Abstract
Glaciers retreat rapidly and create newly exposed terrestrial and aquatic habitats in glacier forefields, where primary succession proceeds synchronously in glacier forefields. Here, we introduced the “Dual-Domain Primary Succession” concept to examine the parallel yet distinct primary succession processes in soil and stream ecosystems within glacier forefields, by focusing on Hailuogou Glacier and Urumqi Glacier No.1 in China. Findings showed that soil bacterial communities exhibited higher α-diversity with a decreasing pattern in Hailuogou Glacier, in contrast to Urumqi Glacier No.1, which displayed lower and unimodally distributed α-diversity along the glacier forefield chronosequence (GFC). A similar pattern emerged in streams, except for an increasing α-diversity trend in Urumqi Glacier No.1 stream along the GFC. Additionally, α-diversity in streams changed more rapidly than in soils for Hailuogou Glacier, but more slowly for Urumqi Glacier No.1. Along GFC, both soil and stream bacterial communities experienced spatial variations, primarily due to species turnover. The succession of community composition was evident at the OTU level, with each module in the co-occurrence network consisting of OTUs enriched at specific successional stages. A substantial number of OTUs shared between paired soil and stream samples showed a decreasing trend along the GFC, while β-diversity increased. The results suggested that bacterial communities have a similar succession pattern but in different pace between soil and stream while having distinct successional trajectories between the studied glaciers. This study highlighted the “Dual-Domain Primary Succession” in glacier forefields, but further studies with more glaciers are necessary to make broader generalizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaciers are vital components of the global hydrological cycle, covering ~ 10% of the Earth’s land surface and containing ~ 75% of its freshwater [1, 2]. As a consequence of climate change, glaciers are retreating at an unprecedented rate worldwide, with many predicted to disappear within decades [3, 4]. Retreating glaciers unveil new terrestrial and aquatic landscapes in glacier forefields [4, 5], creating arenas for ecological succession [6, 7]. These newly exposed habitats, though initially hosting minimal biological activity, are ideal natural laboratories to study the fundamental processes driving biodiversity and ecosystem development. Thus, there have been increasing interests in exploring the primary succession of glacier forefield soils [8, 9], glacier-fed streams [10, 11], and glacier-fed lakes [12, 13]. Studying characteristics of primary succession provides critical insights into how life colonizes, and ecosystems evolve, in response to changing environmental conditions. Glacier forefields are notable for their concurrent creation of soil and stream ecosystems, which develop in parallel yet interact intimately. Despite significant research into individual succession pathways in these environments [4, 8, 14, 15], a comprehensive understanding of their synchronous successions remains elusive.
In glacier forefields, new soil originates from basal sediments left behind as the glacier terminus retreats, along with supraglacial sediments in the case of debris-covered glaciers, both of which are colonized by early-successional microorganisms [6]. These initial microorganisms are essential for soil development and biogeochemical cycles, initiating the formation of terrestrial ecosystems [16, 17]. Over time, vegetation begins to colonize, accelerating soil development by secreting organic acids and accumulating organic matter [5]. The progressive retreat of the glacier exposes new substrate over time, establishing the glacier forefield chronosequence (GFC) — a gradient that reflects increasing substrate exposure time with distance from the glacier terminus [14]. This GFC represents a mixture of exposure time and spatial distance, as areas farther from the glacier terminus have generally been exposed for longer periods, though the exposure timeline is not necessarily linear [18]. Along the GFC, soil microbial communities experience profound shifts driven by changes in both biotic and abiotic conditions [8, 14, 17, 19,20,21].
Accompanying soil ecosystems, glacier-fed streams are a key ecological and geomorphological feature in glacier forefields, serving as biogeochemical channels and biodiversity hotspots [15, 22, 23]. Glacier-fed streams receive materials transported from upstream glacial and terrestrial ecosystems, linking glacial processes and downstream aquatic ecosystems [15, 22]. Flushing from glaciers, streams share a substantial proportion of microorganism with soil. Retreating glaciers results in the lengthening of glacier-fed streams, which are colonized by aquatic biota over time [24, 25] and characterized by longitudinal alterations in hydrological and physicochemical environments [11, 24, 26]. In glacier-fed streams, benthic biofilms are the main contributors to primary production, integrating biogeochemical cycling through nutrient uptake, transfer, and remineralization [27, 28]. Previous studies have indicated that biodiversity in glacier-fed stream biofilms can either decrease or increase with glacier retreat, depending on factors such as changes in hydrology, nutrient availability, and temperature along the stream gradient [11, 23]. In glacier forefields, soil and stream ecosystems develop concurrently, yet limited research has explored how these systems interact and potentially influence each other’s successional pathways. This study addresses this gap by examining synchronous primary succession processes in these two interconnected domains.
Alpine glaciers on Earth can be broadly categorized into continental and maritime glaciers, which differ substantially in many aspects, such as climate patterns, ablation processes, mass balance, and retreat rate [29],O’Neel et al., 2014). Continental glaciers, such as those in the Tianshan Mountains of Central Asia, experience a dry and cold climate and are less sensitive to climate change with low mass exchange and low retreat rates (O’Neel et al., 2014; [30]. In contrast, maritime glaciers, such as those in the Maritime Alps (south-western European Alps) and the Hengduan Mountains (Southeastern Tibetan Plateau), exist in wet and warm climates and are highly sensitive to climate change with higher mass exchange and faster retreat rates [31, 32]. Numerous studies have investigated the primary succession of terrestrial and aquatic ecosystems in glacier forefields [13, 33]. In addition, different succession patterns have been found in soil along GFC between continental and maritime glaciers [34]. While primary succession is a well-studied phenomenon, its synchronous manifestation in adjacent terrestrial and aquatic habitats, especially under the influence of different glacier types, remains underexplored.
In this study, we introduced the concept of “Dual-Domain Primary Succession,” which refers to the synchronous yet distinct development of microbial communities in both soil and stream ecosystems within glacier forefields. This concept is grounded in ecological succession theories that consider how adjacent environments, connected by nutrient and microbial exchanges, might exhibit both independent and interlinked successional trajectories. By focusing on microbial communities, we aim to examine whether shared initial colonizers and subsequent shifts align across soil and stream domains or diverge based on unique habitat conditions. To support this concept, this study examines bacterial communities in glacier forefield soils and associated glacier-fed streams from a typical continental glacier in the Tianshan Mountains of Central Asia and a maritime glacier in the Hengduan Mountains on the southeastern edge of the Qinghai-Tibet Plateau. Expected results for “Dual-Domain Primary Succession” would include similarities in initial microbial communities due to shared colonization sources, followed by habitat-specific differentiation influenced by distinct environmental pressures, leading to markedly different nature and pace of the succession between soil and stream environments as well as between maritime and continental glacier.
Methods
Study Area
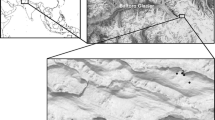
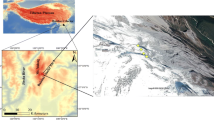
The continental glacier studied is Urumqi Glacier No.1 (43°06′N, 86°49′E), with its terminus at an elevation of 3796 m. It is situated in the eastern Tianshan Mountain Range in Central Asia (Fig. 1). The maritime glacier studied is Hailuogou Glacier (29°34′N, 101°59′E), located on Gongga Mountain, the highest peak in the Hengduan Mountain Range, with its terminus at an elevation of 2942 m (Fig. 1). The Tianshan Mountain Range in China contains 7934 glaciers, covering a total area of 7179 km2 [35]. This accounts for 16.3% of the total number and 13.9% of the total glacier area in China [36]. This glacier region, predominantly influenced by westerly circulation, experiences a temperate continental climate characterized by low precipitation, mostly occurring during the summer months [37]. Accelerated global warming has led to rapid shrinkage of these glaciers [38, 39]. The Urumqi Glacier No.1, for instance, has retreated from an area of 1.95 km2 in 1962 to 1.52 km2 in 2018 [40], with predictions indicating it may nearly disappear by 2100 [41]. The mean annual precipitation of Urumqi Glacier No.1 is 475 mm and the mean annual air temperature is − 4.8 °C [42]. The Second Glacier Inventory of China [35] identified 8607 maritime glaciers, covering an area of 13,203 km2, primarily located in the southern and eastern Qinghai-Tibet Plateau. These glaciers represent 18.6% of the total number and 22.2% of the total glacier area in China. The maritime glaciers on Gongga Mountain are primarily influenced by the southwest and southeast monsoons. These glaciers have been retreating at an average annual rate of 1.04 km2 per year from 1994 to 2021 [43]. Since the 1930s, the Hailuogou Glacier has retreated by 2 km. The Hailuogou Glacier receives an average annual precipitation of 1947 mm and experiences a mean annual air temperature of 4.2 °C [44]. Unlike continental glaciers, the termini of maritime glaciers, such as Hailuogou Glacier, often extend into forested areas.
a The location of the studied glaciers. b Urumqi Glacier No.1, the studied continental glacier. c Glacier-fed stream of the Urumqi Glacier No.1 viewing from the site with 945m from the glacier. d Hailuogou Glacier, the studied maritime glacier. e Glacier-fed stream of the Hailuogou Glacier viewing from the site with 830 m to the glacier. Photos in b–e were taken by Ze Ren
Field Sampling and Chemical Analyses
The sampling activities took place in July and August 2021, focusing on the forefield soils (SO) and glacier-fed streams (ST) of Urumqi Glacier No.1 and Hailuogou Glacier. In each glacier, paired soil and stream samples were collected along the glacier forefield chronosequence (GFC), starting from the glacier terminus (10 m away from the glacier) and extending to areas with well-developed vegetation of climax community (2100 m and 830 m away from the glacier for Urumqi Glacier No.1 and Hailuogou Glacier, respectively). The space-for-time substitution is used to study ecological processes that occur slowly by examining the relationships between ecological variables at sites that are assumed to be at different stages of development [45, 46]. A total of 7 paired soil and stream samples were collected from Urumqi Glacier No.1, and 5 pairs from Hailuogou Glacier. While this scope provided an initial exploration of dual-domain succession, we acknowledge that more comprehensive studies involving multiple sampling campaigns and additional glacier sites are needed to fully validate and generalize the findings. At each stream sampling site, 6 to 9 submerged rocks were randomly sampled across the stream [47]. The benthic biofilms were then thoroughly removed by scrubbing a 4.5 cm diameter area on the upper surface of each rock using a sterilized nylon brush. The slurry on the rock and brush was rinsed using sterile water and collected in an acid-cleaned polyethylene bottle to a volume of 500 mL. From the mixed slurry, 100 mL of the mixed slurry was filtered onto a 0.2-µm membrane filter (polycarbonate, Whatman, UK) in triplicate, which were combined and frozen in dry-ice immediately in the field and used for DNA extraction and sequencing in the laboratory. Additionally, water conductivity (Cond) and pH were measured in situ using a handheld multiparameter instrument (YSI ProPlus, Yellow Springs, Ohio), and 500 mL water was collected in acid-cleaned polyethylene bottles in triplicate for further chemical analyses in the laboratory. The chemical analyses, including total nitrogen (TN), nitrate (NO3−), ammonium (NH4+), total phosphorus (TP), soluble reactive phosphorus (SRP), and dissolved organic carbon (DOC), were conducted according to our previous studies [5, 47]. TN and TP samples were firstly persulfate oxidized, TN was measured using the ion chromatography method (EPA 300.0), and TP was measured using the ascorbic acid colorimetric method (EPA 365.3). Water samples for NO3−, NH4+, SRP, and DOC were first filtered through glass fiber filters (GF/F, Whatman). After filtration, NO3− was measured by ion chromatography (EPA 300.0). NH4+ was determined using the indophenol colorimetric method (EPA 350.1). SRP was quantified with the ascorbic acid colorimetric method (EPA 365.3). DOC was measured using a TOC-Analyzer (TOC-VCPH, Columbia, Maryland). The variations of these stream physicochemical variables along the increasing distance to glacier are shown in Fig. S1.
In glacier forefield, soils are originated from the glacier basal sediments, surface debris, and in situ bedrock pedogenesis [14]. Soil samples were collected from three transects which were perpendicular to the stream bank close to the stream sampling site (5 m from the stream shore). The sampled soils were not subject to regular flooding from the adjacent stream. Topsoil samples (0–10 cm depth) were collected using a soil auger with a 10 cm inner diameter along each transect at three evenly spaced points (1 m apart), chosen to capture the typical variation in soil properties near the stream. The auger was thoroughly cleaned between each sampling point using deionized water and ethanol to prevent contamination. The soil from each site was combined into a composite sample. The use of composite samples was intended to capture the general microbial community characteristics at each site while balancing practical fieldwork constraints, such as time, access, and preservation challenges in these remote and extreme environments. Microbial samples were placed in 45-mL sterile centrifuge tubes and immediately kept frozen on dry ice to preserve their integrity. The remaining soil was placed in sterile bags and transported in a cooler to the field laboratory for further processing. In the laboratory, the soil samples were air-dried at room temperature (approximately 25 °C) for 5 days and then passed through a 2-mm nylon sieve to remove visible roots, residues, and stones. Soil organic carbon (SOC), TN, TP, NO3−, NH4+, SRP, pH, and conductivity were analyzed [48,49,50,51]. SOC was determined using the potassium dichromate oxidation spectrophotometric method (HJ615-2011). TN was assessed using the modified Kjeldahl method (HJ717-2014). TP was measured after microwave extraction with nitric acid, using the ascorbic acid colorimetric method. NO3− and NH4+ were measured using a spectrophotometer after the extraction with 2 M potassium chloride (HJ634-2012). SRP was determined using the ascorbic acid colorimetric method after the extraction with 0.5 M sodium bicarbonate (HJ704-2014). pH was measured using a pH meter with a 1:2.5 soil-to-distilled water ratio. Conductivity was measured using a conductivity meter with a 1:5 soil-to-distilled water ratio. The variations of these soil physicochemical variables along the increasing distance to the glacier were shown in Fig. S1.
DNA Extraction, PCR, and Sequencing
Following the manufacturer’s protocols, DNA was extracted from filters (stream samples, n = 12) and soils (n = 12) using the Magen Hipure Soil DNA Kit (Magen, China). The extracted DNA was purified and quantified, and 20 ng of template DNA was used to generate amplicons. The V3-V4 hypervariable regions of prokaryotic 16S rRNA were amplified using the forward primer 343F (5′-TACGGRAGGCAGCAG-3′) and the reverse primer 798R (5′-AGGGTATCTAATCCT-3′) [52]. PCR amplifications were performed in triplicate for each sample to reduce amplification bias. PCRs were carried out in a 25 µL reaction mixture comprising 2.5 µL of TransStart buffer, 2 µL of dNTPs, 1 µL of each primer, 0.5 µL of TransStart Taq DNA polymerase, and 20 ng of template DNA. The thermal cycling was performed on an ABI GeneAmp® 9700 (USA) with the following program: an initial denaturation at 94 °C for 5 min; 24 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 20 s; followed by a final extension at 72 °C for 5 min. One soil sample failed to amplify during the PCR process. Following PCR, the triplicate products were pooled to create a DNA library, which was then purified and quantified with a Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, USA). This resulted in a total of 23 amplicon libraries. These DNA libraries were multiplexed and sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions.
Double-end sequencing was performed on both positive and negative reads. Paired-end reads were initially processed using Trimmomatic software to identify and remove ambiguous bases (N) and trim sequences with an average quality score below 20, employing a sliding window approach for quality trimming. After this step, paired-end reads were merged using FLASH software with parameters set for a minimum overlap of 10 bp, a maximum overlap of 200 bp, and a maximum mismatch rate of 20%. Further denoising involved excluding reads with ambiguous or homologous sequences and those shorter than 200 bp. Only reads with at least 75% of bases scoring above Q20 were retained. The resulting high-quality sequences were used for operational taxonomic units (OTUs) clustering, which was conducted using VSEARCH 2.4.3 [53] with a sequence similarity threshold set at 97% based on the SILVA 138 database [54]. Singleton OTUs were excluded. We rarefied the data to standardize sequencing depth (21,774 sequences) across samples (Fig. S2), which can help reduce potential biases caused by uneven sequencing effort and improve comparability between samples. This step was taken after confirming significant variation in sequencing depth across samples. The raw sequences have been uploaded to the China National Center for Bioinformation.
Analyses
The differences in α-diversity and β-diversity of bacterial communities across different sample categories (soil and stream samples collected from the studied maritime and continental glacier) were evaluated using the Wilcoxon rank-sum test. Regression analyses were performed between α-diversity and the distance to the glacier to elucidate the successional pattern of α-diversity. The best regression model was selected based on the corrected Akaike Information Criterion (AICc). To illustrate bacterial community differences across sample categories, non-metric multidimensional scaling (NMDS) ordinations were conducted based on Bray–Curtis dissimilarities using the relative abundance of OTUs. The differences between sample categories were further assessed by ADONIS using “vegan 2.5–7” package [55]. To uncover the processes shaping bacterial communities, β-diversity (βsor, Sorensen dissimilarity) can be partitioned into two components: turnover (βturn) and nestedness (βnest) [56, 57]. This partitioning was performed using the “betapart 1.5.4” package [58]. To assess the relationships between α-diversity and β-diversity with environmental variables, we conducted Mantel tests using the “linkET 0.0.7.4” package. To control for type I errors of mantel tests, adjustments for multiple comparisons were applied using the false discovery rate (FDR) method. The bacterial co-occurrence network for each sample category was constructed based on the Pearson correlation between pairs of OTUs (only the OTUs with an average relative abundance of ≥ 0.1% and occurring in more than 80% of the samples). The P-values were adjusted using the FDR method [59]. Only the correlations with Pearson’s R > 0.8 or R < − 0.8 and P < 0.01 were considered for network construction. The topological features of the network were calculated using igraph 1.3.5 [60]. The network visualization was performed in Gephi 0.9.7 [61]. All the statistical analyses were carried out in R 4.1.2 [62].
Results
Alpha-Diversity and Community Composition
After quality filtering, a total of 1192 OTUs were clustered. In general, bacterial communities in forefield soils had a higher α-diversity compared to those in adjacent glacier-fed streams (Fig. 2a). Additionally, bacterial communities in both forefield soils and glacier-fed stream of the Hailuogou Glacier had a higher α-diversity than those of the Urumqi Glacier No.1 (Fig. 2a). Along GFC (measured as the distance from the glacier terminus), regression analysis revealed that OTU richness significantly decreased with increasing distance from the glacier in both soil and stream of the Hailuogou Glacier, with the rate of decrease being faster in stream than in soils (Fig. 2b). Moreover, the number of shared OTUs between paired soil and stream samples also significantly decreased (Fig. 2d). For the Urumqi Glacier No.1, however, OTU richness significantly increased in stream and displayed a unimodal distribution pattern in soils along GFC (Fig. 2c). The number of shared OTUs between paired soil and stream samples in the Urumqi Glacier No.1 did not exhibit a clear pattern along GFC (Fig. 2d).
a Alpha-diversity in glacier forefield soils (SO) and glacier-fed streams (ST) of Hailuogou Glacier and Urumqi Glacier No.1. The differences of α-diversity between different ecosystems were assessed by the Wilcoxon rank-sum test. b, c Spatial patterns of bacterial α-diversity along the increasing distance to glacier. d Longitudinal patterns of shared OTUs and Bray–Curtis distance between paired soil and stream samples along the glacier forefield chronosequence. The grey area represents the 95% confidence interval of the regression
Considering the measured environmental variables, the mantel test showed that the bacterial α-diversity of Hailuogou Glacier soil significantly correlated with SRP, and that of Urumqi Glacier No.1 stream significantly correlated with pH, TN, and NO3− (mantel’s p < 0.05, Fig. 3). However, bacterial α-diversity of Hailuogou Glacier stream and Urumqi Glacier No.1 soil did not show significant relationships with those environmental variables (mantel’s p > 0.05, Fig. 3).
The mantel test shows the relationships between environmental variables and bacterial alpha and beta diversities for different groups of bacterial communities. α-diversity was indicated by OTU richness and β-diversity was assessed using Bray–Curtis distance. Lines indicate the relationships, with line width reflecting Mantel’s r statistic. Pairwise correlations between environmental variables are shown in a color gradient matrix, where the color intensity represents Pearson’s correlation coefficient. The abbreviations for environmental variables are detailed in the “Methods” section
Bacterial communities exhibited different dominant phyla between forefield soils and glacier-fed streams, as well as between different glaciers (Fig. 4). For the Hailuogou Glacier, bacterial communities were dominated by γ-Proteobacteria (average relative abundance of 27.6%), α-Proteobacteria (24.8%), Acidobacteria (13.7%), Bacteroidetes (11.8%), and Actinobacteria (9.1%) in the forefield soils, while dominated by γ-Proteobacteria (32%), α-Proteobacteria (23.1%), Bacteroidetes (20.9%), and Cyanobacteria (13.8%) in the glacier-fed stream. For the Urumqi Glacier No.1, bacterial communities were dominated by Acidobacteria (20.6%), α-Proteobacteria (15.6%), γ-Proteobacteria (14.1%), Cyanobacteria (13.8%), Bacteroidetes (13.5%), Actinobacteria (6.1%), and Gemmatimonadetes (5.1%) in the forefield soil, while dominated by γ-Proteobacteria (31.6%), Bacteroidetes (21.8%), α-Proteobacteria (17.2%), Cyanobacteria (13.9%), and Deinococcus-Thermus (6.3%) in the glacier-fed stream. Along GFC, the relative abundance of these dominant phyla rarely shown significant patterns (Fig. S3). In particular, Actinobacteria decreased while Bacteroidetes increased along GFC in the glacier-fed stream of Hailuogou Glacier. Acidobacteria increased while Bacteroidetes decreased along GFC in the forefield soil of Urumqi Glacier No.1. Gemmatimonadetes and α-Proteobacteria increased, while γ-Proteobacteria decreased along GFC in the glacier-fed stream of Urumqi Glacier No.1. However, in different stages of the succession (along the gradient of increasing distance to glacier), bacterial communities were dominated by different OTUs in forefield soil and glacier-fed streams, as well as in different glaciers (Hailuogou Glacier vs. Urumqi Glacier No.1) (Fig. 4).
Bacterial community composition in the glacier forefield soils and glacier-fed stream of a the Hailuogou Glacier and b the Urumqi Glacier No.1. The chord plots on the left show bacterial community composition at the phylum level. The heatmap on the right shows the distribution of abundant OTUs (OTUs had a relative abundance above 1% in at least one site for each glacier) in the sample sites along the glacier forefield. The distance to glacier increased from SO1/ST1 to SO7/ST7. The colors in the heatmap illustrate the relative abundance of each OTU per sampling site transformed using Z-score
Our findings revealed significant variation in microbial diversity and composition between soils and streams, as well as between the two glacier sites. The high number of shared OTUs between soil and stream samples suggests potential microbial transfer and shared colonization sources, supporting part of the “Dual-Domain” concept. However, observed differences in community structure highlight how environmental factors, such as nutrient availability and pH, drive divergence between these domains.
Beta-Diversity
Non-metric multidimensional scaling (NMDS) analyses revealed significant differences in bacterial communities across different ecosystems, a finding further confirmed by ADONIS results (Fig. 5a). Bacterial communities exhibited a significantly higher β-diversity in glacier-fed stream compared to forefield soils for the Hailuogou Glacier (Fig. 5b), suggesting greater taxonomic heterogeneity in stream bacterial communities for the Hailuogou Glacier. However, no significant differences in β-diversity were found between soil and stream bacterial communities for the Urumqi Glacier No.1 (Fig. 5b). In addition, β-diversity between paired soil and stream samples increased with the distance from the glacier for the Hailuogou Glacier, suggesting community divergence (Fig. 2d).
a Non-metric multidimensional scaling ordinations based on Bray–Curtis dissimilarities. The double-headed arrows show the results of ADONIS between two groups of bacterial communities. b Beta diversities of bacterial communities in the glacier forefield soils and glacier-fed stream of Hailuogou Glacier and Urumqi Glacier No.1. c The turnover (βturn) and nestedness (βnest) components of β-diversity
Considering the variation of environmental variables, mantel test showed that bacterial β-diversity of Urumqi Glacier No.1 soil correlated with most of the measured environmental variables, and that of Hailuogou Glacier soil only correlated with SRP and C:N ratio (mantel’s p < 0.05, Fig. 3). In addition, bacterial β-diversity of glacier-fed stream for both glaciers did not show significant relationships with any of those environmental variables (mantel’s p > 0.05, Fig. 3).
According to β-diversity partitioning, the variations in bacterial communities in forefield soils and glacier-fed streams of Hailuogou Glacier and Urumqi Glacier No.1 were composited differently by turnover (βturn) and nestedness (βnest). For the Hailuogou Glacier, bacterial communities exhibited higher βturn but lower βnest in forefield soils than in the glacier-fed stream (Fig. 5c). For the Urumqi Glacier No.1, however, there were no significant differences between soil and stream bacterial communities in terms of βturn and βnest (Fig. 5c). Moreover, when comparing βturn and βnest, βturn was higher than βnest in soil bacterial communities, while βnest was higher than βturn in stream bacterial communities for the Hailuogou Glacier (Fig. 5c). However, for the Urumqi Glacier No.1, βturn was higher than βnest in both soil and stream bacterial communities (Fig. 5c).
Co-occurrence Network
Bacterial communities in the forefield soil and glacier-fed stream in Hailuogou Glacier and Urumqi Glacier No.1 formed distinct co-occurrence networks (Figs. 6 and 7). According to topological features of the network-level (Table S1), the soil and stream bacterial networks of the Hailuogou Glacier were more complex than that of the Urumqi Glacier No.1. Moreover, the bacterial network of stream biofilm was more complex than the soil one for the Hailuogou Glacier, while it was opposite for the Urumqi Glacier No.1. Moreover, these networks had module structures, with most major modules (the module composed by more than 5% of the nodes) in each network were composed by OTUs highly enriched in a certain site (Figs. 6 and 7). For example, in the soil bacterial network of the Hailuogou Glacier (Figs. 6a and 7a), modules M3, M2, M6, M7, and M1 were composed of OTUs which were enriched in different soil samples from SO1 to SO5 along the GFC, respectively. In the soil bacterial network of the Urumqi Glacier No.1 (Figs. 6c and 7c), modules M4, M1, M5, M2, M7, M6, and M3 were composed of OTUs enriched in different soil samples from SO1 to SO7 along the GFC, respectively. Similar patterns were also found in stream bacterial networks in Hailuogou Glacier and Urumqi Glacier No.1 (Figs. 4d and 6b). These results suggested that the succession of bacterial communities in forefield soils and glacier-fed streams was clearly reflected on the co-occurrence network modules.
Discussion
Glacier forefield soils and streams present a unique opportunity to study ecological succession in response to glacier retreat. Our study in glacier forefields, focusing on the new concept “Dual-Domain Primary Succession,” revealed significant insights into the synchronous yet distinct trajectories of soil and stream ecosystems.
Glacier Forefield Soils
In glacier forefield soils, the Hailuogou Glacier exhibited a significantly higher bacterial α-diversity compared to the Urumqi Glacier No.1, with a decreasing pattern along the glacier forefield chronosequence (GFC) for the Hailuogou Glacier and a unimodal distribution for the Urumqi Glacier No.1. These differences suggest that bacterial communities follow distinct successional trajectories, influenced by local environmental conditions. While pioneer microbial communities in newly deglaciated soils originate from various sources, including supraglacial and subglacial habitats and atmospheric transport [14, 17, 63], the diversity of these communities can be shaped by factors such as the glacier’s climate regime and catchment position [64, 65]. The Hailuogou Glacier, situated at a lower elevation and influenced by monsoon winds, receives microbial inputs enriched by diverse surrounding ecosystems [64, 66]. In contrast, the Urumqi Glacier No.1, located at a higher elevation and influenced by westerly winds crossing arid regions, experiences more stringent environmental selection, which may reduce microbial diversity [67, 68]. These results highlight potential differences in microbial diversity between Hailuogou Glacier and Urumqi Glacier No.1, although distinguishing the exact factors contributing to these patterns requires further research involving additional glacier sites and detailed seasonal sampling.
Along GFC, contrasting successional patterns in soil bacterial communities were evident between the Hailuogou Glacier and the Urumqi Glacier No.1. In glacier forefields, the retreating chronosequence has been identified as a significant factor in shaping microbial diversity and community structure [69]. However, previous studies have yielded conflicting results on whether soil microbial diversity increases, decreases, or remains unchanged along GFC [70,71,72], although increasing α-diversity is a commonly observed successional pattern due to the increasing potential niches, resource availabilities, and habitat heterogeneity [73,74,75]. The reason for the inconsistent patterns is that microbial community succession is strongly and collectively controlled by a wide variety of factors in glacier forefields, such as successional stage, deglaciation time, moisture, vegetation, and nutrients [16, 74]. Moreover, these factors contributed differentially to bacterial community succession at different stages [21, 76]. Along GFC, soil properties exhibited distinct patterns, with a decrease in pH accompanied by increases in nutrients, organic carbon, and moisture levels (Fig. S1) [5, 77], accompanied by a general increase in alpha-diversity and shifting microbial community composition [16, 20, 69]. As vegetation develops, plants appear to compete effectively against soil microbes for available nitrogen in the low-N environment of glacier forefields, limiting microbial growth and productivity [78]. Decreasing diversity arising from interspecific competition also occurs during primary succession in other systems [75]. Previous studies have identified three key processes driving the formation of biological communities after glacier retreat: habitat filtering, biotic interactions, and time-mediated processes [14, 79]. However, consensus on the relative importance of these processes remains elusive, potentially due to their varying significance across different life forms and geographic regions [14, 33]. In our study, the unimodal pattern for the Urumqi Glacier No.1 might suggest that the main driving force could shift from edaphic properties at the early stage to vegetation properties at later stages, with the middle stage experiencing less environmental stress and competition. Conversely, for the Hailuogou Glacier, plants succeed rapidly due to the warm and wet climate [80], and the decreasing bacterial α-diversity might suggest increasing influence of vegetation as inferred from known ecological interactions discussed above [75, 78].
Microbial community succession in glacier forefields progresses along GFC, not only varying in α-diversity but also shifting in community composition. In our study, high species turnover was found in glacier forefield soils for both glaciers, consistent with previous findings [71, 72, 74], indicating that bacterial community changes largely contributed by species turnover. Moreover, when comparing different glaciers, bacterial communities in glacier forefield soils of the Urumqi Glacier No.1 exhibited significantly higher turnover than those of the Hailuogou Glacier. This high turnover might be driven by substantial environment changes in glacier forefield soils during succession, particularly in the early stages [72]. An alternative explanation is that the initial bacteria populations might not be adapted to the developing environments during succession, allowing immigrant populations to occupy the newly created ecological niches [72].
At the phylum level, Proteobacteria, Bacteroidetes, Acidobacteria, and Actinobacteria were the predominant (average relative abundance > 5%) phyla in forefield soils for both glaciers, while Cyanobacteria and Gemmatimonadetes were also predominant for the Urumqi Glacier No.1. These phyla are also reported as predominant in forefield ecosystems of other glaciers, decreasing or increasing their relative abundance over successional time [16, 71, 74]. In our study, the relative abundance of the dominant phyla rarely presented clear succession patterns (significantly correlated with the distance to glacier) in the glacier forefields (Fig. S3). However, the successional pattern of bacterial community composition was evident at the OTU level and particularly confirmed by the co-occurrence network. In natural ecosystems, microorganisms frequently coexist within complex networks characterized by intense interactions, playing essential roles in community assembly [81, 82]. These interactions suggest underlying biological and/or biochemical relationships among microbes [83, 84]. Modularity, in particular, highlights the network’s tendency to form sub-clusters of nodes (OTUs here), reducing high-dimension communities into ecological modules (a group of densely connected nodes) [83, 85]. Modules can reveal ecological properties such as functional interactions, co-occurrence patterns, and shared responses to environmental conditions that are often overlooked when communities are solely studied through taxonomic groupings [8, 86]. In our study, the bacterial community networks showed a clear module structure in the glacier forefield soils for both glaciers. Each module consisted of OTUs enriched at a certain successional stage, revealing the succession pattern of bacterial communities dominated by different taxa along GFC.
Glacier-Fed Streams
In the framework of “Dual-Domain Primary Succession,” glacier-fed streams present a fascinating parallel to adjacent glacier forefield soils, exhibiting similar yet distinct successional trajectories of bacterial communities. Different successional trajectories of bacterial communities were also found in glacier-fed streams between the two studied glaciers. Our study highlights both the synchronicity and differentiation in the successional pathways of microbial communities in these interconnected ecosystems.
Glacier-fed streams start succession in synchronization with their adjacent glacier forefield soils after glacier retreat. Similar to microbial communities in forefield soils, microorganisms in glacier-fed streams also come from various sources, some of which are shared with forefield soils, especially at the early successional stage. The pioneering microbes in glacier-fed streams originate from glaciers transported by meltwater, which contains diverse microbes from supraglacial, englacial, and subglacial habitats [65, 87]. Moreover, this microbial influx can be supplemented by contributions from groundwater [88, 89] and adjacent terrestrial environments [90]. Some of these microorganisms can adhere to benthic substrates, forming biofilms that serve as diversity hotspots in glacier-fed streams [23, 27]. As discussed above, maritime glaciers may harbor a higher diversity of microbes in the glacier per se than continental glaciers [91], initializing a richer microbial assembly in the associated glacier-fed streams. In this study, the glacier-fed stream of the Hailuogou Glacier exhibited a higher bacterial α-diversity in benthic biofilms than that of the Urumqi Glacier No.1.
In addition, the bacterial diversity in benthic biofilms in glacier-fed streams presented distinct longitudinal patterns, decreasing for the Hailuogou Glacier while increasing for the Urumqi Glacier No.1. Along the glacier-fed streams with increasing distance to the glacier, one of the most notable changes is the shift in the relative contributions of various water sources to stream discharge — from predominantly glacial meltwater to increasing influences of groundwater and surface runoff [24, 26, 92]. A concomitant change is the shift of relative contributions of different microbial sources (groundwater and terrestrial) to glacier-fed streams [89]. Accordingly, hydrological alterations dramatically re-structure stream habitat template, such as channel stability and water physicochemical properties (pH, temperature, DOC, nutrient) [24], acting as strong environmental filters for microbial communities in glacier-fed streams [11, 93]. For example, cold-adapted species are displaced by less cryophilic species along glacier-fed stream succession [23, 88]. In our study, the measured physicochemical variables exhibited negligible influences on bacterial α-diversity (Fig. 3), highlighting the importance of hydrological or integrated environmental influences. Due to the intimate hydroecological connections between glaciers and glacier-fed streams, biodiversity in these streams is highly susceptible to glacier retreat [92, 94]. The increasing bacterial diversity in the glacier-fed stream of Urumqi Glacier No.1 aligns with previous studies linking rising biodiversity to enhanced channel stability and warmer water temperatures [11, 23]. In contrast, the glacier-fed stream of Hailuogou Glacier showed a decreasing diversity pattern (Fig. 2b) which may result from an initial high diversity contributed by multiple sources, alongside inferred effects of increasing stream flow, current velocity, and channel instability [95, 96]. The growth of benthic biofilms in glacier-fed streams of the maritime glacier appeared to be controlled primarily by increasingly harsh hydrological conditions [92, 97].
In these glacier-fed streams, Proteobacteria, Bacteroidetes, and Cyanobacteria were the predominant phyla for both glaciers. Deinococcus-Thermus was also predominant for the Urumqi Glacier No.1. These phyla are also abundant in other glacier-fed streams [98, 99]. Some of the phyla showed clear successional patterns. For the Hailuogou Glacier, the relative abundance of Cyanobacteria decreased, while Bacteroidetes increased along the glacier-fed stream. For the Urumqi Glacier No.1, α-Proteobacteria increased the relative abundance while γ-Proteobacteria decreased along the glacier-fed stream. Along GFC, groundwater-meltwater interactions and soil–water interactions affect glacier-fed streams, shaping microbial diversity and community structure, and resulting in distinct microbial communities at various sampling points (different successional stages) along glacier-fed streams [89]. In addition, microbial variation pattern could be due to differences in nutrient requirements and environmental tolerances among microbial communities, where certain taxa may thrive under specific nutrient conditions or flow dynamics, while others may be more sensitive to these environmental changes [100, 101]. Similar to glacier forefield soils, bacterial community composition differences along glacier-fed streams were markedly presented at the OTU level. Bacterial communities were dominated by different OTUs at different successional stages. These successional properties were also confirmed by the module structure of the co-occurrence networks as discussed above of glacier forefield soils.
Differences and Relationships Between Soil and Stream in Glacier Forefields
The concept of “Dual-Domain Primary Succession” emerges prominently in our study, highlighting the interconnected yet distinct successional trajectories of terrestrial and stream ecosystems in glacier forefields. Soil and stream microbial communities in glacier forefields may share some initial taxa due to shared glacial sources. However, the distinct hydrological influences — such as channelized subglacial flow in streams versus distributed hydrological inputs in soils — along with biological hotspots beneath the glacier, likely establish differences in initial community composition, that are further shaped by each environment’s specific conditions. In our study, both differences and relationships were evident between soil and stream ecosystems in glacier forefields.
In glacier forefields, we observed that soils generally exhibited higher bacterial richness compared to adjacent glacier-fed streams. This difference can be attributed to the role of soils as a primary bacterial source for streams, a relationship supported by previous studies [90, 102, 103]. In addition to the similar successional patterns presented in the glacier-fed streams and their adjacent forefield soils, a coupling relationship was evidenced by the high number of shared OTUs between them (Fig. 2d), arising from the same initial microbial sources as well as land–water transfer of microorganisms [90, 104]. Some bacteria generating the stream communities reside in the surrounding terrestrial ecosystem, delivered to stream through surface runoff [104]. This initial overlap in microbial communities underscores the concept of “Dual-Domain Primary Succession,” where terrestrial and aquatic habitats, though distinct, commence their successional pathways from a common microbial foundation.
Interestingly, our findings revealed a significant decrease in shared OTUs between soil and stream samples, particularly for the Hailuogou Glacier along GFC. This pattern suggests that the glacial and terrestrial signatures on stream bacterial communities diminish along the succession of glacier forefield, especially for the Hailuogou Glacier. Additionally, we noted an increase in β-diversity between paired soil and stream samples along the GFC, especially marked in Hailuogou Glacier. The decreasing shared OTUs and increasing β-diversity suggested that bacterial communities in these distinct ecosystems are more divergent during ecological succession, highlighting the core of the “Dual-Domain Primary Succession” concept. It demonstrates that while glacier forefield soils and streams share common microbial sources in the initial succession stages, their ecological pathways increasingly diverge, reflecting the distinct environmental influences and evolutionary pressures in each domain. Contrasting with studies focusing on homogenization of biological communities in similar ecosystem types [23, 94], our research revealed a trend towards differentiation and specialization within the unique contexts of soil and stream ecosystems in glacier forefields. This divergence is a hallmark of the “Dual-Domain Primary Succession,” illustrating the nuanced and complex nature of ecological succession in these environments.
Study Limitations
While our results provide initial support for the “Dual-Domain Primary Succession” concept of microbial communities in glacier forefield soils and streams, several limitations (not all) should be acknowledged. The study was limited to only two glaciers (one maritime and one continental) in a single campaign due to limited funding resources, restricting the generalizability of our conclusions. Examining additional glaciers across various climatic regions and glacier types would provide a more comprehensive assessment of the “Dual-Domain Primary Succession” concept. On the other hand, using composite samples restricts detailed statistical analysis and may overlook fine-scale heterogeneity, although it provides an overview of microbial community composition. This approach was necessary due to time and logistical constraints in remote glacier environments. Future studies should include more individual replicates to enable more robust statistics and capture within-site variability. Additionally, our study measured a subset of environmental parameters, focusing on microbial diversity, selected soil properties, and stream characteristics. However, other factors such as vegetation coverage and more detailed nutrient profiling could further inform microbial dynamics in these habitats. Incorporating vegetation data could enhance our understanding of its influence on microbial community succession, as plant colonization may affect soil properties and microbial community composition through increased organic inputs and root-associated microbial interactions. Moreover, the absence of direct dating methods for soils in this study limits our ability to accurately determine the age of soil substrates along the GFC. Implementing precise dating techniques, such as radiocarbon dating, would allow for a more accurate assessment of substrate age and successional stage, enhancing the understanding of microbial succession over time. For stream biofilm, incorporating hydrograph data can also benefit our understanding of microbial succession in glacier-fed streams. While our study introduces the “Dual-Domain Primary Succession” concept, it should be seen as a starting point for further research rather than a comprehensive validation. These limitations suggest directions for our future research to build on the current findings and provide a more comprehensive view of “dule-domain” ecological succession in glacier forefields.
Conclusion
This study investigated “Dual-Domain Primary Succession” in glacier forefields of the Urumqi Glacier No.1 and Hailuogou Glacier, examining simultaneous yet distinct ecological successions in soil and stream ecosystems following glacier retreat. It unveiled complex ecological dynamics influenced by environmental and biological factors, with unique successional patterns in these interconnected domains. Soil ecosystems showed varying bacterial successional patterns between Hailuogou Glacier and Urumqi Glacier No.1. The Hailuogou Glacier had a higher but declining bacterial α-diversity, while the Urumqi Glacier No.1 displayed a unimodal diversity pattern. These differences highlight the impact of glacier-specific factors like climate and position on microbial diversity, along with high species turnover and distinct microbial structures at various levels. Similarly, glacier-fed streams presented unique successional trajectories. Streams from the Hailuogou Glacier initially had higher bacterial diversity, reducing over time due to hydrological changes, whereas the glacier-fed stream of the Urumqi Glacier No.1 showed increasing bacterial diversity, influenced by water source alterations and stream stability. The study emphasized the interconnected yet divergent evolutionary paths of terrestrial and stream ecosystems, initially sharing microbes but later following different developmental routes due to their ecological processes and habitat characteristics. This divergence was evident in the higher bacterial richness in soils, decreasing shared OTUs, and increasing β-diversity along glacier forelands. The “Dual-Domain Primary Succession” concept offers valuable insights into the distinct pathways of ecological succession in terrestrial and aquatic environments in glacier forefields, underscoring the importance of considering both domains in studies of glacier retreat.
Data Availability
The data have been submitted to the China National Center for Bioinformation (CNCB) with the accession number CRA015148 under the project PRJCA015424. The link is https://ngdc.cncb.ac.cn/gsa/browse/CRA015148.
References
Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B (2009) High microbial activity on glaciers: importance to the global carbon cycle. Glob Change Biol 15:955–960
Farinotti D, Longuevergne L, Moholdt G, Duethmann D, Moelg T, Bolch T et al (2015) Substantial glacier mass loss in the Tien Shan over the past 50 years. Nat Geosci 8:716–723
Gao HK, Feng ZJ, Zhang T, Wang YZ, He XB, Li H et al (2021) Assessing glacier retreat and its impact on water resources in a headwater of Yangtze River based on CMIP6 projections. Sci Total Environ 765:e142774
Milner AM, Khamis K, Battin TJ, Brittain JE, Barrand NE, Fuereder L et al (2017) Glacier shrinkage driving global changes in downstream systems. Proc Natl Acad Sci 114:9770–9778
Ren Z, Gao HK, Luo W, Elser JJ (2022) C:N: P stoichiometry in six distinct habitats of a glacier terminus in the source area of the Yangtze River. Biogeochemistry 158:181–194
Pessi IS, Pushkareva E, Lara Y, Borderie F, Wilmotte A, Elster J (2019) Marked succession of cyanobacterial communities following glacier retreat in the high Arctic. Microb Ecol 77:136–147
Schmidt SK, Reed SC, Nemergut DR, Grandy AS, Cleveland CC, Weintraub MN et al (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proceedings of the Royal Society B: Biological Sciences 275:2793–2802
Ren Z, Gao HK (2022) Abundant and rare soil fungi exhibit distinct succession patterns in the forefield of Dongkemadi glacier on the central Qinghai-Tibet Plateau. Sci Total Environ 828:e154563
Zumsteg A, Luster J, Göransson H, Smittenberg RH, Brunner I, Bernasconi SM et al (2012) Bacterial, archaeal and fungal succession in the forefield of a receding glacier. Microb Ecol 63:552–564
Holt AD, Fellman J, Hood E, Kellerman AM, Raymond P, Stubbins A, et al. 2021. The evolution of stream dissolved organic matter composition following glacier retreat in coastal watersheds of southeast Alaska. Biogeochemistry,1–18.
Ren Z, Gao HK, Elser JJ (2017) Longitudinal variation of microbial communities in benthic biofilms and association with hydrological and physicochemical conditions in glacier-fed streams. Freshwater Science 36:479–490
Modenutti B, Martyniuk N, Bastidas Navarro M, Balseiro E. 2023. Glacial influence affects modularity in bacterial community structure in three deep Andean North-Patagonian lakes. Microbial Ecology.
Sommaruga R (2015) When glaciers and ice sheets melt: consequences for planktonic organisms. J Plankton Res 37:509–518
Ficetola GF, Marta S, Guerrieri A, Cantera I, Bonin A, Cauvy-Fraunié S et al (2024) The development of terrestrial ecosystems emerging after glacier retreat. Nature 632:336–342
Ren Z, Martyniuk N, Oleksy IA, Swain A, Hotaling S (2019) Ecological stoichiometry of the mountain cryosphere. Front Ecol Evol 7:e360
Liu J, Kong W, Xia P, Zhu C, Li X (2021) Prokaryotic community succession in bulk and rhizosphere soils along a high-elevation glacier retreat chronosequence on the Tibetan Plateau. Front Microbiol 12:e736407
Rime T, Hartmann M, Frey B (2016) Potential sources of microbial colonizers in an initial soil ecosystem after retreat of an alpine glacier. ISME J 10:1625–1641
Wojcik R, Eichel J, Bradley JA, Benning LG (2021) How allogenic factors affect succession in glacier forefields. Earth Sci Rev 218:103642
Bradley JA, Singarayer JS, Anesio AM (2014) Microbial community dynamics in the forefield of glaciers. Proceedings of the Royal Society B: Biological Sciences 281:e20140882
Franzetti A, Pittino F, Gandolfi I, Azzoni RS, Diolaiuti G, Smiraglia C et al (2020) Early ecological succession patterns of bacterial, fungal and plant communities along a chronosequence in a recently deglaciated area of the Italian Alps. Fems Microbiology Ecology 96:fiaa165
Jiang Y, Lei Y, Yang Y, Korpelainen H, Niinemets Ü, Li C (2018) Divergent assemblage patterns and driving forces for bacterial and fungal communities along a glacier forefield chronosequence. Soil Biol Biochem 118:207–216
Hotaling S, Hood E, Hamilton TL (2017) Microbial ecology of mountain glacier ecosystems: Biodiversity, ecological connections, and implications of a warming climate. Environ Microbiol 19:2935–2948
Wilhelm L, Singer GA, Fasching C, Battin TJ, Besemer K (2013) Microbial biodiversity in glacier-fed streams. ISME J 7:1651–1660
Milner AM, Brown LE, Hannah DM (2009) Hydroecological response of river systems to shrinking glaciers. Hydrol Process 23:62–77
Robinson CT, Thompson C, Freestone M (2014) Ecosystem development of streams lengthened by rapid glacial recession. Fundam Appl Limnol 185:235–246
Gao HK, Han TD, Liu Y, Zhao QD (2016) Use of auxiliary data of topography, snow and ice to improve model performance in a glacier-dominated catchment in Central Asia. Hydrol Res 48:1418–1437
Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14:251–263
Martyniuk N, Souza MS, Bastidas Navarro M, Balseiro E, Modenutti B (2022) Nutrient limitation affects biofilm enzymatic activities in a glacier-fed river. Hydrobiologia 849:2877–2894
Shi YF, Liu SY (2000) Estimation on the response of glaciers in China to the global warming in the 21st century. Chin Sci Bull 45:668–672
Wang X, Yang T, Xu CY, Xiong L, Shi P, Li Z (2020) The response of runoff components and glacier mass balance to climate change for a glaciated high-mountainous catchment in the Tianshan Mountains. Nat Hazards 104:1239–1258
Rettig L, Kamleitner S, Mozzi P, Ribolini A, Ivy-Ochs S, Rea BR et al (2024) Responses of small mountain glaciers in the Maritime Alps (south-western European Alps) to climatic changes during the Last Glacial Maximum. Quatern Sci Rev 325:108484
Zhao F, Long D, Li X, Huang Q, Han P (2022) Rapid glacier mass loss in the Southeastern Tibetan Plateau since the year 2000 from satellite observations. Remote Sens Environ 270:112853
Cauvy-Fraunié S, Dangles O (2019) A global synthesis of biodiversity responses to glacier retreat. Nature Ecology & Evolution 3:1675–1685
Valle B, Gobbi M, Tognetti M, Borgatti MS, Compostella C, Pantini P et al (2022) Glacial biodiversity of the southernmost glaciers of the European Alps (Clapier and Peirabroc, Italy). J Mt Sci 19:2139–2159
Guo WQ, Liu SY, Yao XJ, Xu JL, Shangguan DH, Wu LZ et al (2015) The Second Glacier Inventory dataset of China (version 1.0). Cold and Arid Regions Science Data Center at Lanzhou. J Glaciol 61:357–372
Liu SY, Yao XJ, Guo WQ, Xu JL, Shangguan DH, Wei JF et al (2015) The contemporary glaciers in China based on the Second Chinese Glacier Inventory. Acta Geogr Sin 70:3–16
Xu M, Kang S, Wu H, Yuan X (2018) Detection of spatio-temporal variability of air temperature and precipitation based on long-term meteorological station observations over Tianshan Mountains, Central Asia. Atmos Res 203:141–163
Aizen VB, Aizen EM, Kuzmichonok VA (2007) Glaciers and hydrological changes in the Tien Shan: simulation and prediction. Environ Res Lett 2:045019
He Y, Yang T, Ji Q, Chen J, Zhao G, Shao W (2015) Glacier variation in response to climate change in Chinese Tianshan Mountains from 1989 to 2012. J Mt Sci 12:1189–1202
Li H, Wang P, Li Z, Jin S, Xu C, Mu J et al (2022) Effect of topography on the changes of Urumqi Glacier No. 1 in the Chinese Tianshan Mountains. J Arid Land 14:719–738
Gao HK, Li H, Duan Z, Ren Z, Meng XY, Pan XC (2018) Modelling glacier variation and its impact on water resource in the Urumqi Glacier No. 1 in Central Asia. Sci Total Environ 644:1160–1170
Mu JX, Li ZQ, Zhang H, Xu CH, Jin S, Liang PB (2019) Mass balance variation of continental glacier and temperate glacier and their response to climate change in western China: taking Urumqi Glacier No.1 and Parlung No.94 Glacier as examples. Arid Land Geography 42:20–29
Zhou S, Sun Z, Sun P (2022) Rapid glacier shrinkage in the Gongga Mountains in the last 27 years. Remote Sensing 14:5397
Wu YH, Li W, Zhou J, Cao Y (2013) Temperature and precipitation variations at two meteorological stations on eastern slope of Gongga Mountain, SW China in the past two decades. J Mt Sci 10:370–377
Lovell R, Collins S, Martin SH, Pigot AL, Phillimore AB (2023) Space-for-time substitutions in climate change ecology and evolution. Biol Rev 98:2243–2270
Pickett ST (1989) Space-for-time substitution as an alternative to long-term studies In Long-term studies in ecology. Springer, New York, NY
Ren Z, Niu DC, Ma PP, Wang Y, Wang ZM, Fu H et al (2020) C:N: P stoichiometry and nutrient limitation of stream biofilms impacted by grassland degradation on the Qinghai-Tibet Plateau. Biogeochemistry 150:31–44
Dancer WS, Eliason R, Lekhakul S (1998) Microwave assisted soil and waste dissolution for estimation of total phosphorus. Commun Soil Sci Plant Anal 29:1997–2006
Luo X, Liu K, Shen Y, Yao G, Yang W, Mortimer PE et al (2021) Fungal community composition and diversity vary with soil horizons in a subtropical forest. Front Microbiol 12:650440
Qian Y, Cheng C, Drouillard K, Zhu Q, Feng H, He S et al (2019) Bioaccumulation and growth characteristics of Vallisneria natans (Lour.) Hara after chronic exposure to metal-contaminated sediments. Environ Sci Pollut Res 26:20510–20519
Wang Y, Ren Z, Ma PP, Wang ZM, Niu DC, Fu H et al (2020) Effects of grassland degradation on ecological stoichiometry of soil ecosystems on the Qinghai-Tibet Plateau. Sci Total Environ 722:e137910
Nossa CW, Oberdorf WE, Yang L, Aas JA, Paster BJ, Desantis TZ et al (2010) Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J Gastroenterol 16:4135–4144
Rognes T, Flouri T, Nichols B, Quince C, Mahe F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, et al. 2020. vegan: Community Ecology Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan.
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143
Podani J, Schmera D (2016) Once again on the components of pairwise beta diversity. Eco Inform 32:63–68
Baselga A, Orme CDL (2012) betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 57:289–300
Csardi MG. 2013. Package ‘igraph’. Available at https://cran.r-project.org/web/packages/igraph/index.html.
Bastian M, Heymann S, Jacomy M. 2009. Gephi: an open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media.
R Core Team. 2020. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org.
Rolli E, Marasco R, Fusi M, Scaglia B, Schubotz F, Mapelli F et al (2022) Environmental micro-niche filtering shapes bacterial pioneer communities during primary colonization of a Himalayas’ glacier forefield. Environ Microbiol 24:5998–6016
Qi J, Ji M, Wang W, Zhang Z, Liu K, Huang Z et al (2022) Effect of Indian monsoon on the glacial airborne bacteria over the Tibetan Plateau. Sci Total Environ 831:154980
Zarsky JD, Kohler TJ, Yde JC, Falteisek L, Lamarche-Gagnon G, Hawkings JR, et al. 2018. Prokaryotic assemblages in suspended and subglacial sediments within a glacierized catchment on Qeqertarsuaq (Disko Island), west Greenland. Fems Microbiology Ecology, 94: fiy100.
Li X, Chen H, Yao M (2020) Microbial emission levels and diversities from different land use types. Environ Int 143:105988
Azzoni RS, Tagliaferri I, Franzetti A, Mayer C, Lambrecht A, Compostella C et al (2018) Bacterial diversity in snow from mid-latitude mountain areas: Alps, Eastern Anatolia, Karakoram and Himalaya. Ann Glaciol 59:10–20
Pan Y, Kalume A, Wang C, Santarpia J (2021) Atmospheric aging processes of bioaerosols under laboratory-controlled conditions: a review. J Aerosol Sci 155:105767
Garrido-Benavent I, Pérez-Ortega S, Durán J, Ascaso C, Pointing SB, Rodríguez-Cielos R et al (2020) Differential colonization and succession of microbial communities in rock and soil substrates on a maritime antarctic glacier forefield. Front Microbiol 11:e126
Fernandez-Martinez MA, Pointing SB, Perez-Ortega S, Arroniz-Crespo M, Green T, Rozzi R et al (2016) Functional ecology of soil microbial communities along a glacier forefield in Tierra del Fuego (Chile). Int Microbiol 19:161–173
Jangid K, Whitman WB, Condron LM, Turner BL, Williams MA (2013) Soil bacterial community succession during long-term ecosystem development. Mol Ecol 22:3415–3424
Schuette UME, Abdo Z, Foster J, Ravel J, Bunge J, Solheim B et al (2010) Bacterial diversity in a glacier foreland of the high Arctic. Mol Ecol 191:54–66
Fierer N, Nemergut D, Knight R, Craine JM (2010) Changes through time: integrating microorganisms into the study of succession. Res Microbiol 161:635–642
Kim M, Jung JY, Laffly D, Kwon HY, Lee YK (2017) Shifts in bacterial community structure during succession in a glacier foreland of the high Arctic. Fems Microbiology Ecology 93:fiw213
Ortiz-álvarez R, Fierer N, de Los RA, Casamayor EO, Barberán A (2018) Consistent changes in the taxonomic structure and functional attributes of bacterial communities during primary succession. ISME J 12:1658–1667
Bai Y, Huang X, Zhou X, Xiang Q, Zhao K, Yu X et al (2019) Variation in denitrifying bacterial communities along a primary succession in the Hailuogou Glacier retreat area. China PeerJ 7:e7356
Schmidt SK, Porazinska D, Concienne BL, Darcy JL, King AJ, Nemergut DR (2016) Biogeochemical stoichiometry reveals P and N limitation across the post-glacial landscape of Denali National Park, Alaska. Ecosystems 19:1164–1177
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Zhou J, Bing H, Wu Y, Yang Z, Wang J, Sun H et al (2016) Rapid weathering processes of a 120-year-old chronosequence in the Hailuogou Glacier foreland, Mt. Gongga. SW China Geoderma 267:78–91
Barberan A, Bates ST, Casamayor EO, Fierer N (2012) Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J 6:343–351
Fuhrman JA (2009) Microbial community structure and its functional implications. Nature 459:193–199
Newman ME (2006) Modularity and community structure in networks. Proc Natl Acad Sci 103:8577–8582
Weiss S, Van Treuren W, Lozupone C, Faust K, Friedman J, Deng Y et al (2016) Correlation detection strategies in microbial data sets vary widely in sensitivity and precision. ISME J 10:1669–1681
Deng Y, Jiang YH, Yang Y, He Z, Luo F, Zhou J (2012) Molecular ecological network analyses. Bmc. Bioinformatics 13:113
Menezes AB, Prendergast-Miller MT, Richardson AE, Toscas P, Farrell M, Macdonald LM et al (2015) Network analysis reveals that bacteria and fungi form modules that correlate independently with soil parameters. Environ Microbiol 17:2677–2689
Dubnick A, Kazemi S, Sharp MJ, Wadham J, Hawkings J, Beaton A et al (2017) Hydrological controls on glacially exported microbial assemblages. J Geophys Res Biogeosci 122:1049–1061
Hotaling S, Foley ME, Zeglin LH, Finn DS, Tronstad LM, Giersch JJ et al (2019) Microbial assemblages reflect environmental heterogeneity in alpine streams. Glob Change Biol 25:2576–2590
Purkamo L, DBMacdonaldCousins OAC (2022) Following the flow-microbial ecology in surface- and groundwaters in the glacial forefield of a rapidly retreating glacier in Iceland. Environ Microbiol 24:5840–5858
Crump BC, Amaral-Zettler LA, Kling GW (2012) Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J 6:1629–1639
Zhang C, Ren Z (2023) The role of subsurface ice in sustaining bacteria in continental and maritime glaciers. Sci Total Environ 896:165324
Brighenti S, Tolotti M, Bruno MC, Wharton G, Pusch MT, Bertoldi W (2019) Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: a review. Sci Total Environ 675:542–559
Brandani J, Peter H, Busi SB, Kohler TJ, Fodelianakis S, Ezzat L et al (2022) Spatial patterns of benthic biofilm diversity among streams draining proglacial floodplains. Front Microbiol 13:e948165
Jacobsen D, Milner AM, Brown LE, Dangles O (2012) Biodiversity under threat in glacier-fed river systems. Nat Clim Chang 2:361–364
Brittain JE, Milner AM (2001) Ecology of glacier-fed rivers: current status and concepts. Freshw Biol 46:1571–1578
Moore RD, Fleming SW, Menounos B, Wheate R, Fountain A, Stahl K et al (2009) Glacier change in western North America: influences on hydrology, geomorphic hazards and water quality. Hydrological Processes: An International Journal 23:42–61
Rinke K, Robinson CT, Uehlinger U (2001) A note on abiotic factors that constrain periphyton growth in alpine glacier streams. Int Rev Hydrobiol 86:361–366
Gu Z, Liu K, Pedersen MW, Wang F, Chen Y, Zeng C et al (2021) Community assembly processes underlying the temporal dynamics of glacial stream and lake bacterial communities. The Science of the Total Environment 761:143178
Kong W, Liu J, Ji M, Yue L, Kang S, Morgan-Kiss RM (2019) Autotrophic microbial community succession from glacier terminus to downstream waters on the Tibetan Plateau. Fems Microbiology Ecology 95:fiz074
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Zeglin LH (2015) Stream microbial diversity in response to environmental changes: review and synthesis of existing research. Front Microbiol 6:454
Caillon F, Schelker J (2020) Dynamic transfer of soil bacteria and dissolved organic carbon into small streams during hydrological events. Aquat Sci 82:e41
Hermans SM, Buckley HL, Case BS, Lear G (2020) Connecting through space and time: catchment-scale distributions of bacteria in soil, stream water and sediment. Environ Microbiol 22:1000–1010
Ruiz-Gonzalez C, Pablo Nino-Garcia J, Del Giorgio PA (2015) Terrestrial origin of bacterial communities in complex boreal freshwater networks. Ecol Lett 18:1198–1206
Archer S, Lee KC, Caruso T, Maki T, Lee CK, Cary SC et al (2019) Airborne microbial transport limitation to isolated Antarctic soil habitats. Nat Microbiol 4:925–932
Edwards A, Rassner SME, Anesio AM, Worgan HJ, Irvine-Fynn TDL, Williams HW, et al. 2013. Contrasts between the cryoconite and ice-marginal bacterial communities of Svalbard glaciers. Polar Research, 32.
O’Neel S, Hood E, Arendt A, Sass L (2014) Assessing streamflow sensitivity to variations in glacier mass balance. Clim Change 123:329–341
Santibanez PA, Maselli OJ, Greenwood MC, Grieman MM, Saltzman ES, Mcconnell JR et al (2018) Prokaryotes in the WAIS Divide ice core reflect source and transport changes between Last Glacial Maximum and the early Holocene. Glob Change Biol 24:2182–2197
Smets W, Moretti S, Denys S, Lebeer S (2016) Airborne bacteria in the atmosphere: presence, purpose, and potential. Atmos Environ 139:214–221
Stibal M, Schostag M, Cameron KA, Hansen LH, Chandler DM, Wadham JL et al (2015) Different bulk and active bacterial communities in cryoconite from the margin and interior of the Greenland ice sheet. Environmental Microbiology Reports 7:293–300
Acknowledgements
We are grateful to Cheng Zhang and Kang Ma for their invaluable assistance in both fieldwork and laboratory work.
Funding
This study was supported by the International Partnership Program of Chinese Academy of Sciences (045GJHZ2024015FN), the National Natural Science Foundation of China (42301132, 91851201), the Key Laboratory of Polar Science, MNR, Polar Research Institute of China (KP202101), and the MNR Key Laboratory of Marine Eco-Environmental Science and Technology, China (MEEST-2022–03).
Author information
Authors and Affiliations
Contributions
Z.R: conceptualization, methodology, formal analysis, investigation, writing - original draft, writing - review & editing, visualization. funding acquisition. HK.G: investigation, resources, writing - review & editing. N.M: methodology, writing - review & editing. H.R: investigation, writing - review & editing. X.X: validation, writing - review & editing. W.L: conceptualization, visualization, writing - review & editing, funding acquisition.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, Z., Gao, H., Martyniuk, N. et al. Dual-Domain Primary Succession of Bacteria in Glacier Forefield Streams and Soils of a Maritime and Continental Glacier. Microb Ecol 88, 5 (2025). https://doi.org/10.1007/s00248-024-02486-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02486-w