraa−/− mutants compared with WT. The results revealed significant enrichment of fatty acid metabolism and PPAR signaling pathways
Regulation of PPAR signaling pathway genes by Roraa and its role in lipid metabolism
To explore the regulatory role of Roraa in the PPAR signaling pathway, we conducted quantitative analyses of key genes within the pathway (Fig. 7 and Supplementary Fig. 9). The results indicated that the expression of the cpt2 and fabp2 genes decreased in the roraa−/− mutants, whereas me1 gene expression primarily increased. Additionally, the expression of the fads2 gene significantly decreased under DD conditions (Fig. 7E–H). These changes suggest that Roraa may play a crucial role in the regulation of various genes within the PPAR signaling pathway.
Roraa directly regulates core genes of the PPAR signaling pathway, influencing PPAR signaling activity. A–D Validation of changes in the expression of me1, cpt2, fads2, and fabp2 in WT and roraa mutants under LD conditions via real-time quantitative PCR. E–H Validation of changes in the expression of me1, cpt2, fads2, and fabp2 iin WT and roraa mutants under DD conditions via real-time quantitative PCR. The JTK cycle was used to determine whether gene expression was rhythmic, and differences in expression levels between the WT and mutant strains were analyzed at each time point. I–L Dual-luciferase reporter assays were used to detect the regulatory effects of Roraa on the activation of the me1, cpt2, fads2, and fabp2 promoters. Red represents mutants, whereas black represents WT; p values indicate results from JTK cycle rhythmicity analysis, with p < 0.05 indicating rhythmicity and p > 0.05 indicating a lack of rhythmicity. The method for difference analysis was Student's t test
To further verify the regulatory mechanism of Roraa in the PPAR signaling pathway, we conducted in vitro cell experiments. The results indicated that Roraa significantly regulated the expression of cpt2, fabp2, me1, and fads2 and that the regulation of fabp2 by Roraa was mediated through the RORE element (Fig. 7I–L).
In summary, these results indicate that Roraa influences lipid metabolism by regulating key genes within the PPAR signaling pathway. The regulatory role of Roraa in lipid metabolism is likely achieved through the modulation of the expression of these genes (Fig. 8).
Model illustrating the regulatory role of Roraa in circadian rhythms and metabolism. This diagram depicts the interactions between the core circadian clock components (Bmal1 and Clock) and various downstream genes, including roraa, as well as their influence on metabolic pathways. Key regulatory elements, such as E-boxes, D-boxes, and ROREs, are highlighted, indicating their involvement in the transcriptional regulation of target genes. Roraa has been shown to directly influence the expression of per2 via the RORE, linking circadian control to metabolic processes. Additionally, the pathways affected by Roraa, including the PPAR signaling pathway and Ras signaling pathway, are illustrated, emphasizing its role in lipid metabolism and overall metabolic homeostasis
Discussion
Zebrafish (Danio rerio) has emerged as an excellent model organism and has been successfully used in studies related to circadian rhythms [18] and other biomedical fields [19]. This study reveals the significant role of roraa in regulating circadian rhythms and metabolism in zebrafish. Through quantitative PCR and in situ hybridization, we confirmed the rhythmic expression of roraa and elucidated the mechanisms underlying its gene oscillation. After the construction of roraa mutants, we demonstrated that the loss of roraa leads to disruptions in circadian rhythms, as evidenced by the reduced expression of core clock genes and impaired behavioral rhythms. Additionally, we found that roraa can directly regulate the expression of per2 through the RORE in the per2 promoter. This finding enriches our understanding of the regulatory network governing circadian rhythms, particularly highlighting the new role of Rora in feedback mechanisms. Furthermore, our study examined the regulation of metabolism-related genes, such as cpt2 and fabp2, by Roraa, revealing that its loss disrupts the expression of these genes and further supporting the crucial role of Rora in maintaining metabolic homeostasis.
Our study demonstrated the rhythmic expression of the roraa gene in zebrafish and confirmed that this expression is precisely regulated by multiple elements, including E-boxes, D-boxes, and RORE elements. Core clock transcription factors such as Bmal1 and Clock activate the expression of roraa by binding to E-box elements [20], whereas D-box elements may enhance this regulation through interactions with other transcription factors [21]. Moreover, RORE elements serve as binding sites for Rora and other orphan nuclear receptors, directly regulating the expression of roraa. Compared with studies in mice, the complexity of the Rora gene family in zebrafish may result in differences in regulatory mechanisms [22], due to zebrafish possess two Rora genes (roraa and rorab) [17], and this study focused solely on roraa, which may influence the regulation of circadian rhythms. After Rora is deleted in mice, the expression of clock genes such as Bmal1 and Clock is significantly reduced. It also causes changes in the mice's activity patterns, weakens the circadian regularity of their activities, and leads to circadian activity disorders [23]. These data are similar to those of zebrafish. This finding indicates that Rora has conserved functions across species. In zebrafish, there are two rora genes. The phenotypes of zebrafish with rorab knockout or double knockout of rora also need further exploration.
Roraa directly regulates the expression of per2 through the RORE element, providing a new perspective on the mechanisms of circadian rhythm regulation. While previous studies have confirmed the importance of Rora in circadian biology [23], no studies have reported its ability to influence the transcription of per2 via the RORE. Research indicates that Rora can affect the circadian feedback loop by modulating the expression of Bmal1 and Clock, highlighting its importance in regulating the expression of circadian rhythm genes [23, 24]. In mouse models, the absence of Rora leads to a significant reduction in circadian genes such as Bmal1 and Clock, impacting the stability of overall biological rhythms [23, 25], which is consistent with our findings and suggests that the role of Roraa in zebrafish may be conserved [26]. In our previous studies, we found that Per2 can directly bind to Rora and regulate the expression of bmal1b through RORE [27]. In mammals, the regulation of this loop is achieved by REV-ERBα [28]. The findings of this study reveal that Rora directly regulates the expression of per2, adding a crucial node to the regulation of the core circadian clock loop. This provides new perspectives for a more in-depth understanding of the precise 24-h cycle regulation of the core circadian clock loop.
Transcriptomic data analysis revealed that the deletion of roraa results in significant changes in several metabolic pathways, including fatty acid and glucose metabolism. The relationship between circadian rhythms and metabolism is close, with the circadian clock regulating physiological processes’ day‒night cycles, including metabolic rate [3], energy balance [29], and food intake [30]. Studies have shown that disruptions in circadian rhythms are linked to metabolic diseases such as obesity and diabetes, emphasizing the importance of maintaining normal circadian function [31]. In previously study, Rora also plays a direct and critical role in metabolic regulation [32]. The literature indicates that Rora can influence fatty acid oxidation and synthesis by directly modulating the expression of lipid metabolism-related genes [8], however, many mechanisms remain unknown. In our study, transcriptomic data revealed a significant decrease in the expression of fatty acid metabolism-related genes (such as cpt2 and fabp2) after roraa deletion, Additional research has proven that Roraa can directly affect the expression of these genes via transcriptional regulation. These results underscore the importance of Roraa in maintaining metabolic homeostasis, particularly within the regulatory network linking circadian rhythms and metabolism.
Cpt2 and Fabp2 are important core genes in the PPAR signaling pathway [33]. Cpt2, also known as carnitine palmitoyltransferase 2, mainly functions in the transport of long—chain fatty acids, transferring long—chain fatty acids from the cytoplasm to the mitochondria for β—oxidation to provide energy for cells [34]. Fabp2, or fatty acid—binding protein 2, is mainly functions to bind and transport fatty acids, facilitating the absorption, transport, and metabolism of fatty acids [35]. The significant decrease in the expression of these genes after the deletion of roraa indicates that the deletion of roraa disrupts the PPAR signaling pathway. The peroxisome proliferator-activated receptor (PPAR) family plays a central role in regulating lipid metabolism, energy balance, and inflammatory responses [36], with PPARγ and PPARδ being particularly important in fatty acid oxidation and energy metabolism [37]. Previous studies have shown that RORα negatively regulates the transcriptional network of PPARγ to control hepatic lipid homeostasis [8]. This negative regulation indicates that RORα can inhibit PPARγ activity, thereby affecting lipid synthesis and storage. Roraa directly regulates the expression of cpt2 and fabp2, indicating that Roraa can directly participate in the transport, absorption, and metabolism of long-chain fatty acids through this pathway, and further regulate the metabolic process of long-chain fatty acids.
Limitation
The main limitations of this article include, firstly, although zebrafish are widely used as model organisms, their physiological and genetic differences from humans may limit the generalizability of the research findings; we have already validated the experimental data in mice. Secondly, while the article discusses the mechanism by which Roraa regulates the expression of per2 through the RORE element, the regulatory networks within the organism are far more complex than revealed, and there may be other unexplored factors involved, requiring further validation. Lastly, there is a homologous gene to roraa in zebrafish, rorab, which necessitates future studies involving the double knockout of roraa and rorab to further investigate how they co-regulate physiological processes.
Conclusion
The article highlight the critical role of the Roraa gene in regulating circadian rhythms and metabolic processes in zebrafish. Key findings demonstrate that Roraa directly influences the expression of the per2 gene via the RORE element in its promoter, underscoring its pivotal role in the circadian machinery. Additionally, the disruption of Roraa alters metabolic pathways, particularly affecting lipid metabolism and the PPAR signaling pathway, which points to its significant impact on metabolic homeostasis.
Data availability
All data and materials used in this study are available from the corresponding author upon request.
References
Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. https://doi.org/10.1038/s41580-019-0179-2
Rosbash M (2021) Circadian rhythms and the transcriptional feedback loop (nobel lecture)*. Angew Chem Int Ed Engl 60:8650–8666. https://doi.org/10.1002/anie.202015199
Reinke H, Asher G (2019) Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20:227–241. https://doi.org/10.1038/s41580-018-0096-9
Partch CL, Green CB, Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24:90–99. https://doi.org/10.1016/j.tcb.2013.07.002
Guillaumond F, Dardente H, Giguère V, Cermakian N (2005) Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20:391–403. https://doi.org/10.1177/0748730405277232
Fagiani F, Di Marino D, Romagnoli A et al (2022) Molecular regulations of circadian rhythm and implications for physiology and diseases. Sig Transduct Target Ther 7:1–20. https://doi.org/10.1038/s41392-022-00899-y
Lee JM, Kim H, Baek SH (2021) Unraveling the physiological roles of retinoic acid receptor-related orphan receptor α. Exp Mol Med 53:1278–1286. https://doi.org/10.1038/s12276-021-00679-8
Kim K, Boo K, Yu YS et al (2017) RORα controls hepatic lipid homeostasis via negative regulation of PPARγ transcriptional network. Nat Commun 8:162. https://doi.org/10.1038/s41467-017-00215-1
Byun J-K, Choi Y-K, Kang YN et al (2015) Retinoic acid-related orphan receptor alpha reprograms glucose metabolism in glutamine-deficient hepatoma cells. Hepatology 61:953–964. https://doi.org/10.1002/hep.27577
Sun Y, Liu C-H, SanGiovanni JP et al (2015) Nuclear receptor RORα regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc Natl Acad Sci U S A 112:10401–10406. https://doi.org/10.1073/pnas.1504387112
Han Y-H, Kim H-J, Na H et al (2017) RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis. Cell Rep 20:124–135. https://doi.org/10.1016/j.celrep.2017.06.017
Kostrouchova M, Krause M, Kostrouch Z, Rall JE (1998) CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development 125:1617–1626. https://doi.org/10.1242/dev.125.9.1617
Carney GE, Wade AA, Sapra R et al (1997) DHR3, an ecdysone-inducible early-late gene encoding a Drosophila nuclear receptor, is required for embryogenesis. Proc Natl Acad Sci U S A 94:12024–12029. https://doi.org/10.1073/pnas.94.22.12024
Bakkers J (2011) Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91:279–288. https://doi.org/10.1093/cvr/cvr098
Choi T-Y, Choi T-I, Lee Y-R et al (2021) Zebrafish as an animal model for biomedical research. Exp Mol Med 53:310–317. https://doi.org/10.1038/s12276-021-00571-5
Vatine G, Vallone D, Gothilf Y, Foulkes NS (2011) It’s time to swim! Zebrafish and the circadian clock. FEBS Lett 585:1485–1494. https://doi.org/10.1016/j.febslet.2011.04.007
Flores MV, Hall C, Jury A et al (2007) The zebrafish retinoid-related orphan receptor (ror) gene family. Gene Expr Patterns 7:535–543. https://doi.org/10.1016/j.modgep.2007.02.001
Lahiri K, Vallone D, Gondi SB et al (2005) Temperature regulates transcription in the zebrafish circadian clock. PLoS Biol 3:e351. https://doi.org/10.1371/journal.pbio.0030351
Dietrich K, Fiedler IA, Kurzyukova A et al (2021) Skeletal biology and disease modeling in zebrafish. J Bone Miner Res 36:436–458. https://doi.org/10.1002/jbmr.4256
Ripperger JA, Schibler U (2006) Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian dbp transcription and chromatin transitions. Nat Genet 38:369–374. https://doi.org/10.1038/ng1738
Yoshitane H, Asano Y, Sagami A et al (2019) Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun Biol. https://doi.org/10.1038/s42003-019-0522-3
Zhong Z, Wang M, Huang G et al (2017) Molecular genetic and genomic analyses of zebrafish circadian rhythmicity. In: Kumar V (ed) Biological Timekeeping: Clocks, Rhythms and Behaviour. Springer India, New Delhi, pp 193–209
Akashi M, Takumi T (2005) The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 12:441–448. https://doi.org/10.1038/nsmb925
Sato TK, Panda S, Miraglia LJ et al (2004) A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43:527–537. https://doi.org/10.1016/j.neuron.2004.07.018
The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor α (rorα)—PubMed. https://pubmed.ncbi.nlm.nih.gov/25544291/. Accessed 6 Oct 2024
Wang M, Zhong Z, Zhong Y et al (2015) The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor α (rorα). J Biol Chem. https://doi.org/10.1074/jbc.M114.605022
Wang M, Zhong Z, Zhong Y et al (2015) The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor α (rorα). J Biol Chem 290:4367–4382. https://doi.org/10.1074/jbc.M114.605022
Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24:345–357. https://doi.org/10.1101/gad.564110
Feeney KA, Hansen LL, Putker M et al (2016) Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532:375–379. https://doi.org/10.1038/nature17407
Challet E (2019) The circadian regulation of food intake. Nat Rev Endocrinol 15:393–405. https://doi.org/10.1038/s41574-019-0210-x
Maury E (2019) Off the clock: from circadian disruption to metabolic disease. Int J Mol Sci 20:1597. https://doi.org/10.3390/ijms20071597
Billon C, Sitaula S, Burris TP (2017) Metabolic characterization of a novel RORα knockout mouse model without ataxia. Front Endocrinol (Lausanne) 8:141. https://doi.org/10.3389/fendo.2017.00141
Kersten S (2014) Integrated physiology and systems biology of PPARα. Mol Metabol 3:354–371. https://doi.org/10.1016/j.molmet.2014.02.002
Bonnefont J-P, Djouadi F, Prip-Buus C et al (2004) Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 25:495–520. https://doi.org/10.1016/j.mam.2004.06.004
Huang X, Zhou Y, Sun Y, Wang Q (2022) Intestinal fatty acid binding protein: a rising therapeutic target in lipid metabolism. Prog Lipid Res 87:101178. https://doi.org/10.1016/j.plipres.2022.101178
Han L, Shen W-J, Bittner S et al (2017) PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol 13:279–296. https://doi.org/10.2217/fca-2017-0019
Wagner N, Wagner K-D (2020) PPAR beta/delta and the hallmarks of cancer. Cells 9:1133. https://doi.org/10.3390/cells9051133
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2019YFA0800400), the National Natural Science Foundation of China (NSFC) (# 31571204), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Funding
National Natural Science Foundation of China, 31571204, Chao Liu, National Key R& D Program of China, 2019YFA0800400, Yingbin Zhong.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M., Liu, Y., Zhong, Z. et al. Direct regulation of Per2 by Roraa: insights into circadian and metabolic interplay in zebrafish. Cell. Mol. Life Sci. 82, 195 (2025). https://doi.org/10.1007/s00018-025-05696-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-025-05696-8