Abstract
There is little evidence, particularly in Germany, on the epidemiology and the cytoreductive management of polycythemia vera (PV). We performed an observational study based on anonymized health claims data to provide estimates of the epidemiology of PV in Germany, to describe the use of cytoreductive drugs in patients with PV, and to assess the occurrence of thromboembolic events (TEs) in prevalent patients on continuous treatment with relevant cytoreductive drugs over time. For the year 2021, we estimated a PV prevalence of 28.6 per 100,000 and an incidence of 3.3 per 100,000 in the German adult population (≥ 18 years). We identified 83.2% of prevalent patients in 2021 as being at high risk for thromboembolic complications, based on age (≥ 60 years) and/or history of TEs. Contrary to treatment guidelines, 43.6% of these high-risk patients did not receive cytoreductive drug treatment in 2021. 63.5% of patients in 2021 who were treated with hydroxyurea (but not ruxolitinib) in that year, met our defined proxy criteria for intolerance/resistance to hydroxyurea. Over time, we observed a lower proportion of patients with TEs in patients continuously treated with ruxolitinib compared to patients treated with hydroxyurea who also met our defined proxy criteria for intolerance/resistance to hydroxyurea (35.8% vs. 56.3% after three years). Our findings suggest that currently available cytoreductive therapies are not being fully utilized according to treatment guidelines, which may lead to avoidable thromboembolic complications in this patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Background
Polycythemia vera (PV) is a Philadelphia chromosome-negative myeloproliferative neoplasm characterized by an overproduction of red blood cells, along with an elevated white blood cell and platelet production and an increased risk of thromboembolic events (TEs) [1]. Comorbid cardiovascular risk factors may further increase the risk of TEs and have been associated with worse survival in patients with PV [2]. PV primarily affects older individuals, with a median age at diagnosis of 65 years [3]. Over time, PV may progress to myelofibrosis and in rare instances to acute myeloid leukemia (AML) [4]. Incidence estimates of PV in the US and Europe range from 0.4 to 2.8 per 100,000 with a slightly higher incidence reported in men than in women [3, 5,6,7]. To our knowledge, epidemiologic data specifically for Germany are currently not available.
There is currently no cure for PV, thus treatment is aimed at reducing the risk of TEs, managing clinical symptoms, and preventing complications. Initial treatment typically involves phlebotomy therapy in combination with low-dose acetylsalicylic acid to lower the hematocrit - the volume of red blood cells in the blood. Subsequent therapy decisions are mainly influenced by the patient’s individual risk for thrombosis, with older age and previous thrombosis being main risk factors. According to treatment guidelines, patients aged 60 years or older, or those with a history of arterial or venous thrombosis, are classified as high-risk patients [8, 9]. Cytoreductive therapy (hydroxyurea [HU] or interferon [IFN]-alfa) is recommended for high-risk patients and for low-risk patients in some circumstances (e.g., need of frequent phlebotomy therapy).
A change of therapy is indicated if patients develop intolerance or resistance to the primary therapy, or when severe clinical symptoms remain uncontrolled. Depending on the cytoreductive first-line therapy, possible second-line therapies include HU, IFN-alfa, ruxolitinib (RUX), or busulfan, an off-label option for patients of advanced age if no other treatment option is available [8]. HU is a common first-line treatment of high-risk PV [8, 10]. However, previous studies have shown that effectiveness can vary, and some patients develop intolerance or resistance [11, 12]. Resistance to HU has been linked to poorer outcomes including shorter survival and higher risk of disease transformation [11]. The European LeukaemiaNet (ELN) has proposed consensus criteria for intolerance/resistance, which include inadequate control of hematocrit, platelet count, and leukocyte count, persistent severe or distressing disease-related symptoms (e.g., pruritus), occurrence of bleeding, hematologic and non-hematologic toxicity, and development of non-melanotic skin cancer [9, 13].
In 2015, the European Medicines Agency (EMA) approved the JAK1/JAK2 inhibitor RUX for adult patients with PV who are HU-intolerant/resistant [14]. Clinical studies demonstrated RUX’s superiority over the best available therapy, offering better symptom control, reduced risk of TEs, and longer thromboembolic event-free survival [15,16,17].
Treatment of PV predominantly occurs in the outpatient setting. However, published data on the treatment of patients with PV with cytoreductive drugs often originate from clinical trials and university hospital settings, thus limiting insights into real-world treatment. Observational data detailing routine PV management is scarce.
We performed an analysis based on German health claims data with two aims. First, we wanted to provide estimates of the epidemiology of PV in Germany, characterize the prevalent patient population in terms of risk status and comorbidity burden, including comorbid cardiovascular risk factors (CVRFs), and provide a snapshot of the current treatment with cytoreductive drugs. Second, in a longitudinal analysis, we aimed to assess the occurrence of thromboembolic events (TEs) over time, focusing on patients on continuous treatment with cytoreductive medicines.
Methods
Study design, data source, and observation periods
We conducted this study in a retrospective, non-interventional cohort design. We used anonymized health claims data from the sample database of the Institute for Applied Health Research Berlin (InGef). This data set contains anonymized health claims data from a total of about four million persons insured by the German statutory health insurance (SHI) and is considered representative for the German population in terms of age and sex [18]. The data provides individual-level information across different healthcare sectors over time, covering sociodemographic information as well as information on hospitalizations (including dates of hospital admission and discharge, diagnostic and therapeutic procedures, main and secondary diagnoses), outpatient services (including diagnoses, diagnostic and therapeutic procedures), and outpatient drug prescriptions (including dates of prescription by a physician and dispensation by a pharmacy). All diagnoses in the database are coded according to the German modification of the 10th version of the International Classification of Diseases (ICD-10-GM). Outpatient diagnoses, which also include information on the diagnostic certainty (confirmed, suspected, diagnoses ruled out, and post-diagnosis status), are only recorded quarterly (i.e., four three-month periods per calendar year) in line with the quarterly reimbursement. Outpatient drug prescriptions are coded according to Anatomical Therapeutic Chemical (ATC) codes. Outpatient diagnostic and therapeutic procedures are coded according to doctor’s fee scale (“Einheitlicher Bewertungsmaßstab”, EBM) numbers while inpatient diagnostic and therapeutic procedures are coded according to the German procedure classification (“Operationen- und Prozedurenschlüssel”, OPS) codes.
We analyzed health claims data from January 1, 2014 to December 31, 2022, the most recent available data years available at the time of the study. To apply exclusion and validation criteria for prevalent and incident case identification, the data years 2014 and 2015 served as pre-observation period and 2022 as post-observation period. Results are reported for the period from January 1, 2016 to December 31, 2021.
We estimated the one-year prevalence and the one-year incidence of PV for the calendar years 2016 to 2021. For the year 2021, we additionally described prevalent patients in terms of cytoreductive treatment, specifically examining the proportion of patients with at least one dispensation of pre-defined cytoreductive drugs, and in terms of comorbidities including CVRFs, specifically examining the proportion of patients with at least one pre-defined diagnosis of the respective disease in comparison to a matched control population (selection described below).
In addition, we analyzed the occurrences of thromboembolic events over consecutive half-year periods for up to three years, focusing on patients who had been consistently receiving cytoreductive treatment with either HU or RUX. These patients were categorized into distinct cohorts based on their treatment, with the detailed criteria for cohort selection described below.
Study population
The study population included all insured persons aged 18 years and older who were continuously insured in the two years before the respective reference year, and who were either continuously insured or continuously insured until death during the respective reference year. All populations considered were identified from this population.
Identification of patients with prevalent PV
Individuals were classified as having prevalent PV if they met the following criteria in the respective reference year:
-
(i)
at least one diagnosis of PV (ICD-10-GM code: D45.-) as a main or secondary hospital diagnosis or as a confirmed outpatient diagnosis;
-
(ii)
no diagnosis of osteomyelofibrosis (D47.4), essential (hemorrhagic) thrombocythemia (D47.3), other myeloproliferative neoplasms (C92.1, D47.0, D47.1, D47.5), or other erythrocytosis (D75.0, D75.1) as a hospital main diagnosis or as a diagnosis by a hematology specialist in the outpatient sector.
-
In addition, to validate prevalent case identification, at least one of the following criteria had to be met either in the respective reference year or the two preceding calendar years:
-
(i)
dispensation of a cytoreductive drug (HU or IFN alfa);
-
(ii)
main hospital diagnosis of PV;
-
(iii)
confirmed outpatient diagnosis of PV by a hematology specialist;
-
(iv)
main hospital diagnosis of a TE and a secondary hospital diagnosis of PV during the same hospitalization;
-
(v)
bone marrow puncture performed in the outpatient sector;
-
(vi)
phlebotomy performed in the outpatient sector.
Cytoreductive drugs dispensed by the pharmacy were identified by documented ATC codes, and inpatient administration of cytoreductive drugs was identified by OPS codes. Outpatient procedures (bone marrow puncture and phlebotomy) were identified by documented EBM numbers (for the operationalization see Table S1 in the supplement). TEs were identified by documented ICD-10-GM codes (see Table S2 in the supplement for operationalization).
Identification of patients with incident PV
Individuals were classified as having incident PV if they met the following criteria:
-
(i)
at least one diagnosis of PV as a main or secondary hospital diagnosis or as a confirmed outpatient diagnosis by a hematology specialist in the respective reference year;
-
(ii)
no diagnosis of PV as a main or secondary hospital diagnosis or as a confirmed outpatient diagnosis in the two years prior to the first observable diagnosis of PV;
-
(iii)
no diagnosis of osteomyelofibrosis, essential (hemorrhagic) thrombocythemia, other myeloproliferative neoplasms, or other erythrocytosis by a hematology specialist or as a main hospital diagnosis in the two years prior to the first observable diagnosis of PV.
In addition, at least one of the following criteria had to be met to validate incident case identification:
-
(i)
dispensation of a cytoreductive drug (HU or IFN alfa) in the year after the first observable diagnosis of PV,
-
(ii)
main hospital diagnosis of a TE and a secondary hospital diagnosis of PV during the same hospitalization in the year after the first observable diagnosis of PV;
-
(iii)
bone marrow puncture performed in the outpatient sector within three months before or after the first observable diagnosis of PV;
-
(iv)
phlebotomy therapy in the year after the first observable PV diagnosis.
Estimation of prevalence and incidence
To estimate the one-year prevalence of PV, we divided the number of patients with prevalent PV in a given reference year by the study population in that year. Similarly, to determine the one-year incidence, we divided the number of patients with incident PV in a given reference year by the study population in that year. We then extrapolated the prevalence and incidence estimates to the entire German adult population (≥ 18 years) using direct standardization (by age and sex) based on population estimates for the respective reference year from the German Federal Statistical Office [19].
Characteristics and treatment with cytoreductive drugs of prevalent patients in 2021
For 2021, we stratified prevalent patients according to risk status. Patients with a TE between 2019 and 2021, and those aged 60 years and older in 2021, were classified as high-risk PV patients. The remaining patients were classified as low-risk PV patients.
We further stratified the prevalent patients in 2021 based on the cytoreductive treatment they received in that year. This was done by assessing the proportion of patients who received specific cytoreductive drugs from pharmacies, using disjunctive strata. Among prevalent patients in 2021, we identified those who had at least one dispensation of HU but no dispensation of RUX. Within this group, we identified those patients with signs of HU intolerance/resistance (operationalization described below).
Next, we analyzed the prevalence of CVRFs and other comorbid conditions among the prevalent patients and their various strata. Results were further compared to a matched control population of insured persons without PV. The control population was selected from a pool of individuals within the study population in 2021 who did not meet the prevalent PV case definition and who did not have a diagnosis of osteomyelofibrosis, essential (hemorrhagic) thrombocythemia, other myeloproliferative neoplasms, or other erythrocytosis (as a hospital main diagnosis or as a diagnosis by a hematology specialist in the outpatient sector). The control population was matched to the prevalent patient population in 2021 at a ratio of 10:1 with respect to similar age (age categories: 18–24, 25–29, 30–34, …, 75–79, 80–84, ≥ 85), same sex, and presence of diagnoses of the same CVRF(s) (considering diabetes, obesity, hypercholesterolemia, smoking, and/or arterial hypertension) in 2021 (for operationalization see Table S3 in the supplement).
The prevalence of CVRFs, major bleeding events, and other comorbid conditions was assessed by determining the proportion of prevalent patients and controls with documented diagnoses for specific diseases according to pre-defined ICD-10-GM codes as a main or secondary hospital diagnosis or as a confirmed outpatient diagnosis in 2021 (for operationalization see Table S3 in the supplement). A single diagnosis code was sufficient to identify the respective disease.
HU intolerance/resistance criteria
Most consensus criteria for HU intolerance/resistance rely on clinical parameters that are not available in German health claims data. Therefore, we identified affected patients using proxy measures. We used ICD-10-GM diagnosis codes (as main or secondary hospital diagnosis or as confirmed outpatient diagnosis), mostly including diagnoses that may indicate non-hematologic toxicity, and the EBM therapy number for phlebotomy, which may indicate inadequate hematocrit control (see Table 1 for operationalization). We classified patients as HU intolerant/resistant if they had any of the defined ICD-10-GM codes/EBM number in the same quarter of the year they received HU. This classification was only considered if the patient did not receive RUX during the same period.
Formation of treatment cohorts
To analyze the prevalence of comorbid CVRFs and the occurrence of thromboembolic events over time, we formed multiple treatment cohorts. Patients with prevalent PV who continuously received treatment with HU or RUX between 2016 and 2020 were included in the ‘HU cohorts’ or ‘RUX cohorts’, respectively. To be included in the HU cohorts, patients could not have been dispensed any RUX during the assessed period. Cohort inclusion was independent of previous treatment status, meaning both new and prevalent HU-treated patients were included. The index date was the first observable dispensation of HU or RUX.
A treatment episode was considered ‘continuous’ if HU or RUX was reimbursed subsequently at least twice in 182-day-periods. Patients were observed during the continuous treatment episode.
Within the HU cohorts, sub-cohorts of HU-treated patients for whom we could observe signs of HU intolerance/resistance (as previously defined) in the same quarter of an HU dispensation were selected (‘HUIT/R cohorts’).
Cohorts of varying treatment durations were selected based on the number of consecutive 182-day-periods with documented continuous treatment (364 days [two 182-day-periods], 546 days [three 182-day-periods], … 1,092 days [six 182-day-periods]). In total, 15 treatment cohorts were formed: 5 RUX cohorts, 5 HU cohorts, and 5 HUIT/R cohorts. The cohorts were observed for approximately 1 to 3 years.
Patients could be included in all cohorts for which they met the criteria and could potentially be included in multiple cohorts if they had multiple continuous treatment episodes between 2016 and 2020.
Statistical analyses
In descriptive analyses, we summarized categorical variables by frequency and percentage, while continuous variables are presented as mean ± standard deviation (SD).
Furthermore, we compared results between groups (prevalent patients vs. matched control population; RUX cohorts vs. HU cohorts; RUX cohorts vs. HUIT/R cohorts) using simple significance tests (depending on sample sizes, chi-square test or Fisher’s exact test for categorical variables and t-tests for continuous variables).
All analyses were performed using R Statistical Software. P-values < 0.05 were considered statistically significant and were not adjusted for multiplicity.
Results
Prevalence and incidence
We identified 937 patients with prevalent PV in the database in 2021 (Fig. 1), corresponding to a one-year prevalence of 30.8 per 100,000. When extrapolated to the German population, this equated to n = 19,805 and a prevalence of 28.6 per 100,000 (Table 2). The prevalence was higher in men than women (33.6 per 100,000 vs. 22.1 per 100,000) and increased with age, peaking in those 80 years and older (81.3 per 100,000). A total of 780 (83.2%) patients met our defined criteria for high-risk PV (i.e., TE between 2019 and 2021 or age 60 or older in 2021). In 2021, we identified 107 patients with incident PV in the database, corresponding to a one-year incidence of 3.5 per 100,000. When extrapolated to the German population, this equated to n = 2,286 and an incidence of 3.3 per 100,000 (Table 3). Figure S1 in the supplement shows the selection steps for incident patients in 2021.
Extrapolated to the German population, we estimated that over 17,000 adult persons were affected by PV in Germany each year from 2016 to 2021. This resulted in a prevalence estimate ranging from 25.5 per 100,000 (in 2016) to 29.6 per 100,000 (in 2020) (Table 3). The estimated number of patients with incident PV in the adult German population and the corresponding incidence estimates were higher from 2019 to 2021 compared to our estimates from 2016 to 2018 (Table 3).
Cytoreductive treatment among prevalent patients in 2021
In 2021, nearly half of the prevalent patients (n = 464; 49.5%) did not receive a cytoreductive drug. 337 patients (36.0%) received HU at least once (but no RUX). Among these, 63.5% (n = 214) met our defined criteria for HU intolerance/resistance (Fig. 2). Fewer patients received RUX (n = 121; 12.9%). IFN-alfa (n = 14; 1.5%) and busulfan (n < 5) played minor roles.
Among patients with high-risk PV, 43.6% (340 out of 780 patients) received no cytoreductive drug. Another 321 (41.2%) received HU at least once (but no RUX); of these, 63.2% (n = 203) met our defined criteria for HU intolerance/resistance.
Cardiovascular risk factors and comorbidity burden in prevalent patients in 2021
In 2021, the majority of prevalent patients (n = 802; 85.6%) had at least one comorbid CVRF (Table 4). The most common CVRFs were arterial hypertension (75.7%), followed by hypercholesterolemia/dyslipidemia (42.5%), and diabetes (25.4%).
Comorbid CVRFs were more common among high-risk patients, with 89.6% having at least one CVRF, 81.2% with arterial hypertension, and 46.4% having diabetes.
Certain diseases were more common in patients with prevalent PV than the matched control population of insured persons without PV (Table 5; full results are presented in Table S4 in the supplement). The largest absolute differences were seen in hepatomegaly and splenomegaly (14.9% vs. 1.1%; p < 0.0001), kidney failure (22.8% vs. 15.2%; p < 0.0001), and heart failure (19.9% vs. 12.9%; p < 0.0001). Additionally, non-melanoma skin cancer was also more common in patients with PV than in controls (10.1% vs. 5.5%; p < 0.0001).
TEs (32.8% vs. 18.2%; p < 0.0001) were also more frequently observed in patients with PV than in matched controls (Table 5). Among high-risk patients, 39.4% had at least one TE in 2021 (results shown in Table S5 in the supplement). A higher proportion of high-risk patients who received a cytoreductive drug had at least one TE in 2021 compared to those who did not receive a cytoreductive drug (41.4% vs. 36.8%, respectively).
Cardiovascular risk factors and thromboembolic events over time
Cohort sizes and characteristics for the HUIT/R cohorts and RUX cohorts are shown in Table 6 (cohort sizes, characteristics, and results regarding the occurrence of TEs over time for the HU cohorts are shown in Table S6 in the supplement). Compared to patients in the HUIT/R cohorts, patients in the RUX cohorts were on average between 4.9 (74.0 vs. 69.1 years; 546-day cohorts) to 6.6 (74.9 vs. 68.3 years; 910-day cohorts) years younger. For each observation period, the proportion of patients with 2 and ≥ 3 CVRFs was higher in the HUIT/R cohorts than in the RUX cohorts. Cohorts with longer observation periods tended to have a higher proportion of patients with 2 and ≥ 3 CVRFs.
In the 364-day cohorts, the proportion of patients with obesity was 7.1%-points higher in the HUIT/R cohort than in the RUX cohort (15.8% vs. 8.7%). Similarly, the proportion of patients with hypertension was 8.6%-points higher in the HUIT/R cohort compared to the RUX cohort (80.4% vs. 71.8%). However, in the 1,092-day cohorts, the proportion of patients with hypertension was slightly higher in the RUX cohort (86.8% vs. 85.6%). In contrast, the 1,092-day HUIT/R cohort included a higher proportion of patients with diabetes (24.1% vs. 15.1%) and hypercholesterolemia/dyslipidaemia (41.4% vs. 24.5%) compared to the 1,092-day RUX-cohort.
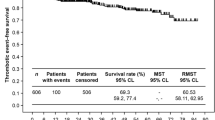
For each observation period, TEs were more common in the HUIT/R cohorts compared to the RUX cohorts (Fig. 3). In the 364-day RUX cohort, 29.1% of patients had at least one TE compared to 45.7% of patients in the 354-day HUIT/R cohort (dif. 16.6%-points, p < 0.05). The absolute difference between the cohorts increased with the length of the observation periods and was highest for the 1,092-day observation period (dif. 20.5%-points, p < 0.05) (Fig. 3).
Discussion
Based on a large representative German health claims database, we provide recent estimates of the prevalence and incidence of PV in the German adult population, and we characterize the prevalent patient population in 2021 in terms of risk status, comorbidity burden, and cytoreductive drug treatment. We further observed patients with PV on continuous cytoreductive treatment with HU or RUX for TEs over time.
Overall, there is little information on the epidemiology of PV available in the literature. In particular, data on the prevalence and incidence in Germany are lacking. Additionally, published studies on the prevalence of PV have yielded heterogeneous results. Based on peer-reviewed literature and online documentation from disease and health registries between 2000 and 2012, Moulard et al. estimated the prevalence of PV in the European Union at 4.96 to 30 per 100,000 [7]. Roaldsnes et al.‘s estimate of 9.2 per 100,000 based on Norwegian cancer registry data is at the lower end of this range [6], while the most recent Orphanet report appears to confirm the upper end of the range at 30.0 per 100,000 [20]. Our estimate for 2021 of 28.6 per 100,000 is also at the upper end of this range.
Published estimates on the incidence of PV in the US and Europe also vary, ranging from 0.4 to 2.8 per 100,000 [3, 5,6,7]. While our incidence estimates for the years 2016 to 2019 are within this range, our estimates for 2020 (3.59 per 100,000) and 2021 (3.30 per 100,000) are higher than those reported. The substantial increase in our estimate for 2020 is mainly due to methodological reasons, as we were only able to include phlebotomy therapy as a criterion to validate incident case identification from 2020 onwards. Phlebotomy (EBM number 13505) has only been billable since April 2020 – in addition to the basic fee – by specialists in internal medicine with a focus on hematology and internal oncology [21]. Thus, we suspect our incidence estimates for 2016 to 2019 and those reported in the literature to be underestimates, rather than our 2020 and 2021 estimates to be overestimates. Our prevalence estimates were also slightly higher in 2020 and 2021, when phlebotomy therapy could be included as a criterion to validate prevalent case identification. However, the difference was less pronounced, which indicates that our algorithm to identify prevalent patients worked well even without considering phlebotomy therapy. It should also be noted that phlebotomy therapy is a first-line treatment irrespective of risk status, thus a more pronounced effect was to be expected when including phlebotomy therapy as a validation criterion for newly diagnosed patients. As PV mainly affects individuals aged 60 and over [22, 23], an increasing trend in prevalence can be expected over the past decade and in the coming years due to changes in the age structure of the German population towards a higher proportion of persons of older age [24]. Increased awareness of PV among physicians may further exacerbate the increasing trend in prevalence in the future. Consistent with several studies [3, 5], we further found PV to be slightly more common in men than in women.
We considered most prevalent patients in 2021 (83.2%) to have high-risk PV, either due to their age (≥ 60 years in 2021) or their recorded history of TEs (criterion checked between 2019 and 2021), which is in line with previous observational studies in which the majority of patients were categorized as high-risk, mainly due to their age [22, 23]. Contrary to current treatment guideline recommendations [8, 9], 43.6% of the patints we categorized as high-risk patients in 2021 did not receive treatment with cytoreductive drugs in that year. This result represents a snapshot of the calendar year 2021, and we cannot rule out the possibility that patients started cytoreductive drug treatment in the following year. However, this finding is consistent with the results of a recent German chart review, which reported that on average only 60.7% of patients with high-risk PV received cytoreductive drug treatment. The authors further noted that the proportion of patients receiving cytoreductive drug treatment varied highly between treatment sites [22]. Next to the control of symptoms, prevention of TEs is the main aim of treatment in PV, as complications from TEs are the most frequent clinical challenge in patients with PV [25, 26]. In this context, much emphasis has been placed on control of the hematocrit below 45% as this target level has been associated with significantly lower rates of major TEs and death [27, 28]. Currently, there is strong consensus that patients with high-risk PV should be treated with cytoreductive drugs to reduce TE risk by controlling blood cell count [9, 27]. While we did observe a lower relative frequency of patients with TE in 2021 in high-risk patients who did not receive cytoreductive treatment compared to high-risk patients who received cytoreductive treatment in that year, it should be noted that due to the cross-sectional design it is not possible to determine the temporality of this finding; for example, patients may have been started on cytoreductive drug treatment only after the respective TE.
In line with previous observational studies [22, 29], the majority of patients with prevalent PV in 2021 (85.6%) had one or more comorbid CVRF(s), with arterial hypertension (75.7%) bein the most prevalent. Thus, it should be noted that most patients can be considered at elevated risk for cardiovascular disease by conventional risk categorization. Several recent studies have explored the association of CVRFs with different outcomes, including the occurrence of TEs, in patients with PV [2, 30, 31]. While CVRFs are currently not being considered for risk classification to inform the therapeutic decisions, the aggressive management of CVRFs is recommended for all patients [27].
Among others, we observed a higher occurrence of non-melanoma skin cancer in patients with PV than matched controls. Increased risk of non-melanoma skin cancer has previously been reported in patients with Philadelphia-negative myeloproliferative neoplasms, including PV, who are treated with HU and RUX [32]. While the underlying mechanisms and the extent to which cytoreductive drugs contribute to the increased risk are not yet fully understood, it has been suggested that prolonged treatment with HU may be a contributing factor [33]. Consequently, guidelines currently recommend regular dermatologic screenings both prior to and throughout the duration of these therapies [8].
In 2021, most prevalent patients on cytoreductive drug treatment received HU, but no RUX. Among these patients, we identified those with signs of HU intolerance/resistance. Since most consensus criteria for HU intolerance/resistance are based on clinical parameters that are not documented in German claims data, in particular constitutional symptoms, we identified affected patients by proxy using ICD-10-GM diagnosis codes, mostly for diagnoses that may indicate non-hematologic toxicity, and claims for phlebotomy, which may indicate inadequate hematocrit control. The operationalization based on documented diagnosis codes and claims for phlebotomy in our study was defined based on the originally proposed consensus criteria [13] in consultation with an expert. Nearly two-thirds (63.5%) of prevalent patients in 2021 who were treated with HU (but not RUX) met our defined proxy criteria for HU intolerance/resistance in that year. This finding is generally consistent with data from other observational studies showing that a considerable proportion of patients may not be adequately controlled with HU. In a prospective observational study of PV patients in the United States, 57% of patients treated with HU for more than three months continued to have an elevated hematocrit level above 45%, 33% continued to receive phlebotomies, and 27% experienced uncontrolled myeloproliferation [34]. In a cross-sectional study from Belgium, 60% of HU-treated patients had elevated values for hematocrit, platelet count, and/or white blood cell count [35]. Similarly, in a multicenter retrospective cohort study, 70% of patiens did not achieve complete response after 12 or more months of therapy with HU, of which 71.3% of patiens nevertheless continued treatment with HU [36].
Our results indicate that, contrary to guideline recommendations, there might be a number of patients whose treatment is not adjusted despite showing signs of potential HU resistance/intolerance. This raises concerns because intolerance and resistance to HU have been linked to adverse outcomes, including an increased risk of thrombosis, lower survival rates, and higher chances of disease progression [11, 37].
RUX has been approved by the European Medicines Agency in 2015 as a second-line therapy for the treatment of adults with PV who are resistant or intolerant to HU, and has shown favorable results in terms of disease control and thromboembolic event-free survival compared to best available therapy [15, 38, 39]. In our analysis, we observed a lower proportion of patients with TEs in patients continuously treated with RUX (RUX cohorts) compared to patients continuously treated with HU who also met our defined proxy criteria for HU intolerance/resistance (HUIT/R cohorts). Some of these differences were statistically significant (1- and 3-year observation periods). However, these results should be interpreted as descriptive only. The analysis was not adjusted for differences in the composition of the cohorts (e.g., in terms age, CVRFs, treatment status [prevalent vs. new-user]). For example, due to small sample sizes, the treatment cohorts were not matched based on criteria known to increase the risk of TEs. Patients in the HUIT/R cohorts were on average older and had more CVRFs than patients in the RUX cohorts, suggesting that younger and healthier patients may be more likely to receive RUX when treatment with HU is unsatisfactory.
Limitations
We want to recognize the following limitations to our study. We considered patients 60 years and over and those with a history of TEs as patients with high-risk PV. However, the pre-observation period for identifying documented diagnoses for TEs was limited to two years. We may have misclassified patients because previous TEs may have been missed due to the short pre-observation period. Criteria indicating HU intolerance/resistance could only be indirectly operationalized by proxy because health claims data do not contain clinical parameters (e.g. haematocrit and symptoms) used in clinical practice to identify HU intolerance/resistance to HU. Comparisons between treatment cohorts were not adjusted for possible confounders, thus, results may be biased and should be interpreted in terms of real-world drug effectiveness. Furthermore, the treatment cohorts were observed for a maximum of three years, which is a relatively short period for patients with PV, for whom a median survival of 14 years after diagnosis has been reported [40].
Conclusions
To our knowledge, this study is the first comprehensive analysis of the epidemiology of PV in Germany. Based on a large representative database we provide new insights into the characteristics of prevalent patients and their current real-life treatment situation. Our findings suggest that currently available cytoreductive therapies are not being fully utilized according to treatment guidelines. Because suboptimal treatment practices may increase the risk of thromboembolic complications in this patient population, further research, particularly studies including clinical data, are warranted to support the findings of this study that cytoreductive therapy in PV patients may not be fully in compliance with guidelines.
Data availability
The data analyzed in this study was retrieved from the Institute for Applied Health Research Berlin (InGef) Research Database and cannot be made available in the manuscript, the supplementary files, or in a public repository due to German data protection laws (Bundesdatenschutzgesetz). To facilitate the replication of results, anonymized data used for this study are stored on a secure drive at the InGef GmbH. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and can be assessed upon request, after written approval (contact: info@ingef.de), if required.
References
Spivak JL (2019) How I treat polycythemia vera. Blood 134:341–352. https://doi.org/10.1182/blood.2018834044
Mancuso S, Santoro M, Accurso V et al (2020) Cardiovascular Risk in Polycythemia Vera: thrombotic risk and survival: can Cytoreductive Therapy be useful in patients with low-risk Polycythemia Vera with Cardiovascular Risk factors? Oncol Res Treat 43:526–530. https://doi.org/10.1159/000509376
Verstovsek S, Yu J, Scherber RM et al (2022) Changes in the incidence and overall survival of patients with myeloproliferative neoplasms between 2002 and 2016 in the United States. Leuk Lymphoma 63:694–702. https://doi.org/10.1080/10428194.2021.1992756
Waksal JA, Wagner NE, Mascarenhas JO (2024) Risk factors for Disease Progression and Treatment goals in Polycythemia Vera. Clin Adv Hematol Oncol 22:31–42
Hultcrantz M, Ravn Landtblom A, Andréasson B et al (2020) Incidence of myeloproliferative neoplasms - trends by subgroup and age in a population-based study in Sweden. J Intern Med 287:448–454. https://doi.org/10.1111/joim.13019
Roaldsnes C, Holst R, Frederiksen H et al (2017) Myeloproliferative neoplasms: trends in incidence, prevalence and survival in Norway. Eur J Haematol 98:85–93. https://doi.org/10.1111/ejh.12788
Moulard O, Mehta J, Fryzek J et al (2014) Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 92:289–297. https://doi.org/10.1111/ejh.12256
Lengfelder E, Baerlocher GM, Döhner K et al (2023) Polycythaemia Vera (PV). https://www.onkopedia.com/de/onkopedia/guidelines/polycythaemia-vera-pv/@@guideline/html/index.html
Marchetti M, Vannucchi AM, Griesshammer M et al (2022) Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol 9:e301–e311. https://doi.org/10.1016/S2352-3026(22)00046-1
Barbui T, Vannucchi AM, Finazzi G et al (2017) A reappraisal of the benefit-risk profile of hydroxyurea in polycythemia vera: a propensity-matched study. Am J Hematol 92:1131–1136. https://doi.org/10.1002/ajh.24851
Alvarez-Larrán A, Pereira A, Cervantes F et al (2012) Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 119:1363–1369. https://doi.org/10.1182/blood-2011-10-387787
Alvarez-Larrán A, Garrote M, Ferrer-Marín F et al (2022) Real-world analysis of main clinical outcomes in patients with polycythemia vera treated with ruxolitinib or best available therapy after developing resistance/intolerance to hydroxyurea. Cancer 128:2441–2448. https://doi.org/10.1002/cncr.34195
Barosi G, Birgegard G, Finazzi G et al (2010) A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol 148:961–963. https://doi.org/10.1111/j.1365-2141.2009.08019.x
Kiladjian J-J, Winton EF, Talpaz M et al (2015) Ruxolitinib for the treatment of patients with polycythemia vera. Expert Rev Hematol 8:391–401. https://doi.org/10.1586/17474086.2015.1045869
Harrison CN, Nangalia J, Boucher R et al (2023) Ruxolitinib Versus Best available therapy for Polycythemia Vera intolerant or resistant to Hydroxycarbamide in a Randomized Trial. J Clin Oncol 41:3534–3544. https://doi.org/10.1200/JCO.22.01935
Kiladjian J-J, Zachee P, Hino M et al (2020) Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol 7:e226–e237. https://doi.org/10.1016/S2352-3026(19)30207-8
Verstovsek S, Vannucchi AM, Griesshammer M et al (2016) Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 101:821–829. https://doi.org/10.3324/haematol.2016.143644
Ludwig M, Enders D, Basedow F et al (2022) Sampling strategy, characteristics and representativeness of the InGef research database. Public Health 206:57–62. https://doi.org/10.1016/j.puhe.2022.02.013
Federal Statistical Office (2021) Current population of Germany. https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/_node.html#1346190. Accessed 13 Dec 2024
Orphanet Report Series (2023) Number 1 | November 2023 Prevalence and incidence of rare diseases: Bibliographic data: Prevalence, incidence or number of published cases listed by diseases (in alphabetical order). https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Accessed 05 Feb 2024
Bundesvereinigung K (2020) EBM-Reform: Übersicht der Auswirkungen je Fachgruppe. https://www.kbv.de/media/sp/EBM-Reform__bersicht_Fachgruppen.pdf. Accessed 05 Feb 2024
Crodel CC, Jentsch-Ullrich K, Reiser M et al (2022) Cytoreductive treatment in real life: a chart review analysis on 1440 patients with polycythemia vera. J Cancer Res Clin Oncol 148:2693–2705. https://doi.org/10.1007/s00432-021-03855-5
Jentsch-Ullrich K, Eberhardt J, Zeremski V et al (2016) Characteristics and treatment of polycythemia vera patients in clinical practice: a multicenter chart review on 1476 individuals in Germany. J Cancer Res Clin Oncol 142:2041–2049. https://doi.org/10.1007/s00432-016-2209-1
Federal Institute for Population Research (2021) Demographic facts and trends in Germany, 2010–2020. Report by the Federal Institute for Population Research (BiB). https://www.bib.bund.de/Publikation/2021/pdf/Demographic-facts-and-trends-in-Germany-2010-2020.pdf . Accessed 05 Feb 2024
Griesshammer M, Kiladjian J-J, Besses C (2019) Thromboembolic events in polycythemia vera. Ann Hematol 98:1071–1082. https://doi.org/10.1007/s00277-019-03625-x
Barbui T, Finazzi G, Falanga A (2013) Myeloproliferative neoplasms and thrombosis. Blood 122:2176–2184. https://doi.org/10.1182/blood-2013-03-460154
Barbui T, Tefferi A, Vannucchi AM et al (2018) Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 32:1057–1069. https://doi.org/10.1038/s41375-018-0077-1
Marchioli R, Finazzi G, Specchia G et al (2013) Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 368:22–33. https://doi.org/10.1056/NEJMoa1208500
Altomare I, Parasuraman S, Paranagama D et al (2021) Real-world dosing patterns of Ruxolitinib in patients with Polycythemia Vera who are resistant to or intolerant of Hydroxyurea. Clin Lymphoma Myeloma Leuk 21:e915–e921. https://doi.org/10.1016/j.clml.2021.06.023
Benevolo G, Elli EM, Bartoletti D et al (2021) Impact of comorbidities and body mass index on the outcome of polycythemia vera patients. Hematol Oncol 39:409–418. https://doi.org/10.1002/hon.2843
Accurso V, Santoro M, Mancuso S et al (2020) Cardiovascular Risk in essential thrombocythemia and Polycythemia Vera: thrombotic risk and survival. Mediterr J Hematol Infect Dis 12:e2020008. https://doi.org/10.4084/MJHID.2020.008
Barbui T, Ghirardi A, Masciulli A et al (2019) Second cancer in Philadelphia negative myeloproliferative neoplasms (MPN-K). A nested case-control study. Leukemia 33:1996–2005. https://doi.org/10.1038/s41375-019-0487-8
Antonioli E, Guglielmelli P, Pieri L et al (2012) Hydroxyurea-related toxicity in 3,411 patients with Ph’-negative MPN. Am J Hematol 87:552–554. https://doi.org/10.1002/ajh.23160
Grunwald MR, Kuter DJ, Altomare I et al (2020) Treatment patterns and blood counts in patients with Polycythemia Vera treated with Hydroxyurea in the United States: an analysis from the REVEAL Study. Clin Lymphoma Myeloma Leuk 20:219–225. https://doi.org/10.1016/j.clml.2019.09.601
Devos T, Beguin Y, Noens L et al (2018) Disease and treatment characteristics of polycythemia vera patients in Belgium: results from a scientific survey. Eur J Haematol 100:361–366. https://doi.org/10.1111/ejh.13022
Palandri F, Rossi E, Auteri G et al (2023) Predictors of response to Hydroxyurea and switch to Ruxolitinib in HU-Resistant Polycythaemia VERA patients: a real-world PV-NET study. Cancers 15:3706. https://doi.org/10.3390/cancers15143706
Alvarez-Larrán A, Kerguelen A, Hernández-Boluda JC et al (2016) Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br J Haematol 172:786–793. https://doi.org/10.1111/bjh.13886
Vannucchi AM, Kiladjian JJ, Griesshammer M et al (2015) Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 372:426–435. https://doi.org/10.1056/NEJMoa1409002
European Medicines Agency (2024) Jakavi ruxolitinib. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi. Accessed 16 Feb 2024
Tefferi A, Vannucchi AM, Barbui T (2018) Polycythemia vera treatment algorithm 2018. Blood cancer J 8:3. https://doi.org/10.1038/s41408-017-0042-7
Funding
Funding was provided by Novartis Pharma GmbH.
Author information
Authors and Affiliations
Contributions
KCM, AM, KM, MF, AH, VW, SG, FA, and HKA were involved in designing the study. BM and RN were involved in carrying out the analyses. All authors were involved in interpreting the results, contributed to the manuscript, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The study was funded by Novartis Pharma GmbH. The authors had complete autonomy for the process of designing the study, carrying out the analyses, interpreting the results, and preparing the manuscript. This also includes the full right to publish the results without limitation. KCM and AH are employees of the IGES Institut GmbH, which is a paid consultant to Novartis Pharma GmbH for conducting the study and preparing the manuscript. At the time of this study, VW and AM were employees of the IGES Institut GmbH. BM and RN are employees of the InGef – Institute for Applied Health Research Berlin GmbH, which was contracted and reimbursed by IGES Institut GmbH for providing the data and carrying out the analyses.KM, MF, and SG are employees of Novartis Pharma GmbH.FA received consulting fees from Abbott, Almirall, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, InGef, Lundbeck, Novartis, NovoNordisk, Shire, and Xcenda.HKA received research support from Bristol-Myers Squibb, Incyte, and Novartis, consulting fees from AbbVie, AOP Health, Bristol-Myers Squibb, GSK, Novartis, and Otsuka, advisory board fees from AbbVie, AOP Health, Blueprint, Bristol-Myers Squibb, GSK, and Novartis, and a travel grant from Alexion.
Ethics approval
Since all patient-level data in the database are anonymized to comply with German data protections regulations and German federal law, approval of an Ethics Committee was not required. The research was conducted in accordance with the Declaration of Helsinki.
Patient consent statement
Since anonymized administrative claims data were used and presented only in aggregate form, informed consent of patients was not required. The pre-existing database did not allow direct identification of patients, and no contact was made with patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 116 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manz, K.C., Mocek, A., Morouj, B. et al. Prevalence, incidence, and thromboembolic events in polycythemia vera: a study based on longitudinal German health claims data. Ann Hematol 104, 347–360 (2025). https://doi.org/10.1007/s00277-025-06192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-025-06192-6