Abstract
The NAD+-dependent aldehyde dehydrogenase AldB from Aromatoleum aromaticum was recombinantly produced in Escherichia coli and biochemically characterized. As suggested by its substrate spectrum, the most probably physiological function of AldB is the oxidation of short aliphatic aldehydes such as acetaldehyde, which occur as intermediates in the degradation of the corresponding alcohols. In addition, we generated some mutant variants in residue Tyr460, which is located at the neck region of the substrate channel and analyzed their effects on the catalytic parameters for different substrates. Single amino acid exchanges at this position revealed profound changes in substrate preference and substrate inhibition of the variants.
Key points
• Small aliphatic aldehydes show the best catalytic efficiency with aldehyde dehydrogenase AldB
• Amino acid exchanges at Y460 results in changed catalytic efficiencies and substrate inhibition
• AldB is a member of a new clade of the aldehyde dehydrogenase superfamily
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The enzyme family of aldehyde dehydrogenases (EC 1.2.1._) consists of NAD(P)-dependent enzymes oxidizing a wide range of aliphatic or aromatic aldehydes to the corresponding acids. They are present in all organisms and typically consist of homodimers or -tetramers of 45- to 60-kDa subunits. The family is currently organized into 24 subclasses (Aldh1-24) which contain most of the known enzymes from animals and plants (Brocker et al. 2013; Hou and Bartels 2015), while microbes contain many more aldehyde dehydrogenases that are mostly not yet affiliated with the established subclasses. The enzymes of this family oxidize aldehydes to organic acids and, depending on the individual enzymes, either exhibit broad substrate spectra or are specific for only few closely related aldehydes (Shortall et al. 2021). While aldehyde oxidation to the corresponding acid is usually thermodynamically irreversible, the aldehyde dehydrogenases family also includes reversible aldehyde dehydrogenases which interconvert aldehydes and activated acids, such as acyl-phosphates, -adenylates or -thioesters with CoA. Some aldehyde dehydrogenases also occur as fusion proteins with alcohol dehydrogenases (e.g., AdhE of E. coli and many other bacteria), proline dehydrogenases (e.g. PutA) or acid kinases (e.g. eukaryotic Δ−1-pyrroline-5-carboxylate synthetase) (Martinelli et al. 2012; Extance et al. 2013; Liu et al. 2017). The structures of aldehyde dehydrogenases are highly conserved and exhibit three major domains per subunit; the first one comprises the N-terminal half of the subunit and is involved in binding NAD(P)+, the following second domain is almost as large as the first one and contains the catalytic center, and finally, a small third domain at the C-terminus is involved in oligomerization (Liu et al. 1997; Shortall et al. 2021). All of these enzymes contain an essential Cys residue in the catalytic center which forms a thiohemiacetal bond with the incoming aldehyde. In most members of the family, the catalytic Cys is deprotonated via a bridging water molecule with a conserved Glu residue to expedite formation of the thiohemiacetal intermediate. This “activated” form of the aldehyde then reacts with a bound NAD(P)+ cofactor, getting oxidized to a covalently bound thioester after hydride abstraction (Shortall et al. 2021). The irreversible aldehyde dehydrogenases then hydrolyze this thioester with the nearby water ligand, releasing the contained energy as heat, while the reversible enzymes convert the bound thioester to another high-energy intermediate, such as an acyl-phosphate (e.g., glyceraldehyde phosphate dehydrogenases or reverse-acting enzymes like glutamyl phosphate reductases) or a CoA-thioester (e.g., AdhE or Clostridium kluyveri aldehyde dehydrogenase) (Smith and Kaplan 1980; Sirover 1999). Further, highly conserved amino acids are involved in NAD(P)+ binding and positioning, as well as in forming an entry channel for the substrates. The latter consists of a “mouth” region on the surface of the N-terminal domain, which opens a funnel-like cavity reaching down to the catalytic Cys as one of the “bottom” residues. Moreover, some “neck” residues flanking this channel on the way toward the active site have been suggested to affect the substrate specificity of the enzymes (Shortall et al. 2021).
We have previously encountered the pdh (ebA4954) and aldB (ebA4625) genes coding for aldehyde dehydrogenases of the Betaproteobacterium Aromatoleum aromaticum in a study on anaerobic phenylalanine degradation, which proceeds with phenylacetaldehyde as intermediate. Phenylacetaldehyde is oxidized to phenylacetate by a dedicated highly specific phenylacetaldehyde dehydrogenase (Pdh), which has been characterized before (Debnar-Daumler et al. 2014). In addition to Pdh, phenylalanine-degrading cells contain varying amounts of an unspecific tungsten-containing aldehyde oxidoreductase (AOR), which apparently acts as backup system to avoid the buildup of toxic aldehyde concentrations (Debnar-Daumler et al. 2014; Schmitt et al. 2017). A Δpdh deletion mutant of A. aromatoleum was created and still revealed growth on phenylalanine in growth media containing tungstate, indicating that Pdh is functionally replaced by the tungsten-containing AOR. While this mutant initially showed a severe growth defect in media without tungstate, a second-site revertant capable of degrading phenylalanine evolved over time. This strain contained a mutation in the aldB gene for a previously unknown aldehyde dehydrogenase and overexpressed both the aldB and adhB genes, which form a common operon (Schmitt et al. 2017). The mutated variant of AldB (Y460C) showed broad substrate specificity and oxidized various aliphatic and aromatic aldehydes including phenylacetaldehyde with NAD+ as redox cofactor (Schmitt et al. 2017). In this study, we purified a recombinant version of wild-type AldB fused with a C-terminal Strep-tag, as well as further mutants with changed residues at position 460, aiming to identify the usual physiological function of AldB and evaluate the effects of the respective mutations on the reactivities with different aldehydes.
Material and methods
Cloning, heterologous gene expression, preparation of cell-free extracts, and protein purification
The gene aldB (ebA4625) from the A. aromaticum strain EbN1 was amplified via PCR from chromosomal DNA using appropriate primers (Table 1) and cloned into the vectors pASG5 and pASG3, using the “Stargate” cloning system (IBA Lifesciences, Göttingen, Germany). The resulting plasmids code for fusion proteins of AldB with N- or C-terminal Strep-tag sequences. Mutants of AldB were generated via site-directed mutagenesis (Braman et al. 1996; Papworth et al. 1996). The pASG3_aldB expression plasmid was used as template with primer pairs containing the desired mutations to create the Y460A, Y460C, and Y460S variants (see primers in Table 1). PCR conditions were adjusted to each individual primer pair. Additional plasmids containing mutagenized aldB genes coding for the Y460F and Y460W variants were generated via inverse PCR and subsequent blunt-end ligation of the wild-type expression plasmid, using forward primers AldBY460F_for3 or AldBY460W_for2 combined with reverse primer AldB_Mut_rev.
To produce the recombinant enzymes, the plasmids were subsequently transformed into E. coli strain DH5α, which was grown in LB medium at 30 °C and induced with added anhydrotetracycline as reported previously (Schmitt et al. 2017). Cells were harvested by centrifugation and resuspended in two volumes of 10 mM Tris/HCl pH 7.5, containing 0.1 mg/ml DNase I and 10% v/v glycerol. Cell-free extracts were prepared by sonification at 4 °C, followed by ultracentrifugation (100,000 × g, 60 min). The activity of AldB was exclusively observed in the soluble fractions. Recombinant proteins were purified from cell-free extracts via affinity chromatography on streptactin columns as published previously (Winiarska et al. 2022; Gemmecker et al. 2024), but with a basal buffer system of 100 mM HEPPS/KOH pH 8.3, containing 100 mM KCl.
Protein chemical methods
The purified proteins were analyzed by SDS-PAGE (13% polyacrylamide) (Laemmli 1970) and native polyacrylamide gel electrophoresis as described before (Gallagher 2018). The molecular mass of native AldB was determined by a Ferguson plot analysis with native gels (6/7/8/10% polyacrylamide; NativeMark, Thermo Fischer) and by crosslinking analysis after treatment with glutaraldehyde or dimethylsuberimidate, as described previously (Schühle et al. 2016). Protein concentrations were determined, as described previously (Bradford 1976).
Enzymatic assays
AldB activity was assayed in 100 mM HEPPS/KOH buffer pH 8.5, containing 100 mM KCl and 2 mM dithioerithritol in a photometric assay by directly following the formation of NADH at 365 nm (ε = 3.4 mM−1 cm−1) at room temperature. The assay contained purified recombinant AldB (10–200 µg/ml) and 1 mM NAD+. The reactions were started by adding the substrate of interest (acetaldehyde 0–2 mM, propionaldehyde 0–3 mM, benzaldehyde 0–2 mM, phenylacetaldehyde 0–2 mM; other substrates were tested at 1 mM).
Phylogenetic analysis
The amino acid sequences of AldB and those of various other members of the aldehyde dehydrogenase family were aligned using Clustal Omega (www. ebi. ac. uk/Tools/msa/clust alo and avermitilis.ls.kitasato-u.ac.jp/clustalo). A neighbor-joining tree was constructed based on the alignment, using the Program iTOL (itol.embl.de/).
AlphaFold model
The structural model of AldB (Uniprot Q5P1R9) was taken from the AlphaFold protein structure database (https://alphafold.com/).
Results
Construction and purification of AldB variants
Because we previously identified a highly overproduced Y460C variant of the aldehyde dehydrogenase AldB, which complemented a Δpdh strain of A. aromaticum for growth on phenylalanine (Schmitt et al. 2017), we were interested in the properties of the original AldB as well as the effects of other mutations in this position. Therefore, we produced wild-type AldB and mutant Y460C but also added mutants Y460F and Y460S as additional structural mimics for the former variants. We also tried to produce variants Y460A and Y460W but did not get them expressed as detectable proteins. We cloned the wild-type gene (ebA4625) into expression vectors pASG5 and pASG3 with added N- or C-terminal Strep-tag fusions, respectively, and generated variants in Y460 from the pASG3-based clone by directed mutagenesis. The successfully produced variants were purified by affinity chromatography from recombinant E. coli cells containing the respective expression plasmids with the cloned aldB gene variants. Both the N- and C-terminally tagged wild-type versions showed the expected molecular masses and molar absorption coefficients at 280 nm. Since they did not show significant difference in their yields or activities, we selected the C-terminal fusion for further characterization and mutagenesis studies. Homogeneous preparations were obtained for the wild-type AldB proteins as well as the Y460F, Y460C, and Y460S variants with yields of 3 to 4 mg protein per 2 L of recombinant E. coli culture, which were used for our further studies.
Molecular properties of AldB
AldB and its variants consisted of a single subunit migrating at about 52 kDa in SDS-PAGE. With expected masses of 56.7 kDa for the subunits (including the Strep-tag), the protein migrates consistently with a previously observed subunit mass of 55 kDa for the untagged Y460C variant purified from A. aromaticum (Schmitt et al. 2017). The native masses were determined by Ferguson plot analysis (Fig. 1A) in native gels, where all proteins exhibited major bands, which were determined at masses of 259 kDa for the wild-type enzyme and between 225 and 241 kDa for the mutant variants. This indicated a homotetrameric composition in all cases, which is also consistent with crosslinking studies (Fig. 1B) and the previously reported data on the Y460C variant (Schmitt et al. 2017). In addition, the proteins also revealed slower-migrating minor bands, which were calculated at masses of 430 to 490 kDa and may represent dimers of the tetramers, which were not observed with other techniques (Fig. 1A). Furthermore, the wild-type protein showed an additional faint band of even higher molecular mass. With the methodology used here, it was not possible to distinguish whether this band corresponds to a trimer of tetramers or even bigger complexes, but similar supercomplex formation has already been observed for other members of the AlDH superfamily (e.g., homotetrameric vs. homododecameric complexes of succinate semialdehyde dehydrogenase in pdb 2W8N and 2W8O).
A Native mass determination via Ferguson plot. Exemplary gel images show migration of AldB variants in 7% and 10% polyacrylamide native PAGE. The standard curve was calculated based on the migration of ovalbumin and BSA. The native mass of the tetramer of the AldB Y460S variant was calculated as 241 kDa in a separate experiment, which did not reveal larger complexes due to lower protein concentration. B Crosslinking of WT-AldB. The protein was incubated with glutaraldehyde and analyzed by SDS-PAGE. The arrows point at the positions of the subunit and the crosslinked derivatives. Lanes: 1, marker proteins; 2, untreated AldB; 3–5, AldB treated with glutaraldehyde for 1, 5, and 15 min, respectively
Catalytic properties of AldB and its variants
The wild-type AldB protein was checked for the conversion of several aldehydes with NAD as electron acceptor and showed the following specific activities: acetaldehyde, 100% (corresponds to 985 mU mg−1); glutaraldehyde, 86%; propionaldehyde, 78%; benzaldehyde, 17%; phenylacetaldehyde, 5%; crotonaldehyde, 2%; and formaldehyde, 15%. Since the strongest recorded activities were observed with small aliphatic aldehydes or glutaraldehyde, these are likely the physiological substrates of AldB, rather than aromatic aldehydes, crotonaldehyde, or formaldehyde. To compare the effect of the introduced mutations on catalytic behavior and substrate preference, we selected acetaldehyde, propionaldehyde, benzaldehyde, and phenylacetaldehyde as representatives for aliphatic and aromatic substrates and performed steady state kinetic analysis with the wild-type enzyme and the three variants (Fig. 2).
Kinetic analysis of AdhB mutants with different substrates. A Wildtype (WT) AldB, B AldBY460F, C AldBY460C, and D AldBY460S. Experiments were done in duplicates and standard deviations are indicated. Codes for the respective substrates and curve fittings used are as follows: acetaldehyde, squares and blue curves; propionaldehyde, triangles and green curves; benzaldehyde, inverted triangles and orange curves; phenylacetaldehyde, circles and red curves. Error bars show the standard deviation of the measurements
As expected from the previous tests, wild-type AldB showed the highest activity with acetaldehyde, followed by that for propionaldehyde and benzaldehyde but exhibited a more than tenfold lower apparent Vmax with phenylacetaldehyde than with acetaldehyde (Table 2). The apparent Km values ranged from very low (7.7 µM for acetaldehyde) to moderate values (23–39 µM for the other substrates), and the resulting catalytic efficiencies indicated reactivities from very high with acetaldehyde, via mediocre with propionaldehyde or benzaldehyde, to low with phenylacetaldehyde (Table 2). Curve fitting indicated strong substrate inhibition with acetaldehyde but successively weaker substrate inhibition with the other substrates (Fig. 2A).
The Y460F variant, which only lacks the phenolic hydroxy group of Tyr460, showed only minor changes in its apparent catalytic parameters. The observed effects included decreased apparent Vmax and increased Km values for acetaldehyde, propionaldehyde, and benzaldehyde, reducing the catalytic efficiencies for their turnover, while the changes were less severe for phenylacetaldehyde, resulting in a similar catalytic efficiency than for the wild-type enzyme (Table 3). In addition, the Y460F variant shows significantly increased substrate inhibition effects for both aliphatic aldehydes, compared to wild-type AldB (Fig. 2B).
The Y460C variant represents the biologically evolved mutant enabling growth on phenylalanine for a Δpdh mutant of A. aromaticum. It exhibited even further decrease of the apparent Vmax and increase of the apparent Km values for acetaldehyde, propionaldehyde, and benzaldehyde, while these parameters stayed almost the same for phenylacetaldehyde as in wild-type AldB (Table 4). Consequently, phenylacetaldehyde shows the best catalytic efficiency of all tested substrates while the turnover of all other substrates is severely restricted (Table 4). In particular, turnover of benzaldehyde is hardly detectable any more, representing a reversal of the activity patterns of wild-type AldB for these substrates (Fig. 2C).
Finally, the Y460S variant showed even less activity with acetaldehyde, propionaldehyde, and benzaldehyde, while the parameters for phenylacetaldehyde oxidation were still not severely affected. This variant even showed higher activity with phenylacetaldehyde than with any other of the analyzed substrates, although the relatively low apparent Km values for the aliphatic aldehydes result in slightly higher catalytic efficiencies for these than for phenylacetaldehyde (Table 5). Another interesting feature is a very strong substrate inhibition by phenylacetaldehyde, which is not shared by the other substrates (Fig. 2D).
Correlation of the observed changes with the predicted structure of AldB
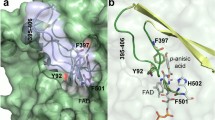
AldB contains all universally conserved amino acids of the aldehyde dehydrogenase family and is predicted to fold into the common structure displayed by these enzymes (Fig. 3A). A high-quality AlphaFold prediction of the structure was available (AF-Q5P1R9-F1-v4) and was used to rationalize the impact of the mutations generated in residue 460. A look into the active site shows the conserved catalytically active Cys299 in close neighborhood with Glu260, another universally conserved active site residue which is expected to initiate the reaction mechanism by deprotonating Cys299 via a shared bridging water ligand (Shortall et al. 2021). A predicted distance of 6.3 Å between these residues would be compatible with this conserved feature of the enzyme family (Fig. 3B). The NAD+ cofactor is expected to close the backside of the active site cavity, binding with its nicotinamide group just below Cys299 in an optimal position to react with the bound aldehyde. We also show two additional amino acid side chains in Fig. 3B, which come close to the active center, namely Tyr460 and Phe466. Phe466, which is almost universally conserved in the enzyme family, is only 3.9 Å away from Cys299 (Fig. 3B) and has an alleged catalytic function of pushing incoming aldehydes toward Cys299, supporting thiohemiacetal formation (Shortall et al. 2021). Tyr460 is further away from Cys299, but close enough to the aromatic ring of Phe466 (3.8 Å) to assist in substrate pushing (Fig. 3B). This assistant function should be only slightly disturbed in the Y460F variant but should be impacted more severely in the Y460C and Y460S variants. In addition, the location of Tyr460 at the neck of the substrate channel and the different observed effects of the mutations for various substrates suggest that Tyr460 is indeed involved in guiding substrates through the channel. Significantly, Tyr460 is uniformly conserved in the aldehyde dehydrogenases of the AldB subbranch but is often replaced by another amino acid in other subfamilies, probably contributing to different substrate specificities (Shortall et al. 2021). Looking at a surface representation of the structural model of AldB, we additionally detected the probable entrance hole of the substrate channel, which leads from the surface directly to Cys299 via Tyr460 (Fig. 3C). Therefore, it appears that Tyr460 serves a dual role as a gatekeeper to restrict access to the active site and as pushing lever (together with Phe466) to facilitate thiohemiacetal formation with the substrate. The observed effects on both the reactivity of certain substrates and substrate inhibition in this study marks this residue as a promising mutagenesis target to change or expand aldehyde dehydrogenase reactivities.
AlphaFold model of AldB. A Predicted overall structure of an AldB subunit, indicating the three functional domains and residues Cys299 and Tyr460. B Zoomed-in image of the active site, showing distances in Å between some of the key residues. The arrow indicates the direction of the substrate channel. C Surface representation showing the entrance of the substrate channel, indicating surrounding amino acids and active site residues residing at the neck (Tyr460) and bottom (Cys299, Glu260, Phe466) of the channel
Phylogenetic analysis of AldB
A phylogenetic tree of the aldehyde dehydrogenase (AlDH) superfamily was prepared from a multiple alignment of representative sequences, indicating that AldB and Pdh, are not closely related, although the latter is complemented by the overproduced AldB Y460C mutant (Fig. 4). Moreover, neither enzyme belongs to one of the previously proposed 24 AlDH families subclades, which are based on the enzymes present in eukaryotes (Hou and Bartels 2015; Islam and Ghosh 2022). However, both enzymes are clearly affiliated to established categories of the conserved protein domain database, which contain verified or predicted enzymes from various bacterial strains. AldB is affiliated to category cd07116, which also contains an acetaldehyde dehydrogenase from Alcaligenes eutrophus (Priefert et al. 1992) or a chloroacetaldehyde dehydrogenase from Xanthobacter autotrophicus (Bergeron et al. 1998) as biochemically characterized members, which fit very well in their biochemical properties to those reported for AldB. In contrast, the Pdh enzyme is a member of category cd07106, which consisted only of translated gene sequences prior to the characterization of Pdh. We also show the affiliation of the other predicted members of the AlDH superfamily encoded in the genome of A. aromaticum in Fig. 4. Working on this paper prompted us to provide an exhaustive overview of the structure–function relationships of the AlDH superfamily enzymes and additional aldehyde dehydrogenase enzymes (Heider and Hege 2025).
Unrooted phylogenetic tree of the aldehyde dehydrogenase family. Recognized subclasses AlDH1-24 are indicated as green branches, the new subbranches containing AldB and Pdh in red and magenta, respectively. Other enzymes of the AlDH superfamily encoded in A. aromaticum are named and marked by orange branches and red asterisks
Discussion
AldB is one of 17 aldehyde dehydrogenases encoded in the genome of A. aromaticum (Table 6). Twelve of these are well understood in their functions, based on similarity to known enzymes of general metabolic pathways, such as glycolysis (gene gapA), amino acid biosynthesis (genes proA, argC, asd, gabA) as well as proline degradation (gene putA for a fusion protein of proline and glutamate semialdehyde dehydrogenases). Furthermore, A. aromaticum contains several well-characterized aldehyde dehydrogenases in the degradation pathways for aerobic benzoate or phenylacetate degradation (genes boxD and paaZ1/2) (Gescher et al. 2006; Teufel et al. 2011), as well as a 4-hydroxybenzaldehyde dehydrogenase in oxygen-independent p-cresol metabolism (gene pchA, Table 6) (Hopper et al. 1991). Furthermore, the nature of the ald gene product as a benzaldehyde dehydrogenase was established by its induction pattern (Wöhlbrand et al. 2007), and the pdh gene product has been identified as specific phenylacetaldehyde dehydrogenase (Debnar-Daumler et al. 2014) (Table 6). Among the remaining five genes, we now designate aldB as the gene for an acetaldehyde dehydrogenase after the substrate converted with the highest catalytic efficiency. While AldB has the highest catalytic efficiencies with small aliphatic aldehydes but also turns over some other substrates, we have recently found that the Fe-dependent alcohol dehydrogenase AdhB encoded in the same operon is almost exclusively specific in oxidizing ethanol or 1-propanol to the corresponding aldehydes (Gemmecker and Heider, unpublished data). Therefore, the major physiological roles of AldB and AdhB are most likely in the degradation pathways of aliphatic alcohols like ethanol or 1-propanol.
In addition to acetaldehyde, AldB shows good reactivity with other small aliphatic aldehydes (such as propionaldehyde, an intermediate of 1-propanol degradation) or with benzaldehyde. Activity of AldB with benzaldehyde may explain why the AdhB protein, which is encoded in the same operon (Schmitt et al. 2017), was observed as substrate-induced protein in cells grown on benzyl alcohol or benzaldehyde (Wöhlbrand et al. 2007). Although the authors observed strong induction of the alternative ald gene and did not detect the presence of AldB in these cells, it may have been overlooked. The presence of some AldB (along with AdhB) would be consistent with a possible backup role to detoxify unwanted build-up of benzaldehyde when the “major” benzaldehyde dehydrogenase Ald does not keep pace with its production or uptake.
Another aspect of AldB biochemistry investigated here are the effects of several mutations in the “neck” residue 460 on substrate specificity and the reaction parameters. We observed that small changes at this position already showed major effects, either on the reactivity with different substrates or on substrate inhibition effects. The investigated variants showed successively decreasing reactivity and increasing substrate inhibition effects with the degree of deviation from the original Tyr460, generally following the series Y > F > C > S (Tables 2–5). The reactions with acetaldehyde and propionaldehyde were only affected by increased Km values and stronger substrate inhibition in the Y460F variant, but lost much more reactivity in the Y460C and Y460 variants, while benzaldehyde turnover was already significantly lower in the Y460F mutant and almost completely lost in the Y460C and Y460 variants. To our surprise, the catalytic parameters for phenylacetaldehyde did not improve very much between wild-type and the mutant AldB variants (especially Y460C) but just did not deteriorate as much as for the other substrates (Tables 2–5). This is especially reflected in the catalytic efficiency values which stayed between 0.83 and 1.7 mU mg−1 µM−1 for phenylacetaldehyde but dropped drastically for the other investigated substrates (e.g., from 70 to 1.3 or from 13 to 0.023 mU mg−1 µM−1 for acetaldehyde and benzaldehyde, respectively), resulting in Y460C exhibiting the best catalytic efficiency for phenylacetaldehyde among the tested substrates. Although the Y460S variant actually showed the highest calculated Vmax value for phenylacetaldehyde and a similar catalytic efficiency as Y460C, it also exhibited much more pronounced substrate inhibition and slightly higher catalytic efficiencies for acetaldehyde and propionaldehyde (due to lower Km values than in the Y460C variant) (Fig. 2D). The changed substrate specificities of the variants observed in this study may become interesting for possible applications of highly specific enzymes oxidizing only some aldehydes (e.g., phenylacetaldehyde) in complex mixtures, while leaving others untouched.
The recorded specific activities of wild-type AldB are consistent with an expected catabolic activity of cells growing on ethanol under aerobic or denitrifying conditions of 30–100 mU (mg protein)−1, assuming doubling times of 10–20 h and yields of 20 or 30 g dry cell mass per mol ethanol under denitrifying or aerobic conditions, respectively. These values are consistent with the recorded specific activities of purified wild-type AldB with acetaldehyde (500–1000 mU mg−1), and even the take-over of phenylacetaldehyde oxidation by the Y460C variant can be explained by the recorded specific activity of 42 mU mg−1 (Table 4), regarding the very strong induction of AldB (plus AdhB) in these cells of up to 50% of the total protein (Schmitt et al. 2017) and some loss of activity connected with heterologous gene expression and purification of the protein.
A remarkably similar effect of an unrelated aldehyde dehydrogenase rescuing the growth defects of E. coli mutants in glyceraldehyde phosphate dehydrogenase or erythrose-phosphate dehydrogenase was recently reported for succinate semialdehyde dehydrogenase, which evolved to mutated variants by adaptive laboratory evolution (He et al. 2024). Therefore, this report reinforces the power of laboratory evolution to obtain new enzyme reactivities and at the same time indicates the strong influence of the amino acid occupying the “neck” region of the substrate channel on substrate specificity and substrate inhibition effects of aldehyde dehydrogenases.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Bergeron H, Labbé D, Turmel C, Lau PCK (1998) Cloning, sequence and expression of a linear plasmid-based and a chromosomal homolog of chloroacetaldehyde dehydrogenase-encoding genes in Xanthobacterautotrophicus GJ10. Gene 207:9–18. https://doi.org/10.1016/S0378-1119(97)00598-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Braman J, Papworth C, Greener A (1996) Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol Biol (Clifton, N.J.) 57:31–44
Brocker C, Vasiliou M, Carpenter S, Carpenter C, Zhang Y, Wang X, Kotchoni SO, Wood AJ, Kirch HH, Kopečný D, Nebert DW, Vasiliou V (2013) Aldehyde dehydrogenase (ALDH) superfamily in plants: gene nomenclature and comparative genomics. Planta 237:189–210. https://doi.org/10.1007/s00425-012-1749-0
Debnar-Daumler C, Seubert A, Schmitt G, Heider J (2014) Simultaneous involvement of a tungsten-containing aldehyde: ferredoxin oxidoreductase and a phenylacetaldehyde dehydrogenase in anaerobic phenylalanine metabolism. J Bacteriol 196:483–492. https://doi.org/10.1128/JB.00980-13
Extance J, Crennell SJ, Eley K, Cripps R, Hough DW, Danson MJ (2013) Structure of a bifunctional alcohol dehydrogenase involved in bioethanol generation in Geobacillusthermoglucosidasius. Acta Crystallogr D Biol Crystallogr 69:2104–2115. https://doi.org/10.1107/S0907444913020349
Gallagher SR (2018) One-dimensional electrophoresis using nondenaturing conditions. Curr Protocol Protein Sci 94:e73. https://doi.org/10.1002/cpps.73
Gemmecker Y, Winiarska A, Hege D, Kahnt J, Seubert A, Szaleniec M, Heider J (2024) A pH-dependent shift of redox cofactor specificity in a benzyl alcohol dehydrogenase of Aromatoleumaromaticum EbN1. Appl Microbiol Biotechnol 108:s00253-s1024
Gescher J, Ismail W, Ölgeschläger E, Eisenreich W, Wörth J, Fuchs G (2006) Aerobic benzoyl-coenzyme A (CoA) catabolic pathway in Azoarcusevansii: conversion of ring cleavage product by 3,4-dehydroadipyl-CoA semialdehyde dehydrogenase. J Bacteriol 188:2919–2927. https://doi.org/10.1128/JB.188.8.2919-2927.2006
He H, Gómez-Coronado PA, Zarzycki J, Barthel S, Kahnt J, Claus P, Klein M, Klose M, Crécy-Lagard V, Schindler D, Paczia N, Glatter T, Erb TJ (2024) Adaptive laboratory evolution recruits the promiscuity of succinate semialdehyde dehydrogenase to repair different metabolic deficiencies. Nat Commun 15:8898
Heider J, Hege D (2025) The aldehyde dehydrogenase superfamilies: correlations and deviations in structure and function. Appl Microbiol Biotechnol. https://www.doi.org/10.1007/s00253-025-13467-5
Hopper DJ, Bossert ID, Rhodes-Roberts ME (1991) p-Cresol methylhydroxylase from a denitrifying bacterium involved in anaerobic degradation of p-cresol. J Bacteriol 173:1298–1301. https://doi.org/10.1128/jb.173.3.1298-1301.1991
Hou Q, Bartels D (2015) Comparative study of the aldehyde dehydrogenase (ALDH) gene superfamily in the glycophyte Arabidopsis thaliana and Eutrema halophytes. Ann Bot 115:465–479
Islam MS, Ghosh A (2022) Evolution, family expansion, and functional diversification of plant aldehyde dehydrogenases. Gene 829:146522
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Liu LK, Becker DF, Tanner JJ (2017) Structure, function, and mechanism of proline utilization A (PutA). Arch Biochem Biophys 632:142–157
Liu ZJ, Sun YJ, Rose J, Chung YJ, Hsiao CD, Chang WR, Kuo I, Perozich J, Lindahl R, Hempel J, Wang BC (1997) The first structure of an aldehyde dehydrogenase reveals novel interactions between NAD and the Rossmann fold. Nat Struct Biol 4:317–326. https://doi.org/10.1038/nsb0497-317
Martinelli D, Häberle J, Rubio V, Giunta C, Hausser I, Carrozzo R, Gougeard N, Marco-Marín C, Goffredo BM, Meschini MC, Bevivino E, Boenzi S, Colafati GS, Brancati F, Baumgartner MR, Dionisi-Vici C (2012) Understanding pyrroline-5-carboxylate synthetase deficiency: clinical, molecular, functional, and expression studies, structure-based analysis, and novel therapy with arginine. J Inherit Metab Dis 35:761–776. https://doi.org/10.1007/s10545-011-9411-8
Priefert H, Kruger N, Jendrossek D, Schmidt B, Steinbuchel A (1992) Identification and molecular characterization of the gene coding for acetaldehyde dehydrogenase II (acoD) of Alcaligenes eutrophus. J Bacteriol 174:899–907. https://doi.org/10.1128/jb.174.3.899-907.1992
Rabus R, Kube M, Heider J, Beck A, Heitmann K, Widdel F, Reinhardt R (2005) The genome sequence of an anaerobic aromatic-degrading denitrifying bacterium, strain EbN1. Arch Microbiol 183:27–36. https://doi.org/10.1007/s00203-004-0742-9
Schmitt G, Arndt F, Kahnt J, Heider J (2017) Adaptations to a loss-of-function mutation in the betaproteobacterium Aromatoleumaromaticum: recruitment of alternative enzymes for anaerobic phenylalanine degradation. J Bacteriol 199:e00383-e417. https://doi.org/10.1128/JB.00383-17
Schühle K, Nies J, Heider J (2016) An indoleacetate-CoA ligase and a phenylsuccinyl-CoA transferase involved in anaerobic metabolism of auxin. Environ Microbiol 18:3120–3132. https://doi.org/10.1111/1462-2920.13347
Shortall K, Djeghader A, Magner E, Soulimane T (2021) Insights into aldehyde dehydrogenase enzymes: a structural perspective. Front Mol Biosci 8:659550
Sirover MA (1999) New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta - Protein Struct Mol Enzymol 1432:159–184
Smith LT, Kaplan NO (1980) Purification, properties, and kinetic mechanism of coenzyme A-linked aldehyde dehydrogenase from Clostridium kluyveri. Arch Biochem Biophys 203:663–675. https://doi.org/10.1016/0003-9861(80)90224-6
Teufel R, Gantert C, Voss M, Eisenreich W, Haehnel W, Fuchs G (2011) Studies on the mechanism of ring hydrolysis in phenylacetate degradation: a metabolic branching point. J Biol Chem 286:11021–11034. https://doi.org/10.1074/jbc.M110.196667
Winiarska A, Hege D, Gemmecker Y, Kryściak-Czerwenka J, Seubert A, Heider J, Szaleniec M (2022) Tungsten Enzyme using hydrogen as an electron donor to reduce carboxylic acids and NAD+. ACS Catal 12:8707–8717. https://doi.org/10.1021/acscatal.2c02147
Wöhlbrand L, Kallerhoff B, Lange D, Hufnagef P, Thiermann J, Reinhardt R, Rabus R (2007) Functional proteomic view of metabolic regulation in “Aromatoleumaromaticum” strain EbN1. Proteomics 7:2222–2239. https://doi.org/10.1002/pmic.200600987
Acknowledgements
Manuel Schreiner is acknowledged for his contributions in the initial phase of this study.
Funding
This work was supported in part by the Deutsche Forschungsgemeinschaft (DFG grant He2190/15–1).
Author information
Authors and Affiliations
Contributions
Conceptualisation, J. H.; methodology D. H., Y. G., I. S., P. O., and G. S.; software, D. H., Y. G., and J. H.; validation and formal analysis, Y. G. and J. H.; investigation, D. H., Y. G., I. S. P. O., and J. H.; resources, J. H.; data curation, D. H. and J. H.; writing–original draft preparation, J. H.; writing–review and editing, J. H., D. H., and Y. G.; visualization, J. H. and D. H.; supervision, G. S. and J. H.; project administration and funding acquisition, J. H. All authors have agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hege, D., Gemmecker, Y., Schall, I. et al. Single amino acid exchanges affect the substrate preference of an acetaldehyde dehydrogenase. Appl Microbiol Biotechnol 109, 103 (2025). https://doi.org/10.1007/s00253-025-13468-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-025-13468-4