Abstract
Factors associated with outcomes of chimeric antigen receptor (CAR)-T cell therapy in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) have not been fully elucidated. We explored the impact of the prelymphodepletion (pre-LD) lymphocyte to monocyte ratio (LMR) and its ratio to lactate dehydrogenase (LDH) (LMR/LDH) on the efficacy and prognosis of 60 patients with R/R DLBCL undergoing CAR-T cell therapy. The optimal cutoff values for pre-LD LMR and LMR/LDH were 3.583 and 0.0103, respectively. The overall response rate (ORR)s were higher in patients with high pre-LD LMR or LMR/LDH than those with low pre-LD LMR or LMR/LDH (ORR, 100% vs. 65.79%, P = 0.006 and 96.15% vs. 38.24%, P < 0.0001, respectively). Pre-LD LMR/LDH was an independent factor associated with ORR (P = 0.010, odds ratio = 18.757; 95% confidence interval [CI] 2.046–171.975) by multivariate logistic regression analysis. Patients with high pre-LD LMR/LDH had significantly longer progression-free survival (PFS) (median PFS, 29.73 vs. 2.47 months, P < 0.0001) and overall survival (OS) (median OS, not reached vs. 7.4 months, P = 0.0002) than those with low pre-LD LMR/LDH. Multivariate Cox regression analysis showed that pre-LD LMR/LDH and ORR were independent factors affecting PFS (P = 0.030, hazard ratio [HR] = 2.561; 95% CI 1.093–5.999 and P = 0.024, HR = 2.202; 95% CI 1.22–4.369, respectively); pre-LD LMR/LDH was an independent factor affecting OS (P = 0.029, HR = 3.331; 95% CI 1.131–9.807). In conclusion, the pre-LD LMR/LDH was an independent factor associated with ORR and an independent prognostic factor in patients with R/R DLBCL undergoing CAR-T cell therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Chimeric antigen receptor (CAR) -T cell therapy has achieved significant efficacy for patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). CD19-targeted CAR-T cells have achieved a complete response (CR) rate of 40–60% and an overall response rate (ORR) of approximately 80% [1,2,3]. Dual targeting of CD19 and CD20 or CD22 CAR-T cells have also shown promising results in R/R DLBCL patients [4,5,6,7]. However, 20–30% of patients with R/R DLBCL have no response after CAR-T cell therapy, and more than one-third of patients eventually relapse. Exploring indicators to effectively predict the efficacy and outcome of CAR-T cell therapy and intervening in advance could help to improve prognosis.
It has been reported that the expansion and persistence or the immunophenotype of CAR-T cells were associated with the outcome of B-cell lymphoma patients undergoing CAR-T cell therapy [8]. Accumulated studied have confirmed the prognostic value of tumor burden at baseline or early response for CAR-T cell therapy in large B-cell lymphoma [9,10,11]. Vercellino et al. reported that extranodal involvement sites were risk factors for early progression in R/R DLBCL patients after CAR-T cell therapy [12]. However, effective predictors are still less, and some were debatable.
The tumor immune microenvironment has been proved to play a significant role in promoting the growth and survival of lymphoma cells in recent years [13, 14]. Lymphocyte/monocyte ratio (LMR), which may present host immune status, is an effective predictive indicator for prognosis in patients with DLBCL, classical Hodgkin's lymphoma and follicular lymphoma [15,16,17]. LMR to lactate dehydrogenase (LDH) ratio (LMR/LDH), which are related to host immune status and tumor burden, have been shown to be prognostic markers for various types of cancer, including DLBCL [18]. It is unclear whether prelymphodepletion (pre-LD) LMR and LMR/LDH levels impact the outcome of patients with R/R DLBCL that receiving CAR-T cell therapy. This study is aimed to explore the prognostic value of pre-LD LMR and LMR/LDH in patients with R/R DLBCL undergoing CAR-T cell therapy.
Study design and patients
We conducted a retrospective study of consecutive patients with R/R DLBCL who underwent CAR-T cell therapy at the Department of Hematology, the Affiliated Hospital of Xuzhou Medical University between January, 2017, and November, 2020 through participation in prospective clinical trials (Clinical Trial Registry: NCT02782351, NCT03207178, NCT02903810 and NCT02794961). Patients with primary central nervous system lymphoma was excluded. The data cutoff for the analysis was November 1, 2023. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. Informed consent were obtained from all patients.
Patients underwent leukapheresis to collect peripheral blood mononuclear cells (PBMCs) for the preparation of CAR-T cells about 14 to 21 days before CAR-T cell therapy. Patients received lymphodepletion chemotherapy consisting of fludarabine (30 mg/m2 on days -5 to -3) and cyclophosphamide (750 mg/m2 on day -5) before CAR-T cell infusion. On day 0, patients received infusions of CD19 CAR-T cells alone, or in combined with anti-CD22 or anti-CD20 CAR-T cells, or CD20 CAR-T cells alone at a median dose of 2 + 10E6 cells/kg (ranging from 0.8 to 6 + 10E6 cells/kg).
Clinical data involved patients' age, gender, Eastern Cooperative Oncology Group (ECOG) performance status score, Ann Arbor stage, B symptoms, International Prognostic Index (IPI) score, previous therapies, central nervous system invasion, bone marrow invasion, bulky disease, complete blood count and LDH before lymphodepletion preconditioning. Calculate the LMR as the ratio of absolute lymphocyte count (ALC) to absolute monocyte count (AMC), and LMR/LDH as the ratio of LMR to LDH.
Evaluation criteria for response and toxicity
Efficacy were evaluated according to 2014 Lugano efficacy evaluation criteria [19]. Responses were assessed as CR, partial response (PR), stable disease (SD), and disease progression (PD). The ORR includes the rates of CR and PR. Cytokine release syndrome (CRS) were graded according to the Lee criteria [20]. Progression-free survival (PFS) was defined as the time from the date of CAR T-cell infusion to disease progression or death due to any cause, or the end of follow-up. Overall survival (OS) was defined as the time from the date of CAR T-cell infusion to the death or the end of follow-up.
Statistical analysis
Statistical analysis was conducted using SPSS 26.0 software. The receiver operating characteristic (ROC) curve was used to determine the optimal cutoff values of LMR and LMR/LDH to predict a response, and patients were divided into high and low groups according to these cutoff values. The variance inflation factor (VIF) was introduced to assess the multi-collinearity among variables in the multivariate model, and the VIF values of these variables were less than 5. Continuous variables were analyzed using t-tests or Wilcoxon rank-sum tests, and the comparison of rates between groups was conducted using chi-square tests or Fisher's exact method. Logistic regression analysis was used to analyze factors affecting ORR. The inverse Kaplan–Meier method was used to calculate the follow-up time. Kaplan–Meier method and log-rank test to assess differences in PFS and OS between groups. Cox proportional hazards model was used for univariate and multivariate analysis to assess the prognostic factors. The variables with significant difference in univariate analysis were included in the multivariate analysis. P value less than 0.05 was considered statistically significant.
Results
Patients’ clinical characteristics
A total of 60 patients with R/R DLBCL were enrolled, including 6 with transformed DLBCL. The baseline characteristics are listed in Table 1. The median age was 47 years (range 21 to 70). Fifty-four patients (90%) were in Ann Arbor stage III-IV status, and 32 patients (53.33%) had an IPI score of 3–5. Central nervous system involvement was observed in 6 patients (10%) and bone marrow involvement in 15 patients (25%). Nineteen patients (31.67%) had bulky disease, which was defined as a mass of at least 10 cm in largest diameter. Thirty-nine patients (65%) had received at least three lines of prior therapy before CAR-T cell therapy. Nineteen patients (31.67%) received CD19 CAR-T cell infusion, 8 (13.33%) received CD20 CAR-T cell infusion, 16 (26.67%) received CD19 and CD20 CAR-T cell infusion, and 17 (28.33%) received CD19 and CD22 CAR-T cell infusion.
Factors associated with pre-LD LMR and LMR/LDH
The cutoff value of pre-LD LMR and LMR/LDH was 3.583 (area under the curve [AUC], 0.709; 95% confidence interval [CI] 0.579 to 0.840; P = 0.007) and 0.0103 (AUC, 0.760; 95% CI 0.635 to 0.884; P = 0.001) with the optimum specificity and sensitivity, respectively. According to the optimal cut-off values, 47 patients were included in the low pre-LD LMR group (≤ 3.583), and 13 patients were included in the high pre-LD LMR group (> 3.583). Thirty-four patients had a low pre-LD LMR/LDH (≤ 0.0103), and 26 patients had a high pre-LD LMR/LDH (> 0.0103).
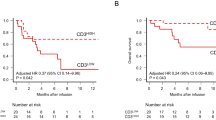
There were no significant differences in ECOG score, Ann Arbor stage, IPI score, B symptoms, central nervous system involvement, bone marrow invasion, bulky disease, etc. between patients with low pre-LD LMR and those with high pre-LD LMR. The ORR was higher in patients with high pre-LD LMR than in patients with low pre-LD LMR (100% [13/13] vs. 53.19% [25/47]; P = 0.006). There were more males in patients with low pre-LD LMR/LDH, compared with patients with high pre-LD LMR/LDH (82.35% [28/34] vs. 50% [13/26], P = 0.008). The other clinical characteristics were comparable between patients with low pre-LD LMR/LDH and those with high pre-LD LMR/LDH. Patients with high pre-LD LMR/LDH achieved higher ORR than patients with low pre-LD LMR/LDH (96.15% [25/26] vs. 38.24% [13/34]; P < 0.0001; Table 1).
Impact of pre-LD LMR and LMR/LDH on response
On univariate analysis, the ORR was lower in patients with IPI score 3–5 than patients with IPI score 0–2 (50.00% [16/32] vs. 78.57% [22/28], P = 0.022), higher in patients with high pre-LD LMR and LMR/LDH than in patients with low pre-LD LMR and LMR/LDH (P = 0.006 and P < 0.0001, respectively). There were no significant differences in gender, age, Ann Arbor Stage, ECOG score, central nervous system invasion, bone marrow invasion, bulky disease, time since diagnosis to CAR-T cell infusion, previous therapy lines, and CAR-T cell targets between patients who responded and those did not respond. Meanwhile, the severity of cytokine release syndrome (CRS) was not associated with ORR. Multivariate analysis results showed that pre-LD LMR/LDH was an independent factor associated with ORR (P = 0.010, odds ratio [OR] = 18.757; 95% confidence interval [CI] 2.046–171.975; Table 2).
Survival analysis based on pre-LD LMR and LMR/LDH
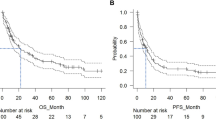
Among all 60 patients, the median follow-up was 39.1 months. The median PFS was 4.93 months, and median OS was 25.87 months. The patients with high pre-LD LMR seemed to have a longer PFS and OS than those with low pre-LD LMR; however, there were no significant differences (Fig. 1A, B). Compared with patients with low pre-LD LMR/LDH, patients with high pre-LD LMR/LDH had longer PFS (median PFS: 29.73 months vs. 2.47 months, P < 0.0001) and OS (median OS: not reached vs. 7.4 months, P = 0.0002; Fig. 1 C, D).
Progression-free survival (PFS) and overall survival (OS) based on pre-LD LMR and LMR/LDH. Panel A shows Kaplan–Meier curves of PFS and Panel B shows Kaplan–Meier curves of OS, according to LMR. Panel C shows Kaplan–Meier curves of PFS and Panel D shows Kaplan–Meier curves of OS, according to LMR. Tick marks indicate the time of data censoring at the last follow-up
The impact of pre-LD LMR/LDH on long-term outcomes
Univariate analysis showed that the time since diagnosis to CAR-T cell therapy (P = 0.025), pre-LD LDH (P = 0.0003), pre-LD LMR/LDH (P < 0.0001), and ORR (P < 0.0001) were associated with PFS after CAR-T cell therapy. However, gender, age, Ann Arbor stage, ECOG score, IPI score, central nervous system invasion, bone marrow invasion, bulky disease, previous lines of treatment, and pre-LD LMR level were not associated with PFS. There were no significant differences in PFS and OS among patients receiving different CAR-T cell products by log-rank test. Multivariate analysis results showed that pre-LD LMR/LDH and ORR were independent factors affecting PFS (P = 0.030, hazard ratio [HR] = 2.561; 95% CI 1.093–5.999 and P = 0.024, HR 2.202; 95% CI 1.22–4.369, respectively; Table 3).
Pre-LD LDH in peripheral blood (P = 0.011), pre-LD LMR/LDH (P = 0.001), and ORR (P = 0.008) were associated with OS after CAR-T cell therapy by univariate analysis. And pre-LD LMR/LDH was an independent prognostic factor for OS by multivariate analysis (P = 0.029, HR = 3.331; 95% CI 1.131–9.807; Table 3).
Discussion
Predictive biomarkers are currently lacking for CAR-T cell therapy in R/R DLBCL. The original IPI score and its modified versions, which are based primarily on clinical features and tumor burden, routinely used as a prognostic and predictive tool for patients with DLBCL. The molecular subtype, eg. germinal center B-cell and non-germinal center B-cell subtype of DLBCL are proved to be associated with prognosis [21]. The predict role of above indicators was explored in patients with R/R DLBCL undergoing CAR-T cell therapy, but the conclusion was debatable [12, 22, 23], which supports the needed of effective and convenient prognostic factors. In our study, high pre-LD LMR/LDH was associated with longer survival, providing evidence that the pre-LD LMR/LDH is a prognostic marker for patients with R/R DLBCL undergoing CAR-T cell therapy.
Recent evidence shows that the tumor immune microenvironment plays a critical role in CAR-T cell resistance and relapse. The host immunity and circulating immune cell function participate in regulating tumor cell progression and associate with survival. Lymphocytes are involved in the immune surveillance against tumors and are characterized with functional roles in relation to the tumor microenvironment and host immune status in lymphoma patients [24,25,26]. Changes in lymphocyte counts before and after lymphodepletion are closely related to the efficacy of CAR-T cell therapy in patients with R/R large B-cell lymphoma [27], and patients with low pre-LD absolute lymphocyte count (ALC) have poor outcome [28]. Monocytes serve as a principal source of microenvironment infiltrating cells, thus contributing to cancer immune evasion by differentiating into immune regulatory cells [29,30,31]. Pretreatment circulating monocyte count associated with poor prognosis in tumor patients [32, 33]. The LMR, which reflects the degree of systemic inflammation, also reflects the host immune status and the degree of tumor progression [34], as well as the balance between immune-immune escape and anti-tumor immunity. LMR has recently been reported to correlate with survival and a low LMR is therefore associated with a poorer prognosis in various types of malignancies, including DLBCL, classical Hodgkin's lymphoma and follicular lymphoma [15,16,17, 35]. A low LMR is an independent poor prognostic factor for DLBCL patients treated with chemotherapy [26, 35]. In this study, pre-LD LMR count was associated with response rate by univariate analysis rather nor multivariate analysis. Furthermore, we did not find the prognostic value of LMR in survival in patients with R/R DLBCL treated with CAR-T cell therapy, which was suggested that the balance between the host immune system and TEM was not the independent factors affecting response and survival after CAR-T cell therapy. The reason may be that the synergistic effect of LMR pre-LD and some other factors, especially tumor burden, may play important role in the prognosis value.
Mounting evidence suggests that tumor burden assessed by tumor imaging not only represents a prognostic biomarker at baseline, but also a mean to dynamically assess disease response in the context of CD19 CAR-T cell therapy [36]. As a markers of tumor burden, LDH is one of the risk factors in the IPI score and has been proven to be associated with poor prognosis in DLBCL. It is reported that LDH is an independent factor for early relapse after CAR-T cell therapy [12], and is associated with PFS [37]. In our study, univariate analysis results showed that pre-LD LDH was associated with PFS and OS in patients after CAR-T cell therapy. However, LDH did not serve as a prognostic indicator by multivariate analysis. Nevertheless, our results, together with the previous studies, underscore the importance of tumor burden pre-LD in the efficacy of CAR-T cell therapy. Combining LMR with LDH together, which reflects the host immunity and tumor burden, respectively, may help to accurately assess the anti-tumor immunity.
Studies showed that LMR/LDH predicts survival in patients with DLBCL [38]. Whether there is a prognostic value of LMR/LDH for CAR-T cell therapy in patients with DLBCL is not reported yet. Here we present the preliminary results, focusing on the potential prognostic value of pre-LD LMR/LDH in CAR-T cell therapy. We found that low pre-LD LMR/LDH was an independent factor affecting the response rates of CAR-T cell therapy in 60 patients with R/R DLBCL, which may be due to the reason that patients with low LMR/LDH had weak anti-tumor immune response and high tumor burden. IPI score was associated with the response rate of CAR-T cell therapy without independent prognosis value, while Ann Arbor stage and bulky disease, did not affect the efficacy of CAR-T cell therapy as reported previously [39]. Pre-LD LMR/LDH is an independent prognostic factor affecting PFS and OS by both univariate and multivariate analysis in this study, emphasizing the importance role of host immune response to tumors on clinical outcomes and providing evidence for the potential value of pre-LD LMR/LDH as an immune-related prognostic marker for R/R DLBCL undergoing CAR-T cell therapy. In our study, IPI score was also not an independent predictor of PFS and OS. This is consistent with other studies that showed that IPI was not sufficient to distinguish DLBCL with different prognosis in the era of rituximab [40, 41]. In addition, the ORR is also an independent prognostic factor affecting patient PFS and OS, which could be attributed to the fact that responders exhibit better long-term outcomes to CAR-T cell therapy. Moreover, patients with clinical response had high pre-LD LMR/LDH, which reflect host anti-lymphoma immunity.
Our study has several limitations that should be considered. First, this is a retrospective study with a limited sample size from a single center, therefore the results may not be generalizable. Second, we did not analyze the impact of molecular heterogeneity on prognosis, including molecular, genetic, and microenvironmental factors, which are associated with clinical outcomes in DLBCL. Future larger sample size prospective studies are needed to validate the predictive role of pre-LD LMR/LDH in patients with R/R DLBCL undergoing CAR-T cell therapy.
In conclusion, pre-LD LMR/LDH was an independent factor associated with the response rate, and an independent factor affecting PFS and OS in patients with R/R DLBCL undergoing CAR-T cell therapy. Our study showed the promising prognostic value of pre-LD LMR/LDH on CAR-T cell therapy in patients with R/R DLBCL in clinical practice.
Data availability
No datasets were generated or analysed during the current study.
References
Abramson JS, Palomba ML, Gordon LI et al (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396(10254):839–852. https://doi.org/10.1016/S0140-6736(20)31366-0
Schuster SJ, Bishop MR, Tam CS et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56. https://doi.org/10.1056/NEJMoa1804980
Locke FL, Ghobadi A, Jacobson CA et al (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20(1):31–42. https://doi.org/10.1016/S1470-2045(18)30864-7
Zhou H, Luo Y, Zhu S et al (2018) The efficacy and safety of anti-CD19/CD20 chimeric antigen receptor-T cells immunotherapy in relapsed or refractory B-cell malignancies: a meta-analysis. BMC Cancer 18:929. https://doi.org/10.1186/s12885-018-4817-4
Tong C, Zhang Y, Liu Y et al (2020) Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood J Am Soc Hematol 136(14):1632–1644. https://doi.org/10.1182/blood.2020005278
Roddie C, Lekakis LJ, Marzolini MAV et al (2023) Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma. Blood J Am Soc Hematol 141(20):2470–2482. https://doi.org/10.1182/blood.2022018598
Spiegel JY, Patel S, Muffly L et al (2021) CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med 27(8):1419–1431. https://doi.org/10.1038/s41591-021-01436-0
Cappell KM, Sherry RM, Yang JC et al (2020) Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol 38(32):3805–3815. https://doi.org/10.1200/JCO.20.01467
Georgi TW, Kurch L, Franke GN et al (2023) Prognostic value of baseline and early response FDG-PET/CT in patients with refractory and relapsed aggressive B-cell lymphoma undergoing CAR-T cell therapy. J Cancer Res Clin 149(9):6131–6138. https://doi.org/10.1007/s00432-023-04587-4
Winkelmann M, Blumenberg V, Rejeski K et al (2023) Prognostic value of the International Metabolic Prognostic Index for lymphoma patients receiving chimeric antigen receptor T-cell therapy. Eur J Nucl Med Mol I 50(5):1406–1413. https://doi.org/10.1007/s00259-022-06075-2
Leithner D, Flynn JR, Devlin SM et al (2024) Conventional and novel [18F] FDG PET/CT features as predictors of CAR-T cell therapy outcome in large B-cell lymphoma. J Hematol Oncol 17(1):21. https://doi.org/10.1186/s13045-024-01540-x
Vercellino L, Di Blasi R, Kanoun S et al (2020) Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 4(22):5607–5615. https://doi.org/10.1182/bloodadvances.2020003001
Tamma R, Ingravallo G, Gaudio F et al (2020) STAT3, tumor microenvironment, and microvessel density in diffuse large B cell lymphomas. Leukemia Lymphoma 61(3):567–574. https://doi.org/10.1080/10428194.2019.1678154
Scott DW, Gascoyne RD (2014) The tumour microenvironment in B cell lymphomas. Nat Rev Cancer 14(8):517–534. https://doi.org/10.1038/nrc3774
Wang J, Gao K, Lei W et al (2016) Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget 8(3):5414. https://doi.org/10.18632/oncotarget.14289
Hasenclever D, Diehl V, Armitage JO et al (1998) A prognostic score for advanced Hodgkin’s disease. New Engl J Med 339(21):1506–1514. https://doi.org/10.1056/NEJM199811193392104
Marcheselli L, Bari A, Anastasia A et al (2015) Prognostic roles of absolute monocyte and absolute lymphocyte counts in patients with advanced-stage follicular lymphoma in the rituximab era: an analysis from the FOLL 05 trial of the Fondazione Italiana Linfomi. Br J Haematol 169(4):544–551. https://doi.org/10.1111/bjh.13332
Wu XB, Hou SL, Liu H (2021) Systemic immune inflammation index, ratio of lymphocytes to monocytes, lactate dehydrogenase and prognosis of diffuse large B-cell lymphoma patients. World J Clin Cases 9(32):9825–9834. https://doi.org/10.12998/wjcc.v9.i32.9825
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3067. https://doi.org/10.1200/JCO.2013.54.8800
Lee DW, Gardner R, Porter DL et al (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood J Am Soc Hematol 124(2):188–195. https://doi.org/10.1182/blood-2014-05-552729
Chapuy B, Stewart C, Dunford AJ et al (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24(5):679–690. https://doi.org/10.1038/s41591-018-0016-8
Garcia-Recio M, Wudhikarn K, Pennisi M et al (2021) The International Prognostic Index is associated with outcomes in diffuse large B cell lymphoma after chimeric antigen receptor T cell therapy. Transpl Cell Ther 27(3):233–240. https://doi.org/10.1016/j.jtct.2020.10.022
Jacobson CA, Hunter BD, Redd R et al (2020) Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol 38(27):3095–3106. https://doi.org/10.1200/JCO.19.02103
Porrata LF, Ristow K, Habermann TM et al (2009) Absolute lymphocyte count at the time of first relapse predicts survival in patients with diffuse large B-cell lymphoma. Am J Hematol 84(2):93–97. https://doi.org/10.1002/ajh.21337
Lin Y, Gustafson MP, Bulur PA et al (2011) Immunosuppressive CD14+ HLA-DRlow/− monocytes in B-cell non-Hodgkin lymphoma. Blood J Am Soc Hematol 117(3):872–881. https://doi.org/10.1182/blood-2010-05-283820
Porrata LF, Ristow K, Habermann TM et al (2012) Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leukemia Lymphoma 53(11):2159–2165. https://doi.org/10.3109/10428194.2012.690605
Strati P, Jallouk AP, Sun R et al (2022) Impact of conditioning chemotherapy on lymphocyte kinetics and outcomes in LBCL patients treated with CAR T-cell therapy. Leukemia 36(11):2669–2677. https://doi.org/10.1038/s41375-022-01704-z
Lu Y, Zhu H, Liu Y et al (2023) Prognostic value of prelymphodepletion absolute lymphocyte counts in relapsed/refractory diffuse large B-cell lymphoma patients treated with chimeric antigen receptor T cells. Front Immunol 14:1155216. https://doi.org/10.3389/fimmu.2023.1155216
Mantovani A, Ponzetta A, Inforzato A et al (2019) Innate immunity, inflammation and tumour progression: double-edged swords. J Intern Med 285(5):524–532. https://doi.org/10.1111/joim.12886
Mantovani A, Marchesi F, Malesci A et al (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61. https://doi.org/10.1016/j.immuni.2014.06.010
Wu Q, Hu T, Zheng E et al (2017) Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine 96(22):e7051. https://doi.org/10.1097/MD.0000000000007051
Ren L, Xu J, Li J et al (2023) A prognostic model incorporating inflammatory cells and cytokines for newly diagnosed multiple myeloma patients. Clin Exp Med 23(6):2583–2591. https://doi.org/10.1007/s10238-023-00992-8
Diakos CI, Charles KA, McMillan DC et al (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):e493–e503. https://doi.org/10.1016/S1470-2045(14)70263-3
Li YL, Pan YY, Jiao Y et al (2014) Peripheral blood lymphocyte/monocyte ratio predicts outcome for patients with diffuse large B cell lymphoma after standard first-line regimens. Ann Hematol 93:617–626. https://doi.org/10.1007/s00277-013-1916-9
Winkelmann M, Bücklein VL, Blumenberg V et al (2022) Lymphoma tumor burden before chimeric antigen receptor T-Cell treatment: RECIL vs. Lugano vs. metabolic tumor assessment. Front Oncol 12:974029. https://doi.org/10.3389/fonc.2022.974029
Hubbeling H, Leithner D, Silverman EA et al (2024) Metabolic tumor volume response after bridging therapy determines chimeric antigen receptor T-cell outcomes in large B-cell lymphoma. Clin Cancer Res 30(22):5083–5093. https://doi.org/10.1158/1078-0432.CCR-24-0830
Ji H, Niu X, Yin L et al (2018) Ratio of immune response to tumor burden predicts survival via regulating functions of lymphocytes and monocytes in diffuse large B-cell lymphoma. Cell Physiol Biochem 45(3):951–961. https://doi.org/10.1159/000487288
Neelapu SS, Locke FL, Bartlett NL et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med 377(26):2531–2544. https://doi.org/10.1056/NEJMoa1707447
Ngo L, Hee SW, Lim LC et al (2008) Prognostic factors in patients with diffuse large B cell lymphoma: before and after the introduction of rituximab. Leukemia Lymphoma 49(3):462–469. https://doi.org/10.1080/10428190701809156
Romero-Garcia S, Moreno-Altamirano MMB, Prado-Garcia H et al (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52. https://doi.org/10.3389/fimmu.2016.00052
Acknowledgements
The authors thank the patients and families who participated in the trials.
Funding
This work was financially supported by the Program of National Natural Science Foundation of China (81930005), and Advanced Program of The Affiliated Hospital of Xuzhou Medical University (PYJH2024202).
Author information
Authors and Affiliations
Contributions
NL and NA were involved in conceptualization, investigation, methodology, formal analysis, supervision, writing-original, writing-revision and edits; SM and FZ contributed to formal analysis, investigation; JC and QK was involved in investigation, formal analysis, conceptualization, methodology; HC, ZY, WS, WC, TQ, DL and ZL contributed to resources, investigation, validation; YW, and KX were involved in conceptualization, funding acquisition, methodology, supervision, writing-review and edits. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., An, N., Ma, S. et al. Lymphocyte/monocyte to lactate dehydrogenase ratio prior to lymphodepletion impact the outcomes of patients with diffused large B cell lymphoma undergoing CAR-T cell therapy. Cancer Immunol Immunother 74, 148 (2025). https://doi.org/10.1007/s00262-025-03987-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-025-03987-4