Abstract
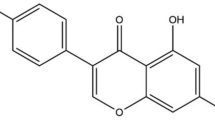
Genistein (GEN) and daidzein (DAI) are soy isoflavones known to bind to estrogen receptors. Overall health effects of GEN and DAI in humans exhibit a dual nature, presenting both health benefits and concerns related to their interaction with the estrogen receptor. The metabolomes of these isoflavones were determined in 28-day oral studies in male and female Wistar rats to elucidate (1) metabolites changes, (2) compare their metabolomes with other compounds and (3) identify toxicological modes of action (MoA). Dose levels for GEN were 1000 and 300 mg/kg bw by gavage and 1000 and 300 ppm (via diet). DAI gavage dose levels were 1000 and 100 mg/kg bw. Results were evaluated using the MetaMap®Tox data base. Both compounds demonstrated metabolome profiles which were associated with estrogenic profiles and compounds, predominantly in females. However, the metabolomes were compound specific with relatively few common metabolite changes. There were no relevant matches between any GEN and any DAI treatment group indicating that both compounds are substantially different from metabolome perspective. Ranking of the metabolome patters for GEN and DAI with ≥ 1000 compounds in the MetaMap®Tox database revealed correlations with estrogenic and other hormonally active compounds. GEN-treated females correlated best with Cabergoline, a dopamine D2 receptor agonist, DAI females with tamoxifen and diethylstilbestrol, suggesting that even their estrogenic activity may be different. Beyond estrogenic effects, the high dose (HD) DAI metabolome indicated altered fatty acid metabolism associated with PPAR-alpha activation. For GEN, there was an indication of ethanolamine-like liver effects. Dose levels without estrogenic effects for GEN were 1000 and 100 mg/kg bw for males and females respectively, there were no estrogenic effects in the feeding studies. For DAI males, the no estrogenic effect level was 300 mg/kg bw, for females < 100 mg/kg bw, suggesting that DAI may be a more potent estrogen than GEN in rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Isoflavones, well-known phytoestrogens, are a group of naturally occurring plant compounds that belong to the flavonoid family and are found in soybeans and other legumes. Soy is the richest and most significant human dietary source of isoflavones. The content in soybeans varies considerably and is influenced by factors, such as origin and maturity. The most common isoflavones in soy expressed as aglycone are DAI (approx. 47% of all isoflavones), GEN (approx. 40% of all isoflavones) and glycitein constituting only a minor part (approx. 13% or all isoflavones) (Forslund and Andersson 2017). Each of these isoflavones is also found as β-glucosides yielding a total of 12 different isomers. Formononetin and biochanin A are also found in soy and are methylated precursors of DAI and GEN, respectively (Forslund and Andersson 2017).
Among the numerous isoflavones in the nature, DAI and GEN have gained significant attention due to their estrogenic activity. The overall health effects of GEN and DAI exhibit a dual nature, presenting both promising health benefits although concerns related to their interaction with the estrogen receptor have been expressed. Both isoflavones exhibit estrogenic effects by binding to estrogen receptors (ERs), particularly ERβ (Kuiper et al. 1998). This interaction results in modulation of gene expression and activation of estrogen-responsive pathways which influence gene expression, and the production of proteins and other molecules involved in various physiological processes. They act as selective estrogen receptor modulators, demonstrating tissue-specific effects. For instance, they may exert estrogenic effects in bone tissue, promoting bone density, while displaying anti-estrogenic effects in breast tissue (Setchell 2001). Studies have suggested that GEN may have a higher affinity for estrogen receptors compared to DAI, and it may exhibit stronger estrogenic effects in certain contexts (Swedenborg et al. 2009). However, the estrogenic effects of these compounds can be tissue-specific, and the relative potency may vary depending on the specific biological system being studied. Additionally, their metabolites, which result from the breakdown of these compounds in the body, can contribute to their overall estrogenic effects (Vitale et al. 2013).
Several studies have indicated protective effects of isoflavones, particularly in the context of hormone-related conditions, such as breast and prostate cancers, osteoporosis, and cardiovascular diseases (Gómez-Zorita et al. 2020; Rietjens et al. 2016). They also bear the potential as alternatives to traditional hormone replacement therapy, particularly in mitigating menopausal symptoms (Messina et al. 2022). Additionally, their antioxidant and anti-inflammatory properties have been implicated in mitigating oxidative stress and inflammation, contributing to overall health benefits (Ruiz-Larrea et al. 1997). Studies suggest that these isoflavones may contribute to cardiovascular health by improving lipid profiles and endothelial function (Taku et al. 2010).
However, alongside these potential benefits, concerns about the toxicological aspects of GEN and DAI have emerged. Research suggests that high exposures to these isoflavones may disrupt endocrine function, posing risks, such as altered reproductive outcomes and thyroid dysfunction (Guelfi et al. 2023; Jefferson 2010). While some studies propose a protective effect against breast cancer due to their anti-estrogenic properties in breast tissue, concerns have been raised about potential dual effects, particularly in estrogen receptor-positive breast cancer (Hilakivi-Clarke et al. 1999). Given the multi-faceted nature of these isoflavones, a better understanding how they change the physiology would provide a better basis for assessing potential risks of these compounds.
‘Omics’ technologies provide a wealth of information on distinct molecular endpoints simultaneously which can improve the safety assessment process (Bugrim et al. 2004). Metabolomics is defined as the analysis of the complete set of endogenous small-molecule metabolites that are caused by specific cellular processes within a biological sample. It determines the concentration and the change of endogenous metabolites by analyzing biological samples such as blood and provides information on changes of the physiological status of an entire organism following exposure to an exogenous compound (Robertson 2005). Metabolomics has been used to identify biomarkers for disease state, drug effect and toxicity (Boudonck et al. 2009; Mattes et al. 2014). The metabolome is placed downstream of the transcriptome and the proteome, integrating regulatory steps of upstream levels of organization, therefore being closer to the actual phenotype (Fukusaki 2015). Metabolomics can also be used for pattern recognition approaches wherein the responses of several signals are collectively used to characterize a particular state or response (Nicholson et al. 1999). Such pattern recognition approaches are most accurate when the reference patterns are based upon a large database of profiles collected under controlled conditions (Strauss et al. 2009; van Ravenzwaay et al. 2015). BASF’s MetaMap®Tox database, which has been developed over the last 20 year and contains the metabolome of more than 1000 compounds, can be used to answer various toxicological questions and allows for a comparison of the metabolome under investigation with all other metabolomes in the data base (Kamp et al. 2012; Montoya et al. 2014).
The present studies were performed to elucidate the metabolic perturbations induced by a 28-day exposure to GEN and DAI in rats and to explore the possibilities of using metabolomics as a research tool to address the following questions: (1) which metabolome changes are produced by GEN and DAI? (2) are the metabolomes of both compounds comparable? (3) how do the metabolomes compare with effects induced by other estrogenic compounds? and (4) can other toxicological MoA be identified? To obtain this information, both compounds have been investigated in 28-day studies in rats with metabolome analysis on days 7, 14, and 28.
Materials and methods
Ethics statement
The studies were approved by the BASF Animal Welfare Body and performed according to the German Animal Welfare Act and EU Directive 2010/63, with the permission of the local authority, the Landesuntersuchungsamt Rheinland-Pfalz (permission numbers G 07-3-001 and 23 177-07/G 18-03-098). The laboratory is AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) certified.
Retention of records
The studies were performed in the spirit of GLP. GLP-relevant records and materials are archived at BASF SE for at least the period specified in the GLP regulation. This includes the study plan, any amendments, raw data, and the study report.
Test substance and administration
In study 1, GEN (purity 98%) was prepared in drinking water containing 1% CMC once before the start of the study and stored deep frozen until the daily use. GEN was administered daily by gavage at dose levels of 300 and 1000 mg kg/bw/day, to 5 animals per sex per treatment group. The control group consisted of 10 animals per sex. The volume administered was 10 mL/kg bw/day. In study 2, GEN (purity 98%) was administered in the diet at concentrations of 1000 ppm and 300 ppm to 5 animals per sex per treatment group. The control group consisted of 10 animals per sex. In study 3, DAI (purity 99.88%,) was administered orally by gavage at doses of 1000 mg/kg bw/day and 100 mg/kg bw/day to 5 animals per sex per treatment group, control groups had 10 animals per sex (Table 1). DAI was suspended in corn oil and administered at a concentration of 5 mg/kg bw/day. Due to reduced food consumption and body weight loss at 1000 mg/kg bw/day, the dose level was reduced to 300 mg/kg bw/day at day 14 of treatment. Diet, ground Kliba mouse/rat maintenance diet “GLP” supplied by Provimi Kliba SA, Switzerland, and drinking water were available ad libitum (except before blood sampling) and regularly assayed for chemical contaminants, including sources of estrogenic activity such as isoflavones, and the presence of micro-organisms.
Animal maintenance
Wistar rats (Crl:WI(Han) were obtained from Charles River Laboratories, Sulzfeld, Germany. At the beginning of the treatment, the age of the animals was 70 ± 1 days. Five animals were housed together per cage. The animals were accommodated in fully air-conditioned rooms in which central air conditioning resulted in uniform temperature range of 20–24 °C, a range of relative humidity of 45–65%. The day/night cycle was 12 h.
Animal examinations
A check for moribund and dead animals was made twice daily from Mondays to Fridays and once daily on Saturdays, Sundays, and public holidays. The animals were checked daily for clinical abnormal signs and changes were documented for each animal. Food consumption was determined on study days 6, 13, 20, and 27. Drinking water consumption was monitored daily by visual checks. Body weights were determined before the start of the administration period to randomize the animals. During the administration period, the body weights were determined on study days 0, 6, 13, 20, and 27.
Blood was taken from the retroorbital venous plexus in the morning from fasted animals on day 7, 14, and 28 following anesthesia with Isoflurane (Isoba, Essex GmbH, Munich, Germany). From each animal, 1 ml of blood was collected with EDTA as anticoagulant (10 μl of a 10% solution). The samples were centrifuged, and the plasma was separated. Blood was sampled and prepared in Eppendorf tubes. The preparation of the samples was done under cooling. All samples were stored covered with a N2 atmosphere at –80 °C and sent (stored in dry ice) to BASF metabolome solutions GmbH, Berlin for metabolome analysis.
Metabolome analysis
Plasma metabolome analyses using Mass Spectrometry were performed on all samples: GC–MS (gas chromatography–mass spectrometry) and LC–MS/MS (liquid chromatography–MS/MS) were used for broad profiling (van Ravenzwaay et al. 2007). For studies 1 and 2 (GEN) for GC–MS analysis, the non-polar fraction was treated with methanol under acidic conditions to yield the fatty acid methyl esters derived from both free fatty acids and hydrolyzed complex lipids. The non-polar and polar fractions were further derivatized with O-methyl-hydroxylamine hydrochloride and pyridine to convert oxo-groups to O-methyl-oximes and subsequently with a silylating agent before analysis (Roessner et al. 2000). For LC–MS analysis, both fractions were reconstituted in appropriate solvent mixtures. HPLC was performed by gradient elution using methanol/water/formic acid on reversed-phase separation columns. Mass spectrometric detection technology was applied which allowed target and high-sensitivity MRM (Multiple Reaction Monitoring) profiling in parallel to a full-screen analysis (WO2003073464).
For study 3, a slightly adapted and improved methodology was used (Kamp et al. 2024). From 60 μL rat plasma, metabolites were extracted with a mixture of methanol, dichloromethane, water, and toluene buffered with ammonium acetate. Internal standards were added to the extraction mixture to ensure reproducible analysis. After centrifugation, an aliquot of the extract was subjected to LC–MS/MS analysis using reverse phase and hydrophilic interaction liquid chromatography (HILIC) followed by MS/MS detection. A second aliquot of the extract was mixed with water resulting in a phase separation. Polar and non-polar phases were analyzed with GC–MS after derivatization. The polar fraction was chromatographed on a DB-XLB column, and the non-polar fraction was chromatographed on a HP-5MS column.
For all studies, the samples were analyzed once in a randomized analytical sequence design to avoid artificial results with respect to analytical shifts. For GC–MS and LC–MS/MS profiling, data were normalized to the median of reference samples. The metabolites were analyzed using the single peak signal of the respective metabolite and a normalization strategy according to the patent WO2007012643A1, resulting in ratio values representing the metabolite change in treated versus control animals.
Steroid hormones, catecholamines, and their metabolites were measured from separate plasma aliquots, 50 µL each, by online SPE-LC–MS/MS (Solid-phase extraction–LC–MS/MS, Symbiosis Pharma Pro Online SPE UHPLC System, Spark Holland) in positive electrospray mode using stable isotope-labeled internal standards (Yamada et al. 2002; Zhang et al. 2011).
Prior to steroid analysis, the samples were extracted with 500 µL MTBE (methyl t-butyl ether) and after evaporation, the residue was derivatized by dissolving in 80 µL acetonitrile/water (9:1), ultrasonication for 3 min, addition of 40 µL dansyl chloride (1 mg/mL in acetonitrile) and 40 µL NaHCO3 (0.2 mol/L), then shaking for 20 min at 60 °C at 400 rpm (Thermomixer) and addition of 75 µL of water after cooling to ambient temperature. Chromatography was performed with a YMC Triart C18 ExRs plus 50 × 2.1 mm × 3 µm HPLC Column at 30 °C using 5% acetonitrile in water with 0.1% formic acid (w/w/w) (A) and acetonitrile with 0.1% formic acid (w/w) (B) (0 min 5% B, 5.5 min 65% B, 8 min 100% B, 9.5 min 100% B, 600 µL/min).
For catecholamine analysis, 50 µL of 0.01 M HCl (internal standard solution) was added and proteins were removed by membrane filter tube centrifugation for 45 min at 13,000 rpm, 4 °C. 50 µL of each NaHCO3 (0.2 mol/L), dansyl chloride (2 mg/mL in acetone), and methanol was added to the filtrate and kept for 12 h at 4 °C prior to analysis. Chromatography was performed with an ODS 7 pH, 60 mm x 2 mm, 4 µm HPLC Column (Alltech Grom) at 30 °C using 5% acetonitrile in water with 0.5% formic acid (w/w/w) (A) and acetonitrile with 0.5% formic acid (w/w) (B) (0 min 40% B, 4 min 52% B, 4.17 min 70% B, 7 min 75% B, 11.67 min 90% B, 12 min 90% B, 300 µL/min). Absolute quantification was achieved by means of stable isotope-labeled standards. All samples were analyzed in a randomized analytical sequence to avoid analytical bias. Data were corrected to internal standards and normalized to the median of reference samples which were derived from a pool generated from sample aliquots of control animals to account for inter- and intra-instrumental variation. For all metabolites, changes were calculated as the ratio of the median of metabolite levels in individual rats in a treatment group relative to median of metabolite levels in rats in a matched control group (time point, dose level, sex).
Metabolome evaluation using MetaMap®Tox
The heteroscedastic t-test ("Welch test") was applied to determine statistical significance of metabolite levels between samples from treated animals and respective controls at p < 0.05. The percentage of significantly changed metabolites was calculated by dividing the number of significantly changed metabolites by the total number of measured metabolites. Test substance-related changes in the metabolome were analyzed as follows:
-
1)
Analysis of specific metabolic changes for each dose group.
-
2)
Similarity analysis of GEN and DAI metabolic profiles with predefined patterns in MetaMap®Tox (> 110 patterns, currently covering 42 MoA) was conducted with an algorithm using a median r value metric. A good match requires that 90% or more of metabolites are significantly changed in the same direction as defined by the pattern (weak match: 75– < 90%; equivocal result 50– < 75%; mismatch: < 50%) (Kamp et al. 2012).
A comparison of the entire metabolomic profiles of GEN and DAI with those of all the compounds available in MetaMap®Tox (ca. 1,000) was performed using Spearman and Pearson correlations. This is obtained by calculating all pairwise coefficients of the entire database stratified by sex (male/female) and dose (high/low). This procedure considers all metabolites. A threshold value of 0.40 for male animals and 0.50 for female animals is equivalent to the 95th percentile of all correlation coefficients. Correlation coefficients at or above these values are considered as providing biologically relevant information. The compounds with the best overall matches are highly likely to share at least part of the toxicity profile of the compound under evaluation.
The MetaMap®Tox database, used for the present metabolome analysis, has been developed by data obtained from many independent studies done over time. These were performed based on the principals of the standardized OECD guideline regulatory studies in the same laboratory using the same experimental set-up. The combination of these independent studies provides information on a broad range of chemicals and active ingredients. It is acknowledged that this information and the resulting predictions of toxicity of evaluated compounds are valid only within the described experimental set-up.
Statistics
For clinical examinations, the two-sided DUNNETT's test was used. The software used for these statistical calculations is part of the GLP certified pathology data system Acopat 2.0 used at BASF and the algorithms were validated in SAS. Principal Component Analysis (PCA) was calculated using R version 4.3.2 (R Core Team, 2023).
Results
Clinical observations, food consumption, and body weight
No animals died or were sacrificed moribund prematurely in the present study.
GEN did not affect food consumption in a biologically relevant way. Occasionally food consumption was a little higher (max 5%) than in controls, however, without a consistent trend. In DAI study at the 1000 mg/kg bw/day dose level, there was a profound reduction in food consumption during the in the second week of administration (ca. 40% in males and 45% in females).
Body weight development of GEN-treated animals was not affected in any of the treatment groups. The HD DAI males and females lost body weight during the first 14 days of the study (16% and 19% respectively). In the 100 mg/kg bw/day group, body weight in males and females was not affected. Considering reduced food consumption and loss of body weight gain in HD group, it was decided to reduce the 1000 mg/kg bw/day dose to 300 mg/kg bw/day from day 14 until the end of the study.
Metabolomics
A table with all metabolite values for GEN and DAI can be found in the supplementary information.
For GEN-treated animals, there was a clear dose response in the number of metabolites changed relative to controls. In male animals, up to 23% of all measured metabolites were changed on day 7 of administration, and in females, this value was 40.2%. For both sexes, the number of altered metabolites was the highest at d7 declining steadily to 14.0% at d28 in males and to 19.2% in females, potentially an adaptation to the treatment. At the lowest concentration of 200 ppm (17–20 mg/kg bw/day), the values were close to the 5% false discovery rate, ranging between 6.3% and 12.1% in males and between 5.1% and 10.2% in females. For DAI, a dose–response was evident, very high values noted for the HD animals (up to 40.8% in males and 45.8% in females, at day 7). These, however, are likely to include a metabolome effect of the reduced food intake and body weight loss. The low dose level (100 mg/kg bw/day) demonstrated clear metabolite changes (see Fig. 1).
The profile strength, calculated as the down-rounded average of absolute medians of all t values, including the absolute number of significantly changed metabolites as well as the magnitude of the respective changes for all days showed similar results (Table 2).
Metabolite changes and classes
As both isoflavones are considered to have a common estrogenic MoA, we first identified those metabolites which had changed in the same direction at two or more time points, separated by sex (Tables 3 and 4).
There were only 7 metabolites which were consistently changed in the same direction for HD-treated males with DAI and GEN. There were no dominant classes of metabolites altered. In contrast, the number of commonly changed metabolites for HD females was higher (27) and the changes were mostly seen in the class of complex lipids and fatty acids (Table 4). The observed reduction in testosterone is most likely related to a feedback mechanism related to the perceived high presence of estrogens, resulting in a reduction of production of sex hormones.
In addition to the common metabolites of the two compounds, we also identified which metabolites are uniquely changed for either GEN or DAI. Tables 5 and 6 show these metabolites for male and female rats treated with the HD of GEN, Tables 7 and 8 for HD DAI-treated males and females, respectively.
There were 31 uniquely changed metabolites for GEN HD-treated males. Most of these belonged to the class of complex lipids and fatty acids and more specifically to the sphingolipids and sphingomyelins, and nearly all of them had reduced values. For females, there were 60 uniquely changed metabolites, specifically complex lipids, particularly fatty acids, dominated the compound specific metabolome changes (Table 6). Like the males, nearly all metabolites had reduced values. The only exception (for both sexes) being a single triacylglycerol (TAG), however not the same one. In addition, a relatively high number of amino acids or related metabolites were altered.
For the HD-treated DAI males and females, a similar picture emerges (Tables 7 and 8).
There were 46 uniquely changed metabolites for DAI HD-treated males. Most of these belonged to the class of complex lipid and fatty acids. Within these groups, the fatty acids and phosphatidylcholines were most prominent, and all of these had reduced values. For females (Table 8) treated with HD DAI, there were 36 uniquely changed metabolites. The classes of metabolites which represented most of these compound specific changes were amino acids, complex lipids, and fatty acids as well as hormones. In contrast to GEN, all fatty acids showed increased values. In addition, various steroid hormones were reduced, again most likely due to a feedback mechanism related to the perceived high presence of estrogens, resulting in a reduction of production of sex hormones.
Identification of toxicological MoA by pattern ranking
GEN
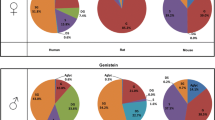
At a dose of 1000 mg/kg bw/day, the comparison of the metabolite changes induced by GEN against the previously established specific metabolite patterns present in MetaMap® Tox resulted for males in 2 weak matches: reduced food consumption at d7 and gonadal steroids agonists (not sign. on day 14). For females, there was one good match: gonadal steroids agonists pattern and three weak matches: prolactin antagonists, endocrine-related effects on ovaries and gonadal steroids agonists patterns. For the 300 mg/kg bw/day group, there were no matches for males. For females, there was a good match with estrogenic agonists and two weak matches for estrogenic agonists and endocrine-related effects on ovaries. For the feeding studies, for males, there was a weak match for oxidative stress in the liver at day 28 for both groups (1000 ppm and 200 ppm). For females, there were no matches at either concentration.
DAI
For the HD males, there were several weak matches, two related to endocrine effects: estrogenic agonists (day7), gonadal steroids agonists (day7) and four which are associated with the liver: oxidative stress (day7), long-chain phthalate-associated changes (day7), PXR agonists, peroxisome proliferators (day7) patterns. For the HD females, there was a weak match with estrogenic agonists (day7) and endocrine-related effects on ovaries (day7). These data indicate that most of the HD matches were observed at d7 but not at day 28 when the dose was reduced to 300 mg/kg bw/day. At the LD for males, there were no matches, and for females, only one weak match, gonadal steroids agonists at d28.
Total profile comparison
The total profile comparison uses the full metabolome profile of the compounds investigated separated by sex and dose level and compares these against > 1000 substance metabolite profiles in the MetaMap®Tox database. A threshold value of 0.40 for male animals and 0.50 for female animals is equivalent to the 95th percentile of all correlation coefficients indicating biologically relevant correlations. The results of the HD treatment groups are presented below and for all LD groups shown in the supplementary information.
GEN-1000 mg/kg bw/day males
The profile comparison of this group (Table 9) demonstrated associations with 28 treatments at a level considered biologically relevant (> 95%). The subsequent evaluation is based on 24 of these treatments, excluding four BASF proprietary ones. Thirteen out of the 24 were associated with hormonal effects. Although several of these were directly connected with estrogenic activity (17-alpha-ethinylestradiole, diethylstilbestrol and raloxifene, an estrogen receptor agonist), the compound which ranked the highest was Mifepristone, a 19-nortestosteron derivative which is a competitive inhibitor of the progesterone receptor. Other hormone-associated compounds with a high ranking are Buserelin and Leuprolide. These drugs stimulate the GnRH receptor in the hypothalamus and would result in an increase in LH (and in females FSH as well), initially increasing testosterone and estradiol production. In humans, at HD levels, this drug is used to desensitize the GnRH receptor, resulting in the opposite effect. The latter compounds, however, were tested in the same experimental set-up, i.e., shared the same control group, which may lead to an overestimation of the correlation coefficient. Another compound with the same MoA is Leuprolide. In addition to estradiol-related activities, there were also some compounds associated with testosterone. These include 17-alpha-methyl-testostrone as well as Anastrozole. The latter inhibits the enzyme aromatase, which converts testosterone into estradiol, resulting in more testosterone and less estradiol.
Among nonendocrine-related compounds was ethanolamine or 2-aminoethanol (MEA) and 3-amino-1-propanol (3AP). Both substances are alkanolamines and known to change lipid metabolism (Sperber et al. 2019). MEA is part of the membrane constituting class of glycerophospholipids as well as a degradation product of the amino acid serine (ECHA SEV 2016) and known to have change choline uptake and metabolism. MEA has been reported to inhibit the uptake of choline in various tissues and organs as mentioned earlier (Lipton et al. 1988; Zha et al. 2011). Finally, Clofibrate (a peroxisome proliferator) was also observed to be associated with the metabolome profile of GEN-1000 mg/kg bw males, which is in line with the observation by Kim et al. (Kim et al. 2004) that GEN could be a PPAR-alpha agonist. Some compounds observed to have metabolome similarities were tested using the same control group, which may have led to bias as they were normalized to the same controls.
Overall, it is concluded that there is good concordance between MoAs indicated in pattern ranking (e.g. hormone-like effects) and the substances identified in the profile comparison. Liver-related toxicity based on pattern ranking appears to be associated with ethanolamine-like effects as well as with lipid metabolism with a suggestion of a peroxisome proliferator-like effect. However, as there were no specific patterns identified for liver toxicity, or peroxisome proliferation, these indications are speculative.
There was no association between 1000 mg/kg bw GEN males and any of the DAI treatment groups.
GEN-1000 mg/kg bw/day females
There were 30 treatments (Table 9) found at a level considered biologically relevant (> 95%). The subsequent evaluation is based on 26 of these compounds, excluding four BASF proprietary ones. Most of these were associated with hormonal (estrogenic) effects. The compound which ranked the highest, after the match with 300 mg/kg bw/day GEN, was Cabergoline, a dopamine D2 receptor agonist. In the hypothalamus–pituitary axis, dopamine (DOPA) acts as a prolactin-inhibiting factor (PIF), and as such Cabergoline is a prolactin-inhibiting drug. Thus, the connection with the weak match “anti-prolactin” like effects is reinforced. The connection with the estrogenic effects of GEN may not be immediately apparent. However, as estradiol increases DOPA synthesis (Pasqualini et al. 1995), as well as enhancing the sensitivity of the DOPA receptor in the brain (Hruska and Silbergeld 1980) and thus increasing the effect of DOPA on the pituitary, a reduction of prolactin is a logical consequence. The second highest ranking compound was norethindrone, a synthetic progestin. Mibolerone and Trenbolone, ranking relatively high are synthetic androgens, which, when dosed at high concentrations also have direct estrogenic activity (Rizza et al. 2014) or following biotransformation (Büttner and Thieme 2010). In this context, it is not surprising that 17-alpha-methyltestosterone is found amongst the compounds which are ranked as biologically significant.
Few compounds were not associated with hormonal effects and did not provide consistent evidence for a particular MoA. Of interest is galactosamine, which is a constituent of some glycoprotein hormones, such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Tolvo et al. 1982). There were two compounds which were tested in the same experimental set-up as GEN, which were excluded based on potential variation bias for this evaluation.
There was no association between 1000 mg/kg bw GEN females and any of the DAI treatment groups.
Comparing the 1000 mg/kg bw GEN-treated females and males, it is observed that there were far more estrogen-associated compounds in females than in males. This is related to the fact that females have more estrogenic receptors and consequently a stronger response is elicited. This is in line with the overall stronger metabolome response in females compared to males.
The results of the total profile comparison for the lower doses of GEN can be found in the supplementary information. As there are fewer compounds with correlations above the threshold and to avoid repletion, these findings are not individually evaluated here. In the discussion section, however the overall results are discussed for all treatment groups. It was observed that there were no compounds at all with good correlations for the 1000 ppm and 300 ppm GEN females.
DAI 1000/300 mg/kg bw/day males
There were 32 treatments found at a level considered biologically relevant, 10 of which were excluded from the evaluation as they were BASF proprietary ones. There was a single compound match with 17-alpha-ethinylestradiol, indicating an estrogenic effect which is in line with the pattern ranking for DAI at this dose level. Most of the remaining compounds are associated with liver effects. Many of these are known to influence fatty acid metabolism and to cause peroxisome proliferation (e.g. mecoprop, clofibrate, diethyl-hexylphthalate) and in line with the pattern ranking. There was no match with GEN. Thus, the overall assessment is made that there are some indications of an estrogenic effect in HD DAI-treated males, but that most metabolome changes indicate an effect on the liver associated with metabolism and in particular with the peroxisome proliferation.
DAI 1000/300 mg/kg bw/day females
There were 31 treatments found at a level considered biologically relevant. The subsequent evaluation is based on 24 of these, excluding seven BASF proprietary ones. Three of the excluded compounds with ranks 1–3 were indicated to influence the liver. This information is considered in the overall assessment. There were 11 compound matches related to hormonal effects, some directly associated with estrogenic activity (17-alpha-ethinylestradiol, HD para-nonylphenol) or modulating such effects (Tamoxifen) while others were related to androgenic effects (Mibolerone and 17-alpha-methyl testosterone). These, however, may also induce estrogenic effects related to metabolism and a weak agonistic effect on the estrogen receptor. Of the remaining compounds, some are associated with liver effects (3 BASF proprietary treatments, Clofibrate, Nitrofen).
There was no match with GEN. Thus, the overall assessment is made that HD DAI has estrogenic effects. In addition, there are also indications for liver effects. However, in contrast to the HD males, the association with peroxisome proliferators is only weak.
PCA
The metabolome changes and a comparison for two substances with known estrogenic activity are shown in the two PCAs for females and males respectively (Figs. 2 and 3). The figures show that for females, the direction of change of both GEN and DAI is like the two positive controls, indicative for a similar MoA. It can also be seen that the low GEN doses are in the same region as the controls. In males, the observed changes do not align with those seen in the positive controls, suggesting that alternative mechanisms of action, rather than estrogenic effects, play a more prominent role.
Discussion
Clinical data indicate that DAI is more toxic than GEN as the 1000 mg/kg bw /day dose (both administered via gavage) could not be maintained for the former for more than 14 days, whereas GEN was virtually non-toxic at this dose. The HD metabolome of both compounds demonstrated that the metabolite changes were rather different with only 7 metabolites consistently changed in the same direction. The higher number of commonly changed metabolites in females is likely to be related to the more pronounced estrogenic effects induced in females. A similar picture was observed in pattern ranking and total profile comparison. The most prominent metabolite changes induced by GEN were associated with complex lipid and fatty acids (males—sphingolipids and sphingomyelins, females—fatty acids) nearly all of which were reduced. Decreases in sphingolipids in HD male animals have been observed with substances associated with estrogenic activity. For DAI, there were more profound differences between males and females, whereas for males, reduced values for fatty acids and phosphatidylcholines were observed. For females, amino acids, complex lipids, hormones and fatty acids (with increased values) were the dominant metabolite classes. The latter metabolite changes are often encountered with substances associated with hepatic enzyme induction and liver toxicity in female animals. In conclusion, the metabolomes of GEN and DAI show few similarities. In females, more commonly changed metabolites were observed most likely related to a more pronounced effect on steroid hormones than in males.
Assessment GEN
Males
At 1000 mg/kg bw/day, there is a good concordance between MoAs indicated in pattern ranking (hormone-like effects) and the substances identified in the profile comparison. Hormone-like effects have an estrogenic component. However, other effects on steroid hormones are also possible. Liver-related toxicity based on pattern ranking appears to be associated with ethanolamine-like liver effects as well as lipid metabolism, with a hint toward a peroxisome proliferator-like effect.
At 300 mg/kg bw/day, there was no indication of estrogenic like effects. There were no good or weak matches with other MoAs. Based on the good compound ranking with MEA and 3AP, an effect on liver functionality like the one noted for the 1000 mg/kg bw/day group may be assumed.
The administration of 1000 ppm GEN (ca. 80 mg/kg bw) had a slightly stronger effect on the metabolome than the 300 mg/kg bw gavage treatment. This may be related to the high number of metabolites changed at day7 (20.3%), which declined rapidly, possibly related to an adaptation to treatment. The treatment did not induce estrogenic effects. Compound ranking and the weak single pattern match suggest the potential for oxidative liver stress. Given the attenuating response over time, this may also be associated with adaptation through enzyme induction to enhance GEN biotransformation.
For the 200 ppm group (17 mg/kg bw), it was concluded that there were only marginal effects. There was no compound confirmation related to the weak match with the putative pattern for oxidative stress in the liver on day 28. There was no indication of a steroid hormone-like effect.
In conclusion, for males, only at a dose of 1000 mg/kg bw, an estrogenic-like effect was observed. The no-observed metabolomic effect level for estrogenic activity was 300 mg/kg bw. Liver-related effects were indicated at doses of 80 mg/kg bw and higher. The no-observed metabolomic effect level for liver-related effects was 17 mg/kg bw.
Females
For the 1000 mg/kg bw-treated females, there were several matches, related with hormones and endocrine activity. These matches were mirrored in the compound ranking in which 74% of these substances were associated with hormonal effects, many connected with estrogenic activity. The compound which ranked the highest was Cabergoline, a dopamine D2 receptor agonist and a prolactin-inhibiting drug. There is evidence indicating that estrogens modulate DOPA-production and -receptor sensitivity in rats (Kirk Clopton and Gordon 1985; Piccardi et al. 1983). Therefore, it is possible that at least part of the GEN-mediated effects is related to an anti-prolactin like effect, which MoA was also suggested in the pattern ranking. There was no other MoA suggested by the metabolome profiling.
For the 300 mg/kg bw-treated females, there was a good concordance between the indicated MoA (estrogenic/ endocrine activity) and compound ranking as 45% of the identified compounds were associated with hormonal effects. As for HD GEN females, the best match was with Cabergoline corroborating the weak match with the pattern anti-prolactin. There was no other MoA suggested by the metabolome profiling.
At 1000 ppm and 200 ppm (100 to 20 mg/kg bw/day, respectively), the metabolome changes were marginal and close natural fluctuation. There were no MoA suggested by pattern ranking nor by compound profile comparison.
In conclusion, for females, at doses of 300 mg/kg bw and higher, an estrogenic-like effect was observed. The no-observed metabolomic effect level for estrogenic activity was 1000 ppm (approximately 100 mg/kg bw). At this dose level, there were also no other MoA suggested.
There was no compound ranking with biological significance between GEN and DAI.
Assessment DAI
Males
In HD DAI-treated males, there were some indications of an estrogenic effect based on a single pattern and a single compound match. More prominently, an effect on the liver, associated with metabolism and with peroxisome proliferation as well some evidence for as PXR and AR agonism was indicated. For the LD-treated males, there were neither good nor weak matches with any pattern. The compound ranking was influenced by the commonly used vehicle corn oil. Therefore, the compounds found with a hormone-like effect in LD males are likely to be an experimental artifact.
In conclusion, for males, only at a dose of 1000 mg/kg bw/day, an estrogenic-like effect was observed with certainty. It was also observed that matches with estrogenic/ hormonal metabolome patterns only occurred at d7. The no-observed metabolomic effect level for estrogenic activity was 300 mg/kg bw/day. The no-observed metabolomic effect level for any effect is < 100 mg/kg bw.
Females
For the HD DAI-treated females, based on pattern matches and compound ranking, it is concluded that there was evidence for estrogenic effects and indications for liver effects. However, in contrast to the HD males, the association with peroxisome proliferators is weak. The LD DAI-dosed females also demonstrated evidence for estrogenic effects based on a pattern match and compound ranking. In addition, a liver-related MoA, possibly peroxisome proliferation, cannot be excluded.
For females, an estrogenic-like effect and liver-associated changes were observed at both dose levels. The no-observed metabolomic effect level for estrogenic activity was < 100 mg/kg bw. The no-observed metabolomic effect level for the liver also was < 100 mg/kg bw.
GEN and DAI: a comparison
Although both compounds are considered to have a common estrogenic MoA, there was never a match between any GEN and any DAI treatment groups in the compound ranking. In addition, there were far more compound-specific metabolic changes than common ones. This indicates that the substances are rather different from a metabolome profile perspective, which may even include their estrogenic MoA. This is also suggested by the very high compound ranking of Cabergoline with GEN, which occurred only for females and only for dose levels where an estrogenic/hormonal effect was observed. For DAI, there was no good compound ranking with Cabergoline. The Cabergoline MoA is indirectly associated with estrogenic receptor agonism via the DOPA pathway, inducing an anti-prolactin like effect (the latter also being indicated in pattern ranking). For the males, the liver effects of DAI are peroxisome proliferation-like, whereas this seems to be less for the GEN-treated males, where an ethanolamine-like effect on the liver appears to have a stronger association.
At a comparable dose level, the overall effects of DAI on both male and female rats are stronger than for GEN which is in line with the overall metabolome profile strength (Table 2). The number of patterns and compound matches related to estrogenic and hormonal activity is higher in females, which is related to the higher number of estrogenic receptors in females compared to males. Clearly identified estrogenic/hormonal effects in GEN-treated males were only observed at 1000 mg/kg bw, the no-observed metabolomic effect (NOMEL) for this MoA being 300 mg/kg bw. Similarly, for DAI-treated males, only the HD-treated group was affected. As matches with estrogenic/hormonal metabolome patterns were only noted at d7 (dose level 1000 mg/mg bw) and not at d14 and d28 (dose reduced to 300 mg/kg bw), it is assessed the that the NOMEL for this effect in males is 300 mg/kg bw. In GEN-treated females, estrogenic/hormonal effects were observed at 1000 and 300 mg/kg bw. The NOMEL for this MoA being 100 mg/kg bw (1000 ppm). For DAI-treated females, estrogenic-like effects were observed at both dose levels, and the NOMEL was < 100 mg/kg bw (Table 10).
In conclusion, females are more sensitive than males, and the observed estrogenic/ hormonal effects of GEN are overall less strong than those induced by DAI. Beyond the estrogenic/ hormonal MoA, the liver is the main target organ. In HD DAI males, this is clearly associated with peroxisome proliferation, and in females, there are no clear indications about the liver MoA, most likely enzyme induction. In the HD GEN males, too, an association with peroxisome proliferation (fatty acid metabolism) is indicated, but less prominently than with DAI. For the GEN-treated females, there was no convincing evidence for other MoA beyond the hormonal ones.
With respect to the overall no-observed metabolomic effect levels, in GEN-treated males, liver-related effects were indicated at doses of 80 mg/kg bw/day and higher. The no-observed metabolomic effect level for liver-related effects was 17 mg/kg bw/day. For GEN-treated females, this value was 100 mg/kg bw (1000 ppm). For DAI-treated males and females, the NOMEL was < 100 mg/kg bw for DAI. A summary of these quantitative conclusions is presented in Table 11.
Human and rat estrogen receptors (ERs) exist as two subtypes ERα and ERβ. The natural ligand for these receptors, 17-β-estradiol can activate these receptors at concentrations in the lower nM range (Kuiper et al. 1998). It was demonstrated that GEN has a 20–30-fold higher affinity for the ERβ (Kuiper et al. 1998; Morito et al. 2001). Quantitative values were provided by (Zhu et al. 2006). The IC50 value for 17β-estradiol was 11.2 nM for human ERα and 8.9 nM for ERβ. The values for GEN being for 199.5 nM for ERα and 11.2 nM for ERβ, an approximate 20-fold difference. For DAI, the IC50 values were determined to be 1000 nM for ERα and 631 nM for ERβ. Thus, DAI would appear to be approximately fivefold less potent for ERα and would have a ca. 50-fold lower binding affinity for ERβ. Similar differences for the two isoflavones were obtained by (Hillerns et al. 2005). For GEN, a value of 0.8 µm was obtained, and for DAI 7.9 µM. In these studies, 17-β-estradiol was far more potent than the isoflavones with a EC50 value of 6 nM.
The results of these molecular studies appear to be at odds with the results of the current study, in which DAI was at least as potent as GEN with respect to inducing estrogenic effects in rats. However, to translate in vitro results to in vivo studies, parameters for absorption, distribution, metabolism, and excretion need to be considered. Both isoflavones are readily absorbed and metabolized. Differences may exist with respect to plasma protein binding. The major contributing factor to the observed differences between in vitro and in vivo studies is most likely related to metabolism (SCCS 2022). Both compounds are well metabolized. However, whereas metabolism of GEN results in a reduction of ER binding, one of the DAI metabolites, equol, was shown to bind to the ER. The binding affinity of equol is like that of GEN and equol is even more potent in inducing ERα transcription (Morito et al. 2001). This indicates that metabolism of DAI may shift the ER receptor activation from ERβ to ERα and increasing its potency. This could explain why the metabolome, as well as the association with compounds in the MetaMap®Tox database for the two isoflavones is rather different. In should also be noted that such differences between molecular potency and in vivo effects pose a significant challenge for appropriate quantitative next-generation risk assessment (Najjar et al. 2024).
Although the European Food Safety Authority (EFSA) has not established a specific tolerable daily intake (TDI) for isoflavones, they concluded that consumption of 35–150 mg of isoflavones per day is unlikely to raise concerns for the general population (EFSA 2015). This range would cover vegan diet consumers for which the isoflavone intake was estimated the be ≥ 45 mg/day (Lee et al. 2019). Health Canada recommends that adults can safely consume up to 25 g of soy protein per day considering overall dietary intake (Benkhedda et al. 2014). The United States Food and Drug Administration approved a health claim that consuming 25 g of soy protein daily may reduce the risk of heart disease ((FDA 2017). The World Health Organization (WHO) indicates that intake of up to 3 mg/kg body weight of isoflavones per day is unlikely to result in adverse effects (WHO 2007). The major regulatory challenge will be to reconcile animal and molecular observations, which are performed for hazard identification, with the human epidemiological data which indicate at least several beneficial health effects of the estrogenic isoflavones. In this human relevance context, (Messina et al. 2022) indicate that these isoflavones should not be classified as endocrine disruptors.
Conclusions
The results of the present study confirm that both GEN and DAI have estrogenic properties. For both compounds in males, this activity is only seen at the very HD level of 1000 mg/kg bw, the NOMEL for GEN being 300 mg/kg bw. For DAI-treated males, associations with estrogenic activity were not found when the 1000 mg/kg bw dose was reduced to 300 mg/kg bw after 14 days of treatment. Considering these values, it is highly unlikely that the two compounds would exert estrogenic effect under real-life situations in male humans.
In females, the estrogenic effects were more pronounced, in terms of the association with predefined patterns and the correlation with the number of estrogenic compounds in the MetaMap®Tox data base. For females dosed with GEN, the NOMEL was 100 mg/kg bw following gavage treatment. There were also no estrogenic effects in the feeding studies. Estrogenic effects for DAI females, however, were still observed at 100 mg/kg bw, indicating that DAI may be a more potent estrogen than GEN in rats.
There were more differences between GEN and DAI than expected based on the literature. There was not a single match between any of the GEN and DAI dose groups, for both sexes, that achieved a biologically meaningful correlation. This is in line with the observation that there were far more compound-specific metabolite changes than common ones for both sexes. GEN-treated females correlated best with Cabergoline, a dopamine D2 receptor agonist, which may suggest a different type of estrogenic activity than DAI, for which estrogenic compound correlations were best for tamoxifen and DES. Beyond the estrogenic effects, the liver was a target organ for both compounds, however with different primary MoAs. In HD GEN males, good correlations were noted for compounds known to cause liver changes, such as ethanolamine and 3-amino-1-propanol. DAI-dosed males were associated with compounds known to influence fatty acid metabolism and to cause peroxisome proliferation by PPAR-alpha activation, and for females, this association was far less pronounced.
Data availability
Data can be requested, however, full information on the metabolites depends on permission by BASF Metabolome Solutions.
References
Benkhedda K, Boudrault C, Sinclair S, Marles RJ, Xiao CW, Underhill L (2014) Health Canada’s proposal to accept a health claim about soy products and cholesterol lowering. Int Food Risk Anal J. https://doi.org/10.5772/59411
Boudonck KJ, Mitchell MW, Német L, Keresztes L, Nyska A, Shinar D, Rosenstock M (2009) Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol 37(3):280–292
Bugrim A, Nikolskaya T, Nikolsky Y (2004) Early prediction of drug metabolism and toxicity: systems biology approach and modeling. Drug Discov Today 9(3):127–135
Büttner A, Thieme D (2010) Side effects of anabolic androgenic steroids: pathological findings and structure-activity relationships. Handb Exp Pharmacol 195:459–484. https://doi.org/10.1007/978-3-540-79088-4_19
ECHA SEV (2016) Stoffbewertung - CoRAP - ECHA. https://echa.europa.eu/de/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table/-/dislist/details/0b0236e18070c23b
EFSA (2015) Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J 13(10):4246. https://doi.org/10.2903/j.efsa.2015.4246
FDA (2017) Food Labelling: health claims; soy protein and coronary heart disease. Federal Register, 21 CRF part 101
Forslund L, Andersson HC (2017) Phytoestrogens in foods on the Nordic market. Nordic Council of Ministers
Fukusaki E (2015) Application of metabolomics for high-resolution phenotype analysis. Rinsho Byori: Jpn J Clin Pathol 63(6):736–745. https://doi.org/10.5702/massspectrometry.s0045
Gómez-Zorita S, González-Arceo M, Fernández-Quintela A, Eseberri I, Trepiana J, Portillo MP (2020) Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients 12(12):1–25. https://doi.org/10.3390/nu12123853
Guelfi G, Pasquariello R, Anipchenko P, Capaccia C, Pennarossa G, Brevini TAL, Gandolfi F, Zerani M, Maranesi M (2023) The role of Genistein in mammalian reproduction. Molecules 28(21):7436. https://doi.org/10.3390/MOLECULES28217436
Hilakivi-Clarke L, Clarke R, Lippman M (1999) The influence of maternal diet on breast cancer risk among female offspring. Nutrition 15(5):392–401
Hillerns PI, Zu Y, Fu Y-J, Wink M (2005) Binding of Phytoestrogens to Rat Uterine Estrogen Receptors and Human Sex Hormone-binding Globulins. Z. Naturforsch, Vol. 60. http://www.znaturforsch.com
Hruska RE, Silbergeld EK (1980) Estrogen treatment enhances dopamine receptor sensitivity in the rat striatum. Eur J Pharmacol 61(4):397–400. https://doi.org/10.1016/0014-2999(80)90081-3
Jefferson WN (2010) Adult ovarian function can be affected by high levels of soy. J Nut 140(12):23225. https://doi.org/10.3945/jn.110.123802
Kamp H, Fabian E, Groeters S, Herold M, Krennrich G, Looser R, Mattes W, Mellert W, Prokoudine A, Ruiz-Noppinger P, Strauss V, Walk T, Wiemer J, Van Ravenzwaay B (2012) Application of in vivo metabolomics to preclinical/toxicological studies: case study on phenytoin-induced systemic toxicity. Bioanalysis 4(18):2291–2301. https://doi.org/10.4155/bio.12.214
Kamp H, AygunKocabas N, Faulhammer F, Synhaeve N, Rushton E, Flick B, Giri V, Sperber S, Higgins LG, Penman MG, van Ravenzwaay B, Rooseboom M (2024) Utility of in vivo metabolomics to support read-across for UVCB substances under REACH. Arch Toxicol 95:755–768. https://doi.org/10.1007/s00204-023-03638-6
Kim S, Shin HJ, Kim SY, Kim JH, Lee YS, Kim DH, Lee MO (2004) Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARα. Mol Cell Endocrinol 220(1–2):51–58. https://doi.org/10.1016/J.MCE.2004.03.011
Kirk Clopton J, Gordon JH (1985) The possible role of 2-hydroxyestradiol in the development of estrogen-induced striatal dopamine receptor hypersensitivity. Brain Res 333(1):1–10. https://doi.org/10.1016/0006-8993(85)90117-9
Kuiper GGJM, Lemmen JG, Carlson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson J-A (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139(10):4252–4264
Lee A, Beaubernard L, Lamothe V, Bennetau-Pelissero C (2019) New Evaluation of isoflavone exposure in the French population. Nutrients 11(10):2308. https://doi.org/10.3390/NU11102308
Lipton BA, Yorek MA, Ginsberg BH (1988) Ethanolamine and choline transport in cultured bovine aortic endothelial cells. J Cell Physiol 137(3):571–576. https://doi.org/10.1002/JCP.1041370325
Mattes W, Davis K, Fabian E, Greenhaw J, Herold M, Looser R, Mellert W, Groeters S, Marxfeld H, Moeller N, Montoya-Parra G, Prokoudine A, Van Ravenzwaay B, Strauss V, Walk T, Kamp H (2014) Detection of hepatotoxicity potential with metabolite profiling (metabolomics) of rat plasma. Toxicol Lett 230(3):467–478. https://doi.org/10.1016/j.toxlet.2014.07.021
Messina M, Mejia SB, Cassidy A, Duncan A, Kurzer M, Nagato C, Ronis M, Rowland I, Sievenpiper J, Barnes S (2022) Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: a technical review of the observational and clinical data. Crit Rev Food Sci Nutr 62(21):5824–5885. https://doi.org/10.1080/10408398.2021.1895054
Montoya GA, Strauss V, Fabian E, Kamp H, Mellert W, Walk T, Looser R, Herold M, Krennrich G, Peter E, Van Ravenzwaay B (2014) Mechanistic analysis of metabolomics patterns in rat plasma during administration of direct thyroid hormone synthesis inhibitors or compounds increasing thyroid hormone clearance. Toxicol Lett 225(2):240–251. https://doi.org/10.1016/j.toxlet.2013.12.010
Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y (2001) Interaction of phytoestrogens with estrogen receptors α and β. In Biol. Pharm. Bull 24(4):351–356
Najjar A, Kühnl J, Lange D, Géniès C, Jacques C, Fabian E, Zifle A, Hewitt NJ, Schepky A (2024) Next-generation risk assessment read-across case study: application of a 10-step framework to derive a safe concentration of daidzein in a body lotion. Front Pharmacol. https://doi.org/10.3389/fphar.2024.1421601
nicholson JK, j. C. Lindon, e. Holmes, (1999) “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29(11):1181–1189
Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V (1995) Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem 65(4):1651–1657. https://doi.org/10.1046/J.1471-4159.1995.65041651.X
Piccardi P, Bernardi F, Rossetti Z, Corsini G (1983) Effect of estrogens on dopamine autoreceptors in male rats. Eur J Pharmacol 91(1):1–9. https://doi.org/10.1016/0014-2999(83)90355-2
Rietjens IMCM, Louisse J, Beekmann K (2016) The potential health effects of dietary phytoestrogens. Br J Pharmacol. https://doi.org/10.1111/bph.v174.11/issuetoc
Rizza P, Barone I, Zito D, Giordano F, Lanzino M, De Amicis F, Mauro L, Sisci D, Catalano S, Wright KD, Gustafsson J, ake, & Andò, S. (2014) Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res. https://doi.org/10.1186/bcr3619
Robertson DG (2005) Metabonomics in toxicology: a review. Toxicol Sci 85(2):809–822
Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Technical advance: simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23:131–142
Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA (1997) Antioxidant activity of phytoestrogenic isoflavones. Free Radical Res 26(1):63–70. https://doi.org/10.3109/10715769709097785
SCCS (2022) Scientific Committee on Consumer Safety SCCS OPINION on Genistein and Daidzein
Setchell KDR (2001) Soy isoflavones—benefits and risks from nature’s selective estrogen receptor modulators (SERMs). J Am Coll Nutr 20:354S-362S. https://doi.org/10.1080/07315724.2001.10719168
Sperber S, Wahl M, Berger F, Kamp H, Lemke O, Starck V, Walk T, Spitzer M, Ravenzwaay BV (2019) Metabolomics as read-across tool: an example with 3-aminopropanol and 2-aminoethanol. Regul Toxicol Pharmacol 108:104442. https://doi.org/10.1016/J.YRTPH.2019.104442
Strauss V, Wiemer J, Leibold E, Kamp H, Walk T, Mellert W, Looser R, Prokoudine A, Fabian E, Krennrich G, Herold M, van Ravenzwaay B (2009) Influence of strain and sex on the metabolic profile of rats in repeated dose toxicological studies. Toxicol Lett 191(1):88–95. https://doi.org/10.1016/j.toxlet.2009.08.004
Swedenborg E, Power KA, Cai W, Pongratz I, Rüegg J (2009) Regulation of estrogen receptor beta activity and implications in health and disease. Cell Mol Life Sci: CMLS 66(24):3873. https://doi.org/10.1007/S00018-009-0118-Z
Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer MS, Mizuno S, Ishimi Y, Watanabe S (2010) Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens 28(10):1971–1982. https://doi.org/10.1097/HJH.0B013E32833C6EDB
Tolvo A, Fujiki Y, Bhavanandan VP, Rathnam P, Saxena BB (1982) Studies on the unique presence of an N-acetylgalactosamine residue in the carbohydrate moieties of human follicle-stimulating hormone. Biochim Biophys Acta BBA - Gen Subj 719(1):1–10. https://doi.org/10.1016/0304-4165(82)90299-9
van Ravenzwaay B, Cunha GCP, Leibold E, Looser R, Mellert W, Prokoudine A, Walk T, Wiemer J (2007) The use of metabolomics for the discovery of new biomarkers of effect. Toxicol Lett 172(1–2):21–28. https://doi.org/10.1016/j.toxlet.2007.05.021
van Ravenzwaay B, Montoya-Para G, Strauss V, Fabian E, Mellert W, Krennrich G, Walk T, Peter E, Looser R, Herold M (2015) The development of a database for metabolomics-looking back on ten years of experience “The development of a database for metabolomics-looking back on ten years of experience.” Int J Biotechnol 14(1):47–68
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metabol Pharmacokinet 38(1):15–25. https://doi.org/10.1007/s13318-012-0112-y
WHO (2007) Protein and amino acid requirements in human nutrition report of a joint WHO/FAO/UNU expert consultation. World Health Organization
Yamada H, Yamahara A, Yasuda S, Abe M, Oguri K, Fukushima S, Ikeda-Wada S (2002) Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J Anal Toxicol 26:17–22
Zha X, Jay FT, Choy PC (2011) Effects of amino acids and ethanolamine on choline uptake and phosphatidylcholine biosynthesis in baby hamster kidney-21 cells. Biochem Cell Biol 70(12):1319–1324. https://doi.org/10.1139/O92-179
Zhang X, Xu H, Zhang H, Guo Y, Dai Z, Chen X (2011) Simultaneous determination of albendazole and its metabolites in fish muscle tissue by stable isotope dilution ultra-performance liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 401:727–734
Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR (2006) Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor a and /3 subtypes: insights into the structural determinants favoring a differential subtype binding. Endocrinology 147(9):4132–4150. https://doi.org/10.1210/en.2006-0113
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Montoya, G., van Ravenzwaay, B., Seefelder, W. et al. Unanticipated differences in the rat plasma metabolome of genistein and daidzein. Arch Toxicol 99, 1387–1406 (2025). https://doi.org/10.1007/s00204-025-03967-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-025-03967-8