Abstract
Neutrophils are integral to the frontline defense against pathogenic bacterial and fungal invasions. Beyond their traditional roles, these cells are increasingly recognized for their dualistic contributions to the pathology of autoimmune and inflammatory diseases, as well as their complex involvement in cancer progression. Neutrophils interact with different disease states, highlighting their potential as therapeutic targets. Within tumor microenvironment (TME), tumor-associated neutrophils (TANs) exhibit a functional dichotomy, capable of either fostering or impeding tumor growth and metastasis. This binary functional potential of TANs, under certain conditions, suggests a reversible state that could transition from tumor-promoting to tumor-eradicating phenotypes. Despite the critical implications of such functional plasticity, systematic studies of TAN behavioral shifts in the context of cancer immunotherapy remain scarce. Herein, we review recent advancements in the understanding of TANs within the TME, highlighting their binary regulatory effects on solid tumors. Leveraging the latest insights from experimental and clinical research, this review elucidates the complex roles of TANs in tumor development and explores their molecular interactions as potential therapeutic targets. The elucidation of these mechanisms holds promise for novel cancer treatment strategies, aiming to improve patient outcomes by manipulating the tumor-promoting or -suppressing functions of TANs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Background
Neutrophils, which arise from hematopoietic stem cells in the bone marrow, have been traditionally acknowledged in immunology for their phagocytic activity and bactericidal capabilities, as well as their role in orchestrating inflammatory responses [1]. Recent research reveals a more complex role for these cells within the tumor milieu. Neutrophil infiltrates in tumors, known as TANs, have emerged as pivotal contributors to cancer progression. TANs facilitate processes such as angiogenesis, enhance the migration of neoplastic cells, and modulate the immune landscape, effectively acting as an immunosuppressive switch that orchestrates the behavior of various immune cell populations [2].
Cancer development results from a complex interplay of genetic predispositions and environmental factors, with certain malignancies linked to harmful lifestyle factors [3]. Within the TME, cancer cells expertly manipulate TANs via a milieu of secreted cytokines, chemokines, and other effector molecules [4]. This interaction propels TANs toward a dichotomous polarization, adopting either an anti-tumoral (N1) or a pro-tumoral (N2) phenotype. N1 TANs are characterized by an upsurge in pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α) and C–C motif chemokine ligand 3 (CCL3), along with enhanced expression of intercellular adhesion molecule-1 (ICAM-1) and reduced arginase activity. CCL3 promotes inflammation and immune responses within the TME [5]. In contrast, N2 TANs exhibit overexpression of chemokines such as CCL2, CCL4, CCL8, CCL12, and CCL17 [6]. Importantly, while CCL3 is involved in pro-inflammatory responses in the N1 phenotype, its role can vary in different contexts, such as contributing to fibrosis in the liver, highlighting the importance of considering specific tumor types and microenvironments.
The roles of neutrophils are shaped by their surrounding environment, influencing their functions in cancer biology. Recent studies have identified mechanisms that affect TAN polarization. For example, in non-small cell lung carcinoma, Smad3 has been recognized as crucial for TAN polarization, especially via TGF-β/Smad3 axis. In experimental models, TANs transition from a predominantly N2 state in wild-type mice to an N1 state in Smad3-knockout (KO) mice, correlating with enhanced neutrophil infiltration and tumor regression. Single-cell analysis reveals that a subset of TANs in the Smad3-KO TME exhibits a mature N1 phenotype, while wild-type TANs remain in an immature N2 state due to Smad3 presence [7]. Another study found that acrolein produced by glioma cells under hypoxia inhibits neutrophil activation and induces an anti-inflammatory phenotype by interacting with Cys310 of protein kinase B (AKT), thereby inhibiting AKT activity [8]. Additionally, research on a dendritic cell-based hepatocellular carcinoma neoantigen nano-vaccine showed that acidic-triggered captopril release can repolarize pro-tumoral N2 neutrophils to an anti-tumoral N1 phenotype [9]. Moreover, melanoma-derived extracellular vesicles (EVs) may activate neutrophils, promoting their migration toward the TME and supporting N2 polarization, thus contributing to tumor progression [10].
The interaction between neutrophils and tumor cells is imperative in regulating tumor growth and progression within the TME. TANs secrete a variety of cytokines, including transforming growth factor β2 (TGF-β2), IL-1β, TNF-α, IL-12, granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), CC family chemokines, and CXC family chemokines, etc. [11]. The release of these cytokines promotes angiogenesis, suppresses anti-tumor immune responses, and creates a supportive environment for tumor cells, facilitating growth and metastasis. During tumor development, each tumor cell undergoes metabolic reprogramming to cope with challenges like hypoxia and nutrient deficiency. In this context, tumor cells can induce an oxidative phenotype in neutrophils through the stem cell factor (SCF) signaling pathway, causing DNA damage in neighboring cells, promoting inflammation, and enhancing tumor cell survival and proliferation [12].
Despite increasing interest in targeting neutrophils for cancer therapy, the molecular details of neutrophil-tumor interactions remain unclear. Understanding these pathways is essential for advancing precision oncology and developing targeted therapies. This review summarizes current insights into the molecular mechanisms by which TANs influence liver, breast, gastric, and colorectal cancers, and highlights the potential of TANs as a focal point for innovative cancer treatment strategies.
Biological characterization and functionality of neutrophils
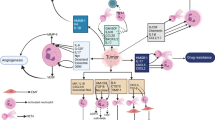
Neutrophils, the predominant class of myeloid lineage cells in human circulation, originate and mature within the bone marrow from hematopoietic progenitors. Their differentiation involves a series of stages where common myeloid progenitors branch into multipotent granulocyte-monocyte progenitors (GMPs). These GMPs then give rise to precursors of neutrophils and monocytes (Fig. 1). A key stage in neutrophil maturation is the expression of CD66b on promyelocytes, followed by increased CD11b and CD16 expression, leading to the formation of band and segmented neutrophils [13]. Within the TME, these cells exhibit remarkable heterogeneity and plasticity. Studies indicate that TGF-β steers TANs toward the N2 phenotype, while its inhibition promotes N1 TANs [14].
The differentiation trajectory of neutrophils and their polarization within the tumor microenvironment. This trajectory encompasses various stages of development. In the tumor microenvironment, TGF-β promotes the polarization of tumor-associated neutrophils (TANs) into a pro-tumoral N2 phenotype. In contrast, the release of TNF-α facilitates the polarization of TANs toward an anti-tumoral N1 phenotype
In solid tumors, neutrophils express specific markers. Immunohistochemical analysis revealed that CCL2+ and CCL17+ cells, which also express the neutrophil marker CD66b, are found throughout hepatocellular carcinoma stroma [15]. CD66b+ TANs and CD8+ tumor-infiltrating lymphocytes (TILs) have prognostic significance in the invasive margins of colorectal cancer (CRC). Additionally, the infiltration of CD163+, CD68+, and CD66b+ cells in gastric cancer tissue was significantly increased and independently associated with gastric cancer prognosis [16].
Neutrophils are classically known for their roles in executing bacterial phagocytosis and mediating inflammatory processes. Their strategies against pathogens include phagocytosis, reactive oxygen species (ROS) generation, protease secretion, and forming neutrophil extracellular traps (NETs). NETs, which consist of DNA, histones, and enzymes, are used by activated neutrophils to capture and eliminate pathogens [17]. Furthermore, neutrophils aid adaptive immunity by presenting antigens through major histocompatibility complex (MHC) class I, facilitating the maturation of naïve CD8+ T cells into cytolytic T cells [18].
The inherent diversity and plasticity of neutrophils underpin the dual potential exhibited by TANs within the TME [13]. N1 TANs can kill cancer cells by releasing ROS and reactive nitrogen species (RNS) and can activate T cells and recruit pro-inflammatory (M1) macrophages. Conversely, N2 TANs promote oncogenesis by releasing matrix metalloproteinase 9 (MMP9), which aids angiogenesis and tumor cell spread, while inhibiting natural killer (NK) cell function. They can also recruit anti-inflammatory (M2) macrophages and regulatory T cells [19].
Neutrophils exhibit a dual role in tumor development. N1 TANs directly kill tumor cells by releasing cellular hydrolases and producing ROS and myeloperoxidase (MPO) through degranulation. They can produce immune factors like TNF-α, activating immune cells such as natural killer cells (NKTs), dendritic cells (DCs), and macrophages [20]. A study discovered that N1 TANs can activate peripheral blood CD4+ T cells by binding their Fc receptors to IgG Fc receptor proteins on tumor cells, leading to tumor cell destruction and reduced tumor growth [21]. On the other hand, N2 TANs foster cancer progression via releasing ROS, cytokines, neutrophil elastase (NE), and NETs, among other factors [22] (Fig. 2).
Molecular mechanisms of TANs in solid tumor development and progression
TANs stimulate angiogenesis, generate NETs, and modulate the immune system to support the growth and dissemination of malignancies. Recent studies highlight the tumor-promoting effects of TANs in high-incidence solid tumors, including liver cancer, breast cancer, CRC, and gastric cancer. This section summarizes the molecular mechanisms by which TANs promote cancer progression in solid tumors (Table 1).
The multifaceted role of neutrophils in hepatocellular carcinoma
Neutrophil proliferation in the TME accelerates tumor angiogenesis, epithelial-mesenchymal transition (EMT), and growth by producing MMP9, NETs, and hepatocyte growth factor, worsening hepatocellular carcinoma and its metastasis [23]. Here, we summarize the critical molecular mechanisms involved in the development of hepatocellular carcinoma.
Immunomodulatory actions of neutrophils via the PD-1/PD-L1 pathway
Programmed death ligand 1 (PD-L1) is a glycoprotein that binds to programmed death receptor 1 (PD-1) [24]. A recent study showed that cancer-associated fibroblasts (CAFs) induce the differentiation of TANs into PD-L1+ neutrophils through the IL-6/STAT3/PD-L1 pathway. Co-culturing purified peripheral CD3+ T cells with HCC-CAF-primed neutrophils significantly reduced T-cell IFNγ production and proliferation (by 40%). Adding PD-L1 neutralizing antibodies to these cultures attenuated T-cell suppression [25]. Notably, neutrophils primarily infiltrate peritumoral tissues in hepatocellular carcinoma patients, where the neutrophil-to-lymphocyte ratio (NLR) is elevated compared to intratumoral tissues, and high levels of PD-L1 expression are observed. Researchers further confirmed a greater prevalence of PD-L1+ neutrophils and PD-1+ T cells in hepatoma-bearing mice, indicating that neutrophils mediate tumor immune escape by upregulating PD-L1 expression [26]. Some studies suggest that tumor cells may reprogram CD10+ALPL+ neutrophils, leading to the “irreversible” exhaustion of T cells [27].
Clinically, safe and effective agents that inhibit STAT3 activation may offer avenues for hepatocellular carcinoma prevention and treatment. For instance, α-mangostin (α-MGT) was found to inhibit STAT3 activation and exert anti-hepatocellular carcinoma effects, significantly suppressing cell proliferation and inducing apoptosis in various cancer cell lines, including HepG2 and SK-Hep-1. Moreover, α-MGT inhibited the expression of STAT3-regulated genes [28], suggesting its potential as a therapeutic agent. Other compounds, like emodin and evodiamine, have also shown promise in inhibiting STAT3 signaling.
The chemokine axis and neutrophil mobilization: exploring the CXCL paradigm in hepatocellular carcinoma
C-X-C motif chemokine ligands (CXCLs) are small cytokines that recruit neutrophils through CXCL5, CXCL2, and CXCL1, modulating T-cell responses. Neutrophil recruitment is influenced by monocytes, macrophages, or epithelial cells [28]. CXCL2 and IL-13 play crucial roles in promoting neutrophil recruitment and upregulating arginase-1 (Arg1), which suppresses T-cell activation and aids tumor progression [29]. CXCL5 binds primarily to the chemokine receptor CXCR2. Although the precise activation mechanisms via CXCR2 are not fully understood, CXCL5 is regulated by TGF-β and Axl signaling, facilitating neutrophil infiltration in HCC patients. Additionally, CXCL5 acts as an effector of TANs, promoting macrophage and regulatory T-cell infiltration through other chemokines like CCL2 and CCL17 [30]. Studies have shown that active serine proteases can downregulate CXCL2 through targeted cleavage, reducing neutrophil recruitment and suppressing hepatocellular carcinoma progression [31]. Moreover, brahma-related gene 1 (Brg1) expression levels demonstrate a positive correlation with CXCL14 and neutrophil infiltration [7]. Recent research indicates that inhibiting CXCR2 can reprogram the TME, transitioning TANs from an N2 to an N1 phenotype [32].
The role of NETs in tumor formation in hepatocellular carcinoma
NETs are extensive, web-like structures released extracellularly and composed of cytosolic, granular, and nuclear proteins. These structures play a significant role in promoting cancer metastasis and immune evasion. In a recent study, researchers demonstrated that neutrophils promote hepatocellular carcinoma metastasis through the enhanced formation of NETs [33], which trigger a tumorous inflammatory response via the activation of the Toll-like receptors 4/9-cyclooxygenase 2 (TLR4/9-COX-2) axis, thereby fueling metastasis [34]. Moreover, in investigations related to non-alcoholic steatohepatitis (NASH)—a condition that can promote liver cancer—researchers found that the inhibition of NET formation, either through treatment with deoxyribonuclease (DNase) or using peptidyl arginine deaminase type IV knockout mice (PAD4–/–), did not inhibit the development of fatty liver but altered the pattern of liver inflammation, ultimately resulting in reduced tumor growth [35]. In a controlled study involving hepatitis B virus (HBV)—infected individuals, researchers observed elevated levels of NETs in serum and tissue specimens from hepatocellular carcinoma patients infected with HBV and concluded that the HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitates the growth and metastasis of hepatocellular carcinoma [36]. Furthermore, recent clinical studies have indicated that detecting NET formation in tumors can effectively predict prognosis and response to immunotherapy in hepatocellular carcinoma patients.
Delving into the P53 tumor suppression mechanism in hepatocellular carcinoma
P53 serves as a critical tumor suppressor, functioning as a transcription factor that regulates DNA repair and induces cell cycle arrest in response to oncogenic stress. Mouse model studies show that significant decreases or chronic hyper-activation of p53 can lead to cancer initiation [37]. p53 inhibits tumor growth by regulating CDC20, essential for cell cycle progression from G2 to M phase. It negatively regulates CDC20 expression by binding its promoter region, preventing premature cell cycle progression. In contrast, mutant p53 fails to regulate CDC20, leading to increased CDC20 levels and uncontrolled cell division. Flow cytometry analyses indicate that CDC20 suppression results in significant G2/M phase cell cycle arrest in HCC cells. High CDC20 expression allows cells with damaged DNA to escape mitosis and evade apoptosis, facilitating hepatocellular carcinoma development [38]. Conversely, the absence of p53 can increase cholesterol esterification through the p53-USP19-SOAT1 signaling axis, thereby facilitating hepatocarcinogenesis [39]. Reticulons (RTNs) facilitate p53 Ser392 phosphorylation via Chk2, inhibiting hepatocellular carcinoma growth and inducing apoptosis [40]. Another study found that ZNF498, a p53 Ser46 phosphorylation suppressor, is highly expressed in hepatocellular carcinoma and correlates positively with advanced disease and poor survival [41]. Moreover, FAT10 has been suggested to promote hepatocellular carcinoma development by mediating p53 degradation [42]. Collectively, p53 is involved in multiple liver cancer development stages, and targeting p53 may provide a novel therapeutic approach.
Navigating the PI3K/Akt/mTOR signaling pathway in hepatocellular carcinoma
The PI3K/Akt/mTOR pathway is crucial for regulating the cell cycle, proliferation, apoptosis, and metabolism, and is activated in many cancers due to dysregulated receptor tyrosine kinases (RTK) [43]. The fatty acid receptor CD36 is implicated in both lipid and glucose metabolism in the liver. The overexpression of CD36 leads to increased glycolytic flux and lactic acid production via activation of the Src/PI3K/AKT signaling axis, which promotes hepatocellular carcinoma growth and metastasis [44]. Additionally, SOS Ras/Rac Guanine Nucleotide Exchange Factor 1 (SOS1) may induce EMT through the PI3K/Akt/mTOR pathway, thereby enhancing the invasion, migration, and metastasis of hepatocellular carcinoma cells [45]. In past studies, researchers found that elevated phosphoinositide-dependent protein kinase 1 (PDK1) expression, valosin-containing protein (VCP) interaction with high mobility group box-1 (HMGB1), and suppressor of cytokine signaling 5 (SOCS5) inhibition can promote hepatocellular carcinoma progression through the PI3K/AKT/mTOR pathway [46]. Long non-coding RNA (lncRNA) has been a hot topic recently. Some studies indicated that 67 dysregulated lncRNAs associated with the PI3K/AKT/mTOR pathway show oncogenic or anti-oncogenic effects in hepatocellular carcinoma by regulating epigenetic, transcriptional, and post-transcriptional levels [47]. Circular RNAs (circ RNAs) are also crucial ncRNAs that are sensitive to the tumor immune response. hsa_circ_0001727 (circZKSCAN1) has been reported to be a tumor-associated circRNA by sponging microRNAs. CircZKSCAN1 encodes circZKSaa, which sensitizes hepatocellular carcinoma cells to sorafenib via ubiquitination of mTOR. Overexpression of circZKSaa inhibits the development of hepatocellular carcinoma through the PI3K/Akt/mTOR pathway [48].
The PI3K/Akt/mTOR pathway is a promising target for anti-cancer compounds. Various dietary compounds, especially flavonoids, have been shown to inhibit this pathway in different cancers [49]. For instance, LY3023414 is a PI3K-AKT-mTOR inhibitor that suppresses human skin squamous cell carcinoma growth both in vitro and in vivo. Moreover, some researchers found that deoxyshikonin, isolated from Arnebia euchroma, inhibited CRC through the PI3K/Akt/mTOR pathway [50]. PI3K serves as a pivotal initiator, contributing to various malignant biological processes, including proliferation, apoptosis, and angiogenesis. Many PI3K inhibitors (PI3Ki) have been validated or advanced into clinical trials as antineoplastic agents. One study revealed that DZW-310 (a novel PI3Ki) significantly diminished hepatocellular carcinoma cell growth by promoting intrinsic apoptosis and G0/G1 phase cell arrest. Furthermore, DZW-310 suppressed angiogenesis by regulating the hypoxia-inducible factor (HIF)-1α/VEGFA axis. DZW-310 shows promise as a therapeutic agent for hepatocellular carcinoma and may expand the clinical applications of PI3K inhibitors in treatment [24].
The role of neutrophils in the metastasis and advancement of breast cancer
Breast cancer is among the three most common cancers worldwide and is a leading cause of death in post-menopausal women, accounting for 23% of all cancer-related fatalities. While only 5–10% of breast cancer cases are linked to genetic factors, 90–95% are influenced by environmental and lifestyle factors, such as ionizing radiation, hormonal therapy, reproductive behaviors (like later childbirth), alcohol consumption, dietary habits, obesity, and physical inactivity [51]. Additionally, the neutrophil-to-lymphocyte ratio (NLR) has been identified as a prognostic marker for poor outcomes in breast cancer. Ordered multinomial logistic regression analysis demonstrated a positive correlation between NLR, carcinoembryonic antigen (CEA), and CA15-3 with tumor-node-metastasis (TNM) staging, based on data from 653 breast cancer patients and 100 patients with breast fibroadenoma [52]. Neutrophils impact breast cancer progression through several complex mechanisms, including the formation of NETs [53].
Mechanistically, NETs enhance the interaction between nuclear factor kappa B (NF-κB) essential modifier (NEMO) and IκB kinase (IKK)α/β, promoting NF-κB activation. One study suggests that NF-κB associates with NETs to establish a positive feedback loop that facilitates breast tumor progression and metastasis [54]. Selective inhibition of NF-κB and PAD4-dependent NETs could serve as an effective therapeutic strategy for breast cancer. Recent research has identified PAD4 as a promising target for tumor therapy, with some studies indicating that highly tumor-targeted PAD4 inhibitors, modified with phenylbutyric acid (PBA), can inhibit tumors in vivo by specifically disrupting the PAD4-H3cit-NETs pathway in neutrophils [55].
γδ T Cells and IL-17A: a dual influence on neutrophil behavior in breast cancer
Γδ T cells, a subtype of innate-like T lymphocytes, play a dual role in the development of breast cancer within the TME. They can secrete interferon (IFN)-γ to directly kill tumor cells, but they also produce interleukin-17A (IL-17A), which promotes tumor growth [56]. Angiogenesis, a critical physiological process and hallmark of cancer, is commonly targeted by antiangiogenic therapies, primarily those directed against the VEGF/VEGFR2 signaling axis. However, such treatments are limited in their efficacy for breast cancer patients, as high-dose anti-VEGFR2 therapy can lead to drug resistance. Research has shown that γδ T cells and neutrophils are actively involved in resistance to high-dose anti-VEGFR2 therapy in mouse models of breast cancer. Notably, high-dose VEGFR2-tyrosine kinase inhibitors have been found to induce γδ T cells to produce IL-17A via activation of the VEGFR1-PI3K-AKT pathway, which subsequently promotes N2-like neutrophil polarization. N2 neutrophils accelerate CD8+ T-cell exhaustion and foster an immunosuppressive microenvironment, thereby facilitating tumor progression [57].
The role of m6A demethylation in breast cancer neutrophils
Senescent neutrophils secrete exosomal piR-17560 in a STAT3-dependent manner, which enhances the expression of fat mass and obesity-associated protein (FTO) in breast cancer. The upregulation of FTO stabilizes and increases the expression of ZEB1, a key transcription factor that initiates EMT in cancer cells, by reducing N6-methyladenosine (m6A) RNA methylation. This alteration contributes to chemoresistance and EMT in tumor cells [58]. Additionally, a novel subset of C5aR1-positive neutrophils has been identified to secrete IL-1β and TNFα, which cooperatively activate extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathways. This activation leads to the phosphorylation of Wilms' tumor 1-associating protein (WTAP) at serine 341, thereby stabilizing the WTAP protein. The stabilization of WTAP further promotes the m6A methylation of enolase 1 (ENO1), influencing the glycolytic activity of breast cancer cells and thereby promoting tumor growth in vivo; this effect is abolished following WTAP silencing [59].
Neutrophils as drivers of gastric cancer progression
Gastric cancer is a leading cause of cancer-related deaths, particularly in low- and middle-income countries. Despite advancements in research, the pathogenesis of gastric cancer remains poorly understood. The presence of neutrophils in tumors has been recognized as an independent and adverse prognostic factor for gastric cancer patients, with studies indicating that an elevated NLR is associated with poor survival outcomes [60]. This section summarizes several pathways through which neutrophils contribute to the progression of gastric cancer.
The GM-CSF-PD-L1 axis in gastric cancer neutrophil regulation
In human gastric cancer tissue samples, it has been observed that gastric cancer cells extend the lifespan of neutrophils, with tumor-derived granulocyte–macrophage colony-stimulating factor (GM-CSF) effectively activating neutrophils and inducing PD-L1 expression via the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signaling pathway. These PD-L1-expressing neutrophils subsequently suppress T-cell function, contributing to the evasion of anti-tumor immunity and promoting gastric cancer progression. Additionally, gastric cancer cell-derived extracellular vesicles (GC-EVs) have been shown to transport high-mobility group box-1 (HMGB1), which activates STAT3 and upregulates PD-L1 expression in neutrophils. Blocking the STAT3 pathway and silencing HMGB1 reverse this GC-EV-induced PD-L1 expression in neutrophils [61]. The prognostic value of the gastric immune prognostic index (GIPI) in patients treated with PD-1/PD-L1 inhibitors is also a growing area of interest.
Neutrophils on EMT in gastric cancer
EMT is a well-characterized process that plays a crucial role in cancer metastasis. In gastric cancer tissues, neutrophils produce IL-17A, and TANs-derived IL-17A promotes the migration, invasiveness, and EMT of gastric cancer cells by activating the JAK2/STAT3 pathway. Inhibition of this pathway, using IL-17A neutralizing antibodies or the JAK2-specific inhibitor AG490, reverses these TAN-induced phenotypes in gastric cancer cells [62]. Furthermore, research has shown that tumor cells activate neutrophils via TGF-β1, leading to the production of the metabolism-regulating signaling molecule FAM3C through Smad2/3 signaling. FAM3C, in turn, promotes EMT in tumor cells via the JNK-ZEB1/Snail signaling pathway [63]. Additionally, the deregulated expression of chemokines, such as CXCL5, in the TME contributes to tumor metastasis by inducing EMT and mediating the pro-tumor activation of neutrophils [64].
The IL-6/STAT3 axis in neutrophil-mediated gastric cancer progression
Cancer-associated fibroblasts (CAFs) are key players in cancer progression, largely through the secretion of growth factors, inflammatory extracellular matrix proteins, and proteases. Mesenchymal stem cells (MSCs), which possess self-renewal and pluripotent differentiation abilities, interact intensively with tumor-infiltrating neutrophils. Studies have shown that gastric cancer-derived MSCs activate neutrophils via the IL-6/STAT3 axis, promoting the migration of gastric cancer cells and the formation of endothelial cell tubes in vitro. The activated neutrophils, in turn, induce the differentiation of normal MSCs into CAFs [25].
The role of NETs and COX-2 in gastric cancer metastasis
NETs are critical in the progression of various cancers. Cyclooxygenase-2 (COX-2) has emerged as a potential target for gastric cancer immunotherapy. In vitro experiments have demonstrated that NETs can activate COX-2 through toll-like receptor 2 (TLR2), thereby enhancing the metastatic potential of gastric cancer cells. Additionally, a liver metastasis model in nude mice has highlighted the pivotal role of NETs and COX-2 in the distant metastasis of gastric cancer [65].
Neutrophil dynamics in CRC
CRC is among the most common causes of cancer-related deaths worldwide, with an increasing number of cases reported each year [66]. The majority of CRC-related deaths are due to distant metastases, particularly liver metastases. Similar to other cancers, a high neutrophil-to-lymphocyte ratio (NLR) has been associated with poor clinical outcomes in CRC patients [67]. TANs exhibit plasticity between an anti-tumoral N1 phenotype and a pro-tumoral N2 phenotype, depending on signals from the surrounding tissue, such as TGF-β. Growing evidence suggests that neutrophils play a dual role in CRC development. Patients with CRC can exhibit elevated levels of NETs both in vivo and in vitro [68], with these NETs primarily localized within the primary tumor sites and at the tumor margins. High IL-8 expression in the CRC microenvironment activates neutrophils and induces NET formation. IL-8, through its receptor CXCR2, stimulates neutrophils to release NETs via Src, ERK, and p38 signaling, with these NETs subsequently upregulating TLR9 pathways to promote cancer progression [69]. A recent mouse study demonstrated that surgical trauma enhances colon cancer cell adhesion and growth on the peritoneal wall via CXCR2 signaling [70]. Moreover, NET-associated carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) has been shown to facilitate the relocation of CRC cells to the liver, both in vitro and in vivo [71]. In a mouse model of liver metastasis and surgical stress, inhibition of NET formation with DNase I reduced postoperative metastasis development. This accumulating evidence positions NETs as a potential therapeutic target for CRC.
Recent studies have also identified that KIAA1199 activates the TGF-β signaling pathway by interacting with TGFBR1/2, stimulating the production of CXCL1 and CXCL3, which facilitate the infiltration of immunosuppressive neutrophils into the liver [72]. Additionally, anterior gradient 2 (AGR2), a protein widely expressed in tumors, including precancerous lesions, primary tumors, and metastatic sites, is increasingly recognized for its role in promoting CRC metastasis [73]. Research has identified TANs as the predominant source of AGR2 in the TME, and TANs have been shown to drive CRC metastasis via the AGR2-CD98hc-xCT axis. Furthermore, in mouse models, the long non-coding RNA MIR4435-2HG (LINC00978) has been found to exert anti-tumor effects by reprogramming neutrophils [74].
The role of neutrophils in different cancer types
Neutrophils exhibit context-dependent roles across various cancer types, demonstrating both pro-tumorigenic and anti-tumorigenic functions shaped by the TME. In HCC, neutrophils contribute to tumor progression through IL-6 and TNF-α-mediated inflammation, promoting angiogenesis and immune suppression via mechanisms involving arginase-1 and ROS [78]. In contrast, breast cancer neutrophils are known to facilitate metastasis primarily through the formation of NETs, which remodel the extracellular matrix (ECM) and can also display direct tumoricidal activity, demonstrating a more aggressive role [79]. Meanwhile, in gastric cancer, neutrophils are associated with poor prognosis, largely fostering immune evasion through matrix metalloproteinase-driven ECM degradation and the secretion of immunosuppressive cytokines [80]. In CRC, neutrophils exhibit a dual role: in early-stage tumors or under specific therapies, they may trigger cytotoxic effects against tumor cells, while in advanced stages, they are more likely to employ ROS and arginase-1 to suppress T-cell activity and promote a pro-metastatic niche. Despite these differences, certain mechanisms are conserved across cancer types [81]. For example, NETs and PD-L1-mediated immune checkpoint inhibition are common pathways through which neutrophils promote metastasis and immune evasion. Additionally, cancer type-specific metabolic adaptations, such as Acod1 expression in breast cancer versus ARG1 in CRC, illustrate how neutrophils can vary in their functional roles based on the tumor context. Microenvironmental drivers also play a significant role in shaping neutrophil functions; for instance, IL-6 is prominent in HCC, while CXCR2 signaling is vital in glioma. These factors underscore the functional plasticity of neutrophils in distinct tumor settings [82]. Ultimately, while neutrophils share mechanisms like ECM remodeling and immune modulation across cancers, their functional plasticity—heavily influenced by TME heterogeneity, disease stage, and therapeutic interventions—highlights their potential as dynamic therapeutic targets or prognostic biomarkers in cancer.
The anti-tumor function of neutrophils
As previously discussed, TGF-β can induce neutrophil differentiation into a pro-tumoral phenotype (N2). By inhibiting TGF-β signaling, TANs can differentiate into an anti-tumor phenotype, characterized by elevated levels of pro-inflammatory cytokines and enhanced cytotoxicity, which induces tumor cell apoptosis and delays tumor growth in vivo [6]. TGF-β is a critical regulator of TANs plasticity, driving their differentiation into a pro-tumoral N2 phenotype characterized by immunosuppressive functions and tissue-remodeling properties. Mechanistically, TGF-β signaling via Smad2/3 phosphorylation suppresses pro-inflammatory pathways while upregulating arginase-1 and MMPs, facilitating tumor angiogenesis and extracellular matrix degradation, thereby promoting metastasis [83]. Inhibition of TGF-β signaling—through genetic knockout of TGF-β receptors or pharmacological agents like galunisertib—shifts TANs toward an anti-tumor N1 phenotype. This transition is marked by increased expression of pro-inflammatory cytokines (e.g., TNF-α, IL-12) and enhanced ROS-mediated cytotoxicity, leading to tumor cell apoptosis and delayed tumor progression in murine models [84].
Low-dose type I interferon (IFN-α) therapy has emerged as a potent inducer of the N1 phenotype. In HCC models, IFN-α stimulates neutrophil STAT1/IRF signaling, upregulating surface markers like ICAM-1 and FAS (factor-related apoptosis), which enhance neutrophil-tumor cell adhesion and direct cytotoxicity [85]. IFN-α-primed N1 neutrophils exhibit heightened production of myeloperoxidase and hydrogen peroxide (H2O2), which induce DNA damage in tumor cells via oxidative stress pathways [86]. Additionally, IFN-α enhances NETs formation, which can directly entrap circulating tumor cells and limit metastatic dissemination in breast cancer models [87].
Anti-tumor neutrophils exert direct cytotoxic effects on tumor cells while also activating adaptive immune responses indirectly. N1 neutrophils have demonstrated anti-tumor efficacy across various tumor models, including hepatocellular carcinoma and breast cancer. For instance, N1 neutrophils can directly kill tumor cells, limit their metastasis, and engage innate immune functions through the secretion of myeloperoxidase (MPO), H2O2, and ROS [88]. Furthermore, N1 neutrophils recruit dendritic cells (DCs), CD8+ T cells, CD4+ T cells, and other immune cells, promoting their proliferation and activation while regulating adaptive immunity through the release of cytokines and chemokines such as IL-8, growth-related oncogene (GRO)-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, IL-12, tumor necrosis factor-alpha (TNF-α), CXCL10, and granulocyte–macrophage colony-stimulating factor (GM-CSF). One of the primary mechanisms by which N1 TANs exert their anti-tumoral effects is by enhancing the activity of cytotoxic T lymphocytes (CTLs). N1 TANs secrete chemokines and cytokines that recruit and activate CTLs, increasing their infiltration into the tumor site and enhancing their ability to target and kill tumor cells. This interaction is crucial, as CTLs are key effector cells in anti-tumor immunity, capable of inducing apoptosis in cancer cells through the release of perforin and granzymes [89]. Additionally, N1 TANs can modulate the activity of other innate immune cells, such as macrophages. By influencing the polarization of macrophages toward the M1 phenotype, which is known for its pro-inflammatory and anti-tumorigenic properties, N1 TANs contribute to the overall anti-tumoral milieu within the TME. This polarization is characterized by the production of pro-inflammatory cytokines and enhanced phagocytic activity, collectively working to suppress tumor growth and metastasis [90]. Furthermore, the interaction between N1 TANs and regulatory T cells (Tregs) is noteworthy. N1 TANs can inhibit the suppressive functions of Tregs, thereby alleviating the immunosuppressive pressure within the TME. This inhibition allows for a more effective immune response by preventing Tregs from dampening the activity of effector T cells and other immune components critical for tumor suppression [91].
In summary, N1 TANs play a multifaceted role in tumor suppression by interacting with and modulating the activity of various immune cells within the TME. These interactions enhance the anti-tumoral immune response, making N1 TANs a critical component of the anti-cancer immune system. A deeper understanding of these interactions could lead to the development of novel therapeutic strategies that harness the potential of N1 TANs in cancer immunotherapy.
The dual roles of NETs across cancer types
NETs play significant roles in cancer biology, exhibiting dual roles that can either promote or inhibit cancer progression. These web-like structures, composed of DNA, histones, and various proteins, are released by neutrophils in response to various stimuli, including cancer cells. Their dual roles in cancer are multifaceted and context-dependent, influencing tumor progression, metastasis, and the TME.
On one hand, NETs can aid cancer progression by promoting tumor cell proliferation, invasion, and metastasis. They facilitate extracellular matrix remodeling, enhance angiogenesis, and create a pro-inflammatory environment that supports tumor growth. NETs trap circulating tumor cells, assisting in their dissemination and establishment at distant sites. Additionally, they can induce EMT in cancer cells, a key process in metastasis, by releasing factors that activate signaling pathways involved in EMT [92].
Conversely, NETs also contribute to anti-tumor immunity by trapping and killing cancer cells through the release of cytotoxic proteins and enzymes. They can interact with other immune cells, such as dendritic cells and macrophages, potentially enhancing the anti-tumor immune response. However, the balance between their pro-tumor and anti-tumor effects is delicate and influenced by various factors, including the type of cancer, the stage of disease, and the specific components of the TME [93].
The dual roles of NETs highlight their potential as therapeutic targets. Modulating NET formation or function could provide novel cancer therapies. For instance, inhibiting NET formation might reduce metastasis, while enhancing their anti-tumor effects could boost immune-mediated tumor clearance. Understanding how NETs influence cancer biology is crucial for developing targeted therapies that can exploit their dual roles to improve cancer treatment outcomes [94].
Targeting tumor-associated neutrophils for cancer therapy
One promising therapeutic strategy involves inhibiting the recruitment of TANs and their downstream signaling pathways using small molecule inhibitors or neutralizing antibodies to impede tumor progression (Table 2). Various monoclonal antibodies (mAbs), small molecule inhibitors, and RNA interference techniques have been employed to block the signaling pathways between TANs and tumors. For example, the accumulation of N2 TANs can be reduced through the use of IL-8 antagonists, significantly delaying tumor growth in murine models [95]. TANs secrete IL-17A via the JAK2/STAT3 signaling pathway, which promotes EMT in gastric cancer cells. Inhibition of IL-17A signaling using polyclonal neutralizing antibodies or targeting the JAK2/STAT3 pathway with the inhibitor AG490 has been shown to reduce TAN-mediated migration and invasion [62]. Additionally, a study found that the tyrosine kinase inhibitor cabozantinib triggers a neutrophil-mediated anti-cancer innate immune response, facilitating tumor clearance [96]. Inhibition of CXCR1/2 can also block the recruitment of immunosuppressive neutrophils, thereby enhancing tumor response to PD-1 therapy [97]. Notably, the injection of β-dextran into normal mice has been found to reprogram TANs to the N1 phenotype, enabling them to suppress tumor growth independently of adaptation, with this tumor-suppressive capability maintained after transplantation into recipient mice [98]. Future research should focus on accurately targeting the clearance of N2 TANs while preserving N1 TANs.
In summary, the diversity of transcription factors and specific proteins contributes to the variable phenotypes of TANs. Among the most extensively studied populations are N1 and N2 neutrophils. IFN-α is a key factor in polarizing neutrophils to the N1 phenotype, enhancing their adhesion, transmigration, phagocytosis, oxidative bursts, deregulation, and NET formation. Conversely, TGF-β drives neutrophil differentiation toward the N2 phenotype, which plays a modulatory role in immune responses. This switch between N1 and N2 phenotypes suggests an antagonistic relationship between IFN-α and TGF-β signaling pathways [84]. Precisely targeting N2 TANs without disrupting the normal immune function of patients with tumors remains a critical issue.
To date, several preclinical and clinical studies have evaluated the efficacy and safety of neutrophil-targeted or -related cancer therapies. For instance, one innovative study explored the combination of peroxynitrite (ONOO–)-mediated radio-sensitization with the polarization of TANs to reverse the immunosuppressive TME. This approach significantly amplified the effectiveness of radiotherapy in orthotopic mouse models of CRC and melanoma [99]. In other research involving mouse models, RCT001, a specific CXCR2 inhibitor, was shown to enhance the efficacy of combined anti-CTLA4 and anti-PD-1 therapies by inhibiting tumor-associated M2 macrophages and TANs [100]. Additionally, Deltex E3 ubiquitin ligase 2 (DTX2) in HCC cells has been identified as a promoter of TAN infiltration and polarization toward a pro-tumor phenotype. In vivo studies using various tumor models demonstrated that treatment with a DTX2 inhibitor not only inhibited tumor growth but also sensitized HCC cells to the therapeutic effects of PD-1 antibodies [101].
Notably, immunotherapies targeting neutrophils can result in unavoidable side effects. Agents such as DS-8273a, capecitabine, and gemcitabine are associated with side effects, including non-selective neutrophil depletion [102]. While selective removal of neutrophils presents a potential therapeutic avenue, this approach may inadvertently eliminate other immune cells, such as monocytes and specific subsets of CD8+ T cells [103]. Specific therapies targeting TANs and circulating neutrophils represent a novel approach for cancer treatment. Nevertheless, further investigation into the precise roles, recruitment pathways, subpopulations, and mechanisms of action of TANs is essential for developing a more reliable and specific treatment model that maximizes therapeutic potential while minimizing adverse effects.
Conclusions and future research
This review sought to elucidate the intricate interactions between TANs and tumor cells, with a particular emphasis on the regulatory role of neutrophils in both promoting and combating cancer (Fig. 2 ). Neutrophils represent a highly heterogeneous population, exhibiting significant phenotypic and functional plasticity. In the TME, the majority of TANs contribute to tumor progression by promoting tumor cell proliferation, metastasis, angiogenesis, tissue remodeling, and immunosuppression. The infiltration of TANs is generally associated with poor prognosis across various malignancies. Numerous factors derived from the TME orchestrate the release, recruitment, and functional polarization of neutrophils. Conversely, neutrophil-secreted factors also play a crucial regulatory role in tumor growth. The effectiveness of tumor cell responses to immunotherapy is influenced not only by the mutation and reprogramming of their own genes but also by the complexity within the TME and the regulation of various cytokines and chemokines. A deeper understanding of the molecules through which TANs interact with the TME, along with the multiple signaling pathways involved, could lead to the reshaping of the TME into one that favors anti-tumor responses. This presents a promising new avenue for targeted tumor immunotherapy. As we move forward in understanding the complex roles of TANs in solid tumors, several key research directions should be prioritized. First, elucidating the molecular drivers behind N1/N2 polarization through advanced techniques such as single-cell multi-omics and spatial profiling will provide deeper insights into the regulatory mechanisms that guide neutrophil behavior within the TME. Additionally, defining the interactions between TANs and various immune and stromal cells is crucial for understanding their functional context. This includes resolving the dual roles of NETs in both metastasis promotion and suppression, as this knowledge may lead to innovative therapeutic strategies. Future research should also focus on metabolic reprogramming strategies for TANs, the development of engineered neutrophil carriers for targeted drug delivery, and the exploration of novel pathways, particularly the interplay between CXCR2 signaling and combinations of TGF-β and IFN-α. Addressing challenges such as clinical translation—including the identification of reliable biomarkers and understanding neutrophil lifespan in the tumor context—is essential for the successful implementation of neutrophil-targeted therapies. Moreover, investigating the influences of the microbiome on neutrophil function and its implications for cancer treatment will be a vital area of research. The integration of artificial intelligence (AI) in modeling TAN behavior could provide predictive insights and facilitate the design of more effective therapies. It is also important to consider the impacts of aging and comorbidities on neutrophil function and cancer progression, as these factors can significantly influence treatment outcomes. Optimizing selective NETosis inhibition could further enhance therapeutic efficacy while minimizing potential side effects. Finally, interdisciplinary approaches that combine immunology, oncology, and bioengineering, along with the development of advanced preclinical models, are critical for harnessing the potential of TANs in cancer immunotherapy. By pursuing these research directions, we can significantly advance our understanding of TANs and develop effective strategies to leverage their functions in treating solid tumors.
In conclusion, unraveling the regulatory pathways governing TAN-tumor interactions will be key to enhancing current immunotherapies by harnessing and manipulating these complex molecular mechanisms.
Data availability
No datasets were generated or analyzed during the current study.
References
Tsai CY, Hsieh SC, Liu CW et al (2021) Cross-talk among polymorphonuclear neutrophils, immune, and non-immune cells via released cytokines, granule proteins, microvesicles, and neutrophil extracellular trap formation: a novel concept of biology and pathobiology for neutrophils. Int J Mol Sci 18:22
Xiong S, Dong L, Cheng L (2021) Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol 14:173
Byrne S, Boyle T, Ahmed M, Lee SH, Benyamin B, Hyppönen E (2023) Lifestyle, genetic risk and incidence of cancer: a prospective cohort study of 13 cancer types. Int J Epidemiol 52:817–826
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Can Res 79:4557–4566
Cheng D, Lian W, Wang T et al (2024) The interplay of Cxcl10(+)/Mmp14(+) monocytes and Ccl3(+) neutrophils proactively mediates silica-induced pulmonary fibrosis. J Hazard Mater 467:133713
Masucci MT, Minopoli M, Carriero MV (2019) Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 9:1146
Chung JY, Tang PC, Chan MK et al (2023) Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat Commun 14:1794
Tsai HC, Tong ZJ, Hwang TL et al (2023) Acrolein produced by glioma cells under hypoxia inhibits neutrophil AKT activity and suppresses anti-tumoral activities. Free Radical Biol Med 207:17–28
Wang Y, Zhao Q, Zhao B et al (2022) Remodeling tumor-associated neutrophils to enhance dendritic cell-based HCC neoantigen nano-vaccine efficiency. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 9:2105631
Guimarães-Bastos D, Frony AC, Barja-Fidalgo C, Moraes JA (2022) Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J Leukoc Biol 111:585–596
Tian S, Chu Y, Hu J et al (2022) Tumour-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc-xCT. Gut 71:2489–2501
Lian X, Yang K, Li R et al (2022) Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer 21:27
Hedrick CC, Malanchi I (2022) Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol 22:173–187
Peng H, Shen J, Long X et al (2022) Local release of TGF-β Inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 9:e2105240
Yin Y, Feng W, Chen J et al (2024) Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside. Exp Hematol Oncol 13:72
Cong X, Zhang Y, Zhu Z et al (2020) CD66b(+) neutrophils and α-SMA(+) fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med 9:2761–2773
Castanheira FVS, Kubes P (2019) Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133:2178–2185
Quinn SM, Cunningham K, Raverdeau M et al (2019) Anti-inflammatory trained immunity mediated by helminth products attenuates the induction of T cell-mediated autoimmune disease. Front Immunol 10:1109
Gershkovitz M, Caspi Y, Fainsod-Levi T et al (2018) TRPM2 mediates neutrophil killing of disseminated tumor cells. Can Res 78:2680–2690
Muqaku B, Pils D, Mader JC et al (2020) Neutrophil extracellular trap formation correlates with favorable overall survival in high grade ovarian cancer. Cancers 12
Sun B, Qin W, Song M et al (2018) Neutrophil suppresses tumor cell proliferation via Fas/Fas ligand pathway mediated cell cycle arrested. Int J Biol Sci 14:2103–2113
Chan YT, Tan HY, Lu Y et al (2023) Pancreatic melatonin enhances anti-tumor immunity in pancreatic adenocarcinoma through regulating tumor-associated neutrophils infiltration and NETosis. Acta Pharmac Sin B 13:1554–1567
Tang J, Yan Z, Feng Q, Yu L, Wang H (2021) The roles of neutrophils in the pathogenesis of liver diseases. Front Immunol 12:625472
Wu Y, Xu X, Liu M et al (2022) DZW-310, a novel phosphoinositide 3-kinase inhibitor, attenuates the angiogenesis and growth of hepatocellular carcinoma cells via PI3K/AKT/mTOR axis. Biochem Pharmacol 201:115093
Cheng Y, Li H, Deng Y et al (2018) Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis 9:422
Chen J, Feng W, Sun M et al (2024) TGF-β1-induced SOX18 elevation promotes hepatocellular carcinoma progression and metastasis through transcriptionally upregulating PD-L1 and CXCL12. Gastroenterology 167:264–280
Meng Y, Ye F, Nie P et al (2023) Immunosuppressive CD10(+)ALPL(+) neutrophils promote resistance to anti-PD-1 therapy in HCC by mediating irreversible exhaustion of T cells. J Hepatol 79:1435–1449
Zhang H, Tan YP, Zhao L et al (2020) Anticancer activity of dietary xanthone α-mangostin against hepatocellular carcinoma by inhibition of STAT3 signaling via stabilization of SHP1. Cell Death Dis 11:63
Xu X, Ye L, Zhang Q et al (2021) Group-2 innate lymphoid cells promote HCC progression through CXCL2-neutrophil-induced immunosuppression. Hepatology (Baltimore, MD) 74:2526–2543
Haider C, Hnat J, Wagner R et al (2019) Transforming growth factor-β and Axl Induce CXCL5 and neutrophil recruitment in hepatocellular carcinoma. Hepatology (Baltimore, MD) 69:222–236
Wang T, Zhou Y, Zhou Z et al (2023) Secreted protease PRSS35 suppresses hepatocellular carcinoma by disabling CXCL2-mediated neutrophil extracellular traps. Nat Commun 14:1513
Leslie J, Mackey JBG, Jamieson T et al (2022) CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 71:2093–2106
Zenlander R, Havervall S, Magnusson M et al (2021) Neutrophil extracellular traps in patients with liver cirrhosis and hepatocellular carcinoma. Sci Rep 11:18025
Yang LY, Luo Q, Lu L et al (2020) Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol 13:3
van der Windt DJ, Sud V, Zhang H et al (2018) Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology (Baltimore, MD) 68:1347–1360
Zhan X, Wu R, Kong XH et al (2023) Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun (London, England) 43:225–245
Nassar-Reis JP, Umeta PF, Stefano JT et al (2023) P53 and VEGF are promising biomarkers for sorafenib efficacy in an experimental model of NASH-related HCC. J Mol Histol 54:473–488
Zhao S, Zhang Y, Lu X et al (2021) CDC20 regulates the cell proliferation and radiosensitivity of P53 mutant HCC cells through the Bcl-2/Bax pathway. Int J Biol Sci 17:3608–3621
Zhu Y, Gu L, Lin X et al (2023) P53 deficiency affects cholesterol esterification to exacerbate hepatocarcinogenesis. Hepatology (Baltimore, MD) 77:1499–1511
Song S, Shi Y, Wu W et al (2021) Reticulon 3-mediated Chk2/p53 activation suppresses hepatocellular carcinogenesis and is blocked by hepatitis B virus. Gut 70:2159–2171
Zhang X, Zheng Q, Yue X et al (2022) ZNF498 promotes hepatocellular carcinogenesis by suppressing p53-mediated apoptosis and ferroptosis via the attenuation of p53 Ser46 phosphorylation. J Exp Clin Cancer Res CR 41:79
Zhang Y, Zuo Z, Liu B et al (2021) FAT10 promotes hepatocellular carcinoma (HCC) carcinogenesis by mediating P53 degradation and acts as a prognostic indicator of HCC. J Gastroint Oncol 12:1823–1837
Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L (2021) Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines 9
Luo X, Zheng E, Wei L et al (2021) The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis 12:328
Li Y, Yin Y, He Y, He K, Li J (2022) SOS1 regulates HCC cell epithelial-mesenchymal transition via the PI3K/AKT/mTOR pathway. Biochem Biophys Res Commun 637:161–169
Pu Z, Duda DG, Zhu Y et al (2022) VCP interaction with HMGB1 promotes hepatocellular carcinoma progression by activating the PI3K/AKT/mTOR pathway. J Transl Med 20:212
Wu Y, Zhang Y, Qin X, Geng H, Zuo D, Zhao Q (2020) PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol Res 160:105195
Song R, Ma S, Xu J et al (2023) A novel polypeptide encoded by the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR. Mol Cancer 22:16
Zughaibi TA, Suhail M, Tarique M, Tabrez S (2021) Targeting PI3K/Akt/mTOR pathway by different flavonoids: a cancer chemopreventive approach. Int J Mol Sci 22
Zhu Y, Zhong Y, Long X et al (2019) Deoxyshikonin isolated from Arnebia euchroma inhibits colorectal cancer by down-regulating the PI3K/Akt/mTOR pathway. Pharm Biol 57:412–423
Xu J, Jiao X, Bayat R (2024) Outcomes of physical exercises on initiation, progression, and treatment of breast cancer. Cell Commun Signal 22:260
Zou H, Liu SH, Yang R, Wu XJ, Cao YP, Huang HF (2022) Combination of neutrophil-to-lymphocyte ratio and red cell distribution width with serum tumor markers for the differential diagnosis of breast cancer and its association with pathological features and molecular types. Clin Breast Cancer 22:e526–e535
Yang C, Wang Z, Li L et al (2021) Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer 9
Zhu B, Zhang X, Sun S, Fu Y, Xie L, Ai P (2021) NF-κB and neutrophil extracellular traps cooperate to promote breast cancer progression and metastasis. Exp Cell Res 405:112707
Zhu D, Lu Y, Hu B et al (2023) Highly-tumor-targeted PAD4 inhibitors with PBA modification inhibit tumors in vivo by specifically inhibiting the PAD4-H3cit-NETs pathway in neutrophils. Eur J Med Chem 258:115619
Silva-Santos B, Mensurado S, Coffelt SB (2019) γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer 19:392–404
Zhang Z, Yang C, Li L et al (2021) “γδT Cell-IL17A-neutrophil” axis drives immunosuppression and confers breast cancer resistance to high-dose anti-VEGFR2 therapy. Front Immunol 12:699478
Ou B, Liu Y, Gao Z et al (2022) Senescent neutrophils-derived exosomal piRNA-17560 promotes chemoresistance and EMT of breast cancer via FTO-mediated m6A demethylation. Cell Death Dis 13:905
Ou B, Liu Y, Yang X, Xu X, Yan Y, Zhang J (2021) C5aR1-positive neutrophils promote breast cancer glycolysis through WTAP-dependent m6A methylation of ENO1. Cell Death Dis 12:737
Onuma S, Hashimoto I, Suematsu H et al (2023) Clinical effects of the neutrophil-to-lymphocyte ratio/serum albumin ratio in patients with gastric cancer after gastrectomy. J Personal Med 13
Shi Y, Zhang J, Mao Z et al (2020) Extracellular vesicles from gastric cancer cells induce PD-L1 expression on neutrophils to suppress T-cell immunity. Front Oncol 10:629
Li S, Cong X, Gao H et al (2019) Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res CR 38:6
Wang Y, Li X, Zhang T et al (2023) Neutrophils promote tumor invasion via FAM3C-mediated epithelial-to-mesenchymal transition in gastric cancer. Int J Biol Sci 19:1352–1368
Mao Z, Zhang J, Shi Y et al (2020) CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis 9:63
Zhang A, Zou X, Yang S, Yang H, Ma Z, Li J (2023) Effect of NETs/COX-2 pathway on immune microenvironment and metastasis in gastric cancer. Front Immunol 14:1177604
Kasprzak A (2021) The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci 22
Xun F, Jiang W, Sha M et al (2024) Neutrophil-to-lymphocyte ratio in colorectal tissue affects prognosis in patients with colorectal cancer. Pathology 56:643–652
Onuma AE, Zhang H, Gil L, Huang H, Tsung A (2020) Surgical stress promotes tumor progression: a focus on the impact of the immune response. J Clin Med 9
Nie M, Yang L, Bi X et al (2019) Neutrophil extracellular traps induced by il8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res Off J Am Assoc Cancer Res 25:1867–1879
Lepsenyi M, Algethami N, Al-Haidari AA et al (2021) CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin Exp Metas 38:401–410
Rayes RF, Vourtzoumis P, Bou Rjeily M et al (2020) Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol (Baltimore, Md: 1950) 204:2285–2294
Wang H, Zhang B, Li R et al (2022) KIAA1199 drives immune suppression to promote colorectal cancer liver metastasis by modulating neutrophil infiltration. Hepatology (Baltimore, MD) 76:967–981
Zhang K, Li Y, Kong X et al (2023) AGR2: a secreted protein worthy of attention in diagnosis and treatment of breast cancer. Front Oncol 13:1195885
Yu H, Chen C, Han F et al (2022) Long noncoding RNA MIR4435-2HG suppresses colorectal cancer initiation and progression by reprogramming neutrophils. Cancer Immunol Res 10:1095–1110
Choudhary HB, Mandlik SK, Mandlik DS (2023) Role of p53 suppression in the pathogenesis of hepatocellular carcinoma. World J Gastrointest Pathophysiol 14:46–70
Liu K, Huang AL, Chen XQ, Wu SG (2024) Patterns of distant metastasis and survival outcomes in de novo metastatic breast cancer according to age groups. Front Endocrinol 15:1385756
Sánchez-Ramírez D, Mendoza-Rodríguez MG, Alemán OR et al (2024) Impact of STAT-signaling pathway on cancer-associated fibroblasts in colorectal cancer and its role in immunosuppression. World J Gastrointest Oncol 16:1705–1724
Arvanitakis K, Mitroulis I, Germanidis G (2021) Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers 13
Xu X, Wang X, Zheng Z et al (2024) Neutrophil extracellular traps in breast cancer: roles in metastasis and beyond. J Cancer 15:3272–3283
Xu X, Chen J, Li W et al (2023) Immunology and immunotherapy in gastric cancer. Clin Exp Med 23:3189–3204
Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y (2019) The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci 20
Zhao Y, Liu Z, Liu G et al (2023) Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1. Cell Metab 35:1688–703.e10
Bertrand-Chapel A, Caligaris C, Fenouil T et al (2022) SMAD2/3 mediate oncogenic effects of TGF-β in the absence of SMAD4. Commun Biol 5:1068
Yang S, Jia J, Wang F et al (2024) Targeting neutrophils: mechanism and advances in cancer therapy. Clin Transl Med 14:e1599
Sagiv JY, Michaeli J, Assi S et al (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10:562–573
Wang Y, Yu X, Song H et al (2018) The STAT-ROS cycle extends IFN-induced cancer cell apoptosis. Int J Oncol 52:305–313
Yang L, Liu Q, Zhang X et al (2020) DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583:133–138
Stakenborg M, Verstockt B, Meroni E et al (2020) Neutrophilic HGF-MET signalling exacerbates intestinal inflammation. J Crohns Colitis 14:1748–1758
Tumbath S, Jiang L, Li X et al (2024) β-Lapachone promotes the recruitment and polarization of tumor-associated neutrophils (TANs) toward an antitumor (N1) phenotype in NQO1-positive cancers. Oncoimmunology 13:2363000
Pernot S, Evrard S, Khatib AM (2022) The give-and-take interaction between the tumor microenvironment and immune cells regulating tumor progression and repression. Front Immunol 13:850856
Scott EN, Gocher AM, Workman CJ, Vignali DAA (2021) Regulatory T cells: barriers of immune infiltration into the tumor microenvironment. Front Immunol 12:702726
Guan X, Guan X, Zhao Z, Yan H (2024) NETs: important players in cancer progression and therapeutic resistance. Exp Cell Res 441:114191
Chan L, Wood GA, Wootton SK, Bridle BW, Karimi K (2023) Neutrophils in dendritic cell-based cancer vaccination: the potential roles of neutrophil extracellular trap formation. Int J Mol Sci 24
Efrimescu CI, Buggy PM, Buggy DJ (2021) Neutrophil extracellular trapping role in cancer, metastases, and cancer-related thrombosis: a narrative review of the current evidence base. Curr Oncol Rep 23:118
He J, Zhou M, Yin J et al (2021) METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Therapy J Am Soc Gene Therapy 29:1821–1837
Di Ceglie I, Carnevale S, Rigatelli A, Grieco G, Molisso P, Jaillon S (2024) Immune cell networking in solid tumors: focus on macrophages and neutrophils. Front Immunol 15:1341390
Kargl J, Zhu X, Zhang H et al (2019) Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight 4
Kalafati L, Kourtzelis I, Schulte-Schrepping J et al (2020) Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183:771–85.e12
Li H, Zeng J, You Q et al (2025) X-ray-activated nanoscintillators integrated with tumor-associated neutrophils polarization for improved radiotherapy in metastatic colorectal cancer. Biomaterials 316:123031
Montemagno C, Jacquel A, Pandiani C et al (2024) Unveiling CXCR2 as a promising therapeutic target in renal cell carcinoma: exploring the immunotherapeutic paradigm shift through its inhibition by RCT001. J Exp Clin Cancer Res CR 43:86
Wu X, Chen J, Chen Y et al (2025) Targeting Deltex E3 ubiquitin ligase 2 inhibits tumor-associated neutrophils and sensitizes hepatocellular carcinoma cells to immunotherapy. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 12:e2408233
Olaoba OT, Yang M, Adelusi TI et al (2024) Targeted therapy for highly desmoplastic and immunosuppressive tumor microenvironment of pancreatic ductal adenocarcinoma. Cancers 16
Chao T, Lu L, Zhang L et al (2021) An inducible model for specific neutrophil depletion by diphtheria toxin in mice. Sci China Life Sci 64:1227–1235
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82472858 and 82002491 to L.W.; 82403413 to Y.L.) and the Natural Science Foundation of Guangdong Province of China (2024A1515012947 and 2020A1515110049 to L.W.; 2025A1515012301 to Y.L.).
Author information
Authors and Affiliations
Contributions
YW collected relevant literature. LW, YL, and XC drafted manuscripts. YW prepared figures and tables. YW, JM, YL, and WC reviewed and made significant revisions to the manuscript. LW, YL, and XC guided the preparation of this manuscript. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Ma, J., Liu, Y. et al. Unraveling the complex role of tumor-associated neutrophils within solid tumors. Cancer Immunol Immunother 74, 210 (2025). https://doi.org/10.1007/s00262-025-04049-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-025-04049-5