Abstract
Purpose
The inflammatory cytokine interleukin (IL)-6 reduces the activity of drug metabolic enzymes and promotes tumor progression. We investigated the effect of IL-6 on the pharmacokinetics of osimertinib and the association between an IL-6 polymorphism and clinical outcomes in 30 patients with non-small cell lung cancer (NSCLC).
Methods
Osimertinib and IL-6 plasma concentrations were measured on day 15 after therapy initiation. The genotype of IL-6 1800796G > C was identified using polymerase chain reaction–restriction fragment length polymorphism. Risk factors affecting overall survival (OS) were assessed by Cox proportional hazard regression analysis.
Results
The IL-6 concentration was significantly correlated with the osimertinib trough plasma concentration (r = 0.423, P = 0.020) and area under the plasma concentration–time curve (r = 0.420, P = 0.021). The IL-6 concentration was significantly higher in patients with the IL-6 rs1800796G allele versus C/C genotype (P = 0.024). OS was significantly shorter in patients with the IL-6 rs1800796G allele versus C/C genotype (median: 15.1 vs. 48.9 months, P = 0.005). Univariate and multivariate analyses indicated that the IL-6 rs1800796G allele is an independent risk factor for OS (crude hazard ratio = 7.07; P = 0.014; adjusted hazard ratio = 6.38; P = 0.021).
Conclusion
A higher IL-6 concentration was associated with reduced metabolic activity of osimertinib, leading to increased osimertinib exposure. As the IL-6 concentration was higher in NSCLC patients with the IL-6 rs1800796G allele, it might be an independent prognostic factor for patients treated with osimertinib.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (EGFR-TKI), has become a first-line treatment for non-small cell lung cancer (NSCLC) with EGFR mutations. In the FLAURA trial, osimertinib prolonged not only progression-free survival (PFS) but also overall survival (OS) compared with first-generation EGFR-TKIs, such as gefitinib and erlotinib (PFS: 18.9 vs. 10.2 months; OS: 38.6 vs. 31.8 months) [1, 2]. Furthermore, osimertinib demonstrated efficacy in patients with EGFR T790M-positive NSCLC who experienced disease progression after EGFR-TKI therapy [3].
Because several TKIs show a correlation between the plasma exposure–response and exposure–toxicity relationships, clinicians need to adjust the dosage based on the plasma concentrations of TKIs [4]. Patients with a high trough plasma concentration (C0) of osimertinib (≥ 211 ng/mL) are reported to have longer PFS than are those with lower concentrations [5]. On the other hand, osimertinib concentrations > 259 ng/mL are reported to increase the risk of toxicity [6]. The steady-state osimertinib concentration shows high inter-individual variability, with the percent coefficient of variation of the area under the plasma concentration–time curve (AUC) ranging from 43.0 to 72.8% [7]. For thus inter-individual variability, polymorphisms in drug-metabolizing enzymes and drug efflux transporters can affect drug pharmacokinetics and help explain the variability in clinical outcomes [8]. Osimertinib is metabolized by the drug-metabolizing enzymes cytochrome P450 (CYP) 3A4 and CYP3A5 [9], and it is a substrate of ATP-binding cassette (ABC) transporters, including P-glycoprotein encoded by ABCB1 and BCRP encoded by ABCG2. The mean osimertinib C0 is significantly lower in patients with the T/T genotype of ABCB1 3435 C > T than in those with the C allele, and ABCB1 3435 C > T and AGCG2 34G > A are risk factors for progression of central nervous system diseases [10]. On the other hand, in contrast to this result [10], we observed no significant differences in the osimertinib plasma concentration according to ABC transporter polymorphisms (ABCB1 3435 C > T, ABCB1 1236 C > T, ABCB1 2677G > T/A, ABCG2 421 C > A) [11]. Therefore, until now, the factors contributing to the inter-individual variability of the osimertinib plasma concentration have not been fully understood.
Inflammatory conditions are major regulators of drug-metabolizing enzymes and transporters [12], which affect drug clearance in cancer patients [13]. Because the inflammatory cytokine interleukin (IL)-6 decreases CYP3A4 metabolic activity [14], it may affect the pharmacokinetics and toxicity of osimertinib. IL-6 promotes tumorigenesis via the JAK/STAT signaling pathway [15]. IL-6 levels are increased in a wide range of cancers, including multiple myeloma, lung cancer, colorectal cancer, renal cell carcinoma, and cervical cancer [16]. Two single nucleotide polymorphisms (rs1800795G > C and rs1800796G > C) in the promoter region of IL-6 are reportedly associated with alterations in the transcriptional activity of IL-6, thereby causing variability in the IL-6 concentration among individuals [17, 18]. IL-6 polymorphisms are correlated with lung cancer risk [19], and the IL-6 rs1800796G > C polymorphism increases cancer susceptibility in Asian populations [20]. However, the influence of the IL-6 rs1800796G > C polymorphism on the therapeutic efficacy of osimertinib is not known.
In this study, we investigated the relationship between the IL-6 concentration and the pharmacokinetics of osimertinib in Japanese patients with NSCLC. In addition, we also examined the effect of the IL-6 rs1800796G > C polymorphism on the clinical outcomes of NSCLC patients treated with osimertinib.
Materials and methods
Patients and protocols
Thirty Japanese patients with NSCLC who were treated with osimertinib (TAGRISSO; AstraZeneca K.K., Osaka, Japan) at Akita University Hospital between July 2016 and July 2023 were enrolled consecutively in this study. The recommended starting dose was 80 mg once daily. Patients from whom blood samples could not be obtained during the osimertinib administration period were excluded from this study. The study was conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Akita University School of Medicine (approval number: 2826), and all patients provided written informed consent for participation in the study. Demographic data, biological data, and clinical events (disease progression or death) were collected retrospectively from medical records.
Sample collection
Whole blood samples were collected just prior to (C0), and at 1, 2, 4, 6, 8, 12, and 24 h on day 15 after, osimertinib administration. Measurements of osimertinib C0 and the IL-6 level were performed at the same time. Plasma was separated from whole blood by centrifugation and stored at − 80 °C until analysis.
Analytical methods
The plasma concentration of osimertinib was measured by high-performance liquid chromatography and the ultraviolet method [11]. The calibration curve generated for the osimertinib concentration in human plasma was linear in the concentration range of 10–1000 ng/mL. The coefficients of variation and accuracies of intra- and inter-day assays at the concentration range of 10–1000 ng/mL were less than 11.4% and within 10.2%, respectively. The limit of quantification of osimertinib was 10 ng/mL.
The plasma concentration of IL-6 was measured using the Quantikine® enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s instructions.
Genotyping
DNA was extracted from peripheral blood samples using the QIAamp Blood Mini Kit (Qiagen, Tokyo, Japan) and was stored at − 80 °C until analysis. Genotyping of the IL-6 1800795G > C and 1800796G > C polymorphisms was performed using polymerase chain reaction–restriction fragment length polymorphism [21]. Because the G/G, G/C, and C/C genotypes of the IL-6 rs1800796G > C polymorphism were detected in 2 (6.7%), 13 (43.3%), and 15 (50.0%) patients, respectively, the patients were divided into two groups: G/G plus G/C (n = 15) and C/C (n = 15) [22]. On the other hand, the G/G, G/C, and C/C genotypes of the IL-6 rs1800795G > C polymorphism were detected in 30 (100%), 0 (0%), and 0 (0%) patients, respectively; thus, this IL-6 polymorphism was not present in our cohort.
Clinical endpoints
In the evaluation of osimertinib efficacy, the primary endpoint was OS, defined as the time from osimertinib treatment initiation to death from any cause. The secondary endpoint was PFS, defined as the time from osimertinib treatment initiation to a documented progression event, either clinical or death from any cause.
Statistical procedures
Descriptive data are expressed as numbers (%) and medians [interquartile range]. Spearman’s rank correlation was used to assess the correlations of continuous values between groups, and all results are expressed as correlation coefficients (r values). Pharmacokinetic analysis of osimertinib was conducted via the standard noncompartmental method using WinNonlin (Pharsight Co., Mountain View, CA, USA; version 5.2). The AUC0 − 24 was calculated using the linear trapezoidal rule. Regarding polymorphisms, the Mann–Whitney U test or Kruskal–Wallis test was used to determine differences in continuous values between two or among three groups, respectively. The Kaplan–Meier method was used to estimate OS and PFS distributions for each group, and the distributions were then compared between groups using the log-rank test. Explanatory variables were initially analyzed as risk factors for OS by univariate analyses, and then these variables were subjected to univariate and multivariate analyses using Cox proportional hazards regression models. The median values of age, weight, body mass index (BMI), IL-6 concentration, and osimertinib C0 were used as the cutoff values. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from the Cox models. Results with a P value < 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics version 27.0 for Windows (SPSS IBM Japan Inc., Tokyo, Japan).
Results
Patient characteristics
The clinical characteristics of the patients are shown in Table 1. Of the 30 patients, 22 (73.3%) were female and 8 (26.7%) were male. Histologically, all patients had adenocarcinoma; 29 (96.7%) patients had stage IV disease at diagnosis and 1 (3.3%) patient stage IIIb. Exon 19 deletion and the exon 21 L858R mutation were observed in 20 (66.7%) and 10 (33.3%) patients, respectively. The G/G, G/C, and C/C genotypes of the IL-6 rs1800796G > C polymorphism were identified in 2 (6.7%), 13 (43.3%), and 15 (50.0%) patients, respectively. The median (quartile 1–3) age, weight, and BMI were 71.5 (62.0–75.0) years, 53.1 (46.3–61.7) kg, and 22.0 (19.5–24.2) kg/m2, respectively. The median IL-6 concentration on day 15 after beginning therapy was 3.00 pg/mL (1.00–6.82 pg/mL). The median C0 and AUC0-24 of osimertinib were 227 ng/mL (122–314 ng/mL) and 7250 ng∙h/mL (4020–8988 ng∙h/mL), respectively.
Association of the osimertinib concentration with the IL-6 level and rs1800796G > C polymorphism
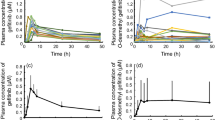
A significant correlation was observed between the IL-6 concentration and osimertinib C0 (r = 0.423, P = 0.020) or AUC0-24 (r = 0.420, P = 0.021) (Fig. 1). The IL-6 concentration was significantly higher in patients with the IL-6 rs1800796G allele than in those with the C/C genotype (median: 4.43 vs. 1.56 pg/mL, P = 0.024; Fig. 2A). There was a significant difference in the IL-6 concentration among the G/G, G/C, and C/C genotypes (median: 17.14 vs. 3.76 vs. 1.56 pg/mL, P = 0.016; Fig. 2B).
Influence of the IL-6 rs1800796G > C polymorphism on the IL-6 level at 15 days after osimertinib therapy initiation. Comparisons of the IL-6 level (A) between the G allele and C/C genotype and (B) among the G/G, G/C, and C/C genotypes. The boxes represent the interquartile ranges, with the bold horizontal lines and numbers next to the boxes indicating the medians. The ends of each whisker (vertical lines) represent the smallest and largest values that were not outliers. Outliers (circles) are the values between 1.5 and 3 quartiles from the end of the box
The C0 of osimertinib was significantly higher in patients with the IL-6 rs1800796G allele compared with the C/C genotype (272 vs. 170 ng/mL, P = 0.044), whereas there was no significant difference in the AUC0-24 of osimertinib in patients with the IL-6 rs1800796G allele versus C/C genotype, although the AUC0-24 tended to be higher in the former (8470 vs. 6593 ng∙h/mL, P = 0.065).
Kaplan–meier curves for OS and PFS according to the IL-6 rs1800796G > C polymorphism
As shown in Fig. 3A, the median OS times of patients with the IL-6 rs1800796G allele and C/C genotype were 15.1 (95% CI: 13.0–17.2) and 48.9 (95% CI: 36.1–61.8) months, respectively, with a significantly shorter OS in the former than the latter patients (P = 0.005). On the other hand, no significant difference in PFS was observed between patients with the IL-6 rs1800796G allele and those with the C/C genotype (median: 16.5 [95% CI: 9.7–23.4] vs. 22.0 [95% CI: 8.5–35.4] months, P = 0.548; Fig. 3B).
Univariate and multivariate analyses of risk factors for OS
The results of the univariate and multivariate analyses using Cox proportional hazard regression models are shown in Table 2. The following candidate variables were evaluated: sex, age, body weight, BMI, EGFR mutation status, osimertinib therapy, smoking, IL-6 rs1800796G > C polymorphism, IL-6 concentration, and osimertinib C0. Univariate analysis identified only the IL-6 rs1800796G allele as a clinical risk factor for OS (crude HR [95% CI] for G allele vs. C/C: 7.07 [1.49–33.6], P = 0.014). In the Cox proportional hazards regression analysis adjusted for age, sex, and EGFR mutation status, the IL-6 rs1800796G allele remained an independent factor influencing OS in NSCLC patients on osimertinib therapy (adjusted HR for G allele vs. C/C: 6.38 (95% CI: 1.32–30.6), P = 0.021).
Discussion
The present study showed that the IL-6 concentration decreased the metabolic activity of osimertinib, leading to an increased plasma concentration of osimertinib. This is the first study to investigate the relationship between the IL-6 concentration and osimertinib pharmacokinetics in patients with EGFR mutation-positive NSCLC. Furthermore, we identified the G allele of the IL-6 rs1800796G > C polymorphism as an independent prognostic factor for a poor clinical outcome in NSCLC patients treated with osimertinib.
The plasma concentration of osimertinib was affected by the IL-6 concentration. CYP3A4 was more sensitive to reduced enzyme activity induced by IL-6 compared with other CYPs, including CYP1A1, CYP2D6, and CYP3A5, in hepatocytes [23, 24]. In an in vitro study, pre-treatment of cultured cells with IL-6 reportedly decreased the expression of CYP3A4 protein and increased toxicity of gefitinib in a concentration-dependent manner [25]. Therefore, similar to an in vitro report of gefitinib [25], the plasma concentration of osimertinib may be increased in a concentration-dependent manner by IL-6. According to physiologically based pharmacokinetic models, the reductions in CYP3A enzyme activity from baseline under a constant level of IL-6 elevation (50–100 pg/mL) are predicted to be 40–52% in the liver and 41–54% in the gut [26]. In the present study, the degree of increase in the plasma concentration of osimertinib induced by inflammation was not evaluated. Therefore, further study is necessary.
We identified the IL-6 rs1800796G > C polymorphism as an independent prognostic predictor of osimertinib clinical outcomes. The IL-6 protein concentration was higher in patients with lung cancer carrying the IL-6 rs1800796G allele compared with the C/C genotype. This result supports previous reports of a higher IL-6 concentration in subjects with the G allele of the IL-6-634G > C polymorphism (rs1800796) [27, 28]. However, in the present study, the IL-6 concentration on day 15 after beginning osimertinib therapy was not an independent prognostic factor for OS. Our data should be interpreted with caution because the median IL-6 concentration early after osimertinib initiation was only 3.0 pg/mL, which is low. In a previous study, NSCLC patients had a higher median IL-6 concentration compared with healthy subjects (15.16 vs. 1.90 pg/mL) [29]. However, according to other reports, the IL-6 concentration was lower at baseline or before lung cancer progression than during disease progression in patients treated with osimertinib [30, 31]. Furthermore, the IL-6 concentration was well correlated with survival time after chemotherapy initiation, compared with before treatment, in NSCLC patients [32]. In our study, we performed measurements at an early time point after osimertinib initiation (day 15), and the IL-6 concentration is expected to increase after day 15 of treatment initiation. A previous report showed that low BMI (< 20 kg/m2) was associated with a shorter OS in osimertinib-treated patients [33]; however, BMI did not affect OS in our study. In contrast, the IL-6 rs1800796G allele was still a significant predictor of poor OS after adjusting for sex, age, and EGFR mutation status in the multivariate analysis.
The present study did not identify the osimertinib C0 as a risk factor influencing clinical outcomes. No correlation was observed between the AUC at an osimertinib concentration of 20–240 mg and the probability of an objective response to osimertinib [34]. Previous studies showed that dividing patients into two groups based on an osimertinib C0 cut-off of 211 versus 226 ng/mL did not reveal a positive correlation between the C0 and OS; however, those studies did not take into consideration other factors that potentially influence OS [5, 6]. In NSCLC cells, IL-6 promotes metastasis by upregulating TIM-4 via the NF-κB pathway [35]. Furthermore, N-glycosylation-defective IL-6 promotes enhanced lung metastasis and osimertinib resistance via the SRC/YAP/SOX2 signaling pathway [36]. A higher IL-6 concentration was observed in osimertinib-resistant cells [30, 31], but the IL-6 inhibitor tocilizumab partially recovered this increase [37]. In patients treated with 80 mg osimertinib, the osimertinib C0 increased from before to after cancer progression [33]. Therefore, while a higher IL-6 concentration increased the plasma concentration of osimertinib, IL-6 is expected to contribute to drug resistance and tumor progression during long-term EGFR-TKI therapy.
Conclusion
In conclusion, the IL-6 concentration affected the plasma concentration of osimertinib. Therefore, systemic inflammatory conditions may increase osimertinib exposure. Additionally, the IL-6 rs1800796G allele was found to be associated with OS in patients with EGFR mutation-positive NSCLC. IL-6 polymorphisms detected before osimertinib therapy may serve as useful prognostic predictors.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ABC:
-
ATP-binding cassette
- AUC:
-
Area under the plasma concentration-time curve
- BMI:
-
Body mass index
- C0 :
-
Trough plasma concentration
- CI:
-
Confidence interval
- CYP:
-
Cytochrome P450
- EGFR:
-
Epidermal growth factor receptor
- HR:
-
Hazard ratio
- IL:
-
Interleukin
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- TKI:
-
Tyrosine kinase inhibitor
References
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113–125. https://doi.org/10.1056/NEJMoa1713137
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41–50. https://doi.org/10.1056/NEJMoa1913662
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376:629–640. https://doi.org/10.1056/NEJMoa1612674
van der Kleij MBA, Guchelaar NAD, Mathijssen RHJ, Versluis J, Huitema ADR, Koolen SLW, Steeghs N (2023) Therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacokinet 62:1333–1364. https://doi.org/10.1007/s40262-023-01293-9
Yamazaki M, Komizo N, Iihara H, Hirose C, Yanase K, Yamada Y, Endo J, Yamashita S, Ohno Y, Todoroki K, Suzuki A, Hayashi H (2023) Relationship between osimertinib concentration and clinical response in Japanese patients with non-small cell lung cancer. Anticancer Res 43:725–732. https://doi.org/10.21873/anticanres.16211
Agema BC, Veerman GDM, Steendam CMJ, Lanser DAC, Preijers T, van der Leest C, Koch BCP, Dingemans AC, Mathijssen RHJ, Koolen SLW (2022) Improving the tolerability of osimertinib by identifying its toxic limit. Ther Adv Med Oncol 14:17588359221103212. https://doi.org/10.1177/17588359221103212
Planchard D, Brown KH, Kim DW, Kim SW, Ohe Y, Felip E, Leese P, Cantarini M, Vishwanathan K, Jänne PA, Ranson M, Dickinson PA (2016) Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol 77:767–776. https://doi.org/10.1007/s00280-016-2992-z
Heersche N, Veerman GDM, de With M, Bins S, Assaraf YG, Dingemans AC, van Schaik RHN, Mathijssen RHJ, Jansman FGA (2022) Clinical implications of germline variations for treatment outcome and drug resistance for small molecule kinase inhibitors in patients with non-small cell lung cancer. Drug Resist Updat 62:100832. https://doi.org/10.1016/j.drup.2022.100832
TAGRISSO® (osimertinib) tablets, for oral use Initial U.S. approval: 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/208065s030lbl.pdf
Veerman GDM, Boosman RJ, Jebbink M, Oomen-de Hoop E, van der Wekken AJ, Bahce I, Hendriks LEL, Croes S, Steendam CMJ, de Jonge E, Koolen SLW, Steeghs N, van Schaik RHN, Smit EF, Dingemans AC, Huitema ADR, Mathijssen RHJ (2023) Influence of germline variations in drug transporters ABCB1 and ABCG2 on intracerebral osimertinib efficacy in patients with non-small cell lung cancer. EClinicalMedicine 59:101955. https://doi.org/10.1016/j.eclinm.2023.101955
Yokota H, Sato K, Sakamoto S, Okuda Y, Fukuda N, Asano M, Takeda M, Nakayama K, Miura M (2022) Effects of CYP3A4/5 and ABC transporter polymorphisms on osimertinib plasma concentrations in Japanese patients with non-small cell lung cancer. Invest New Drugs 40:1254–1262. https://doi.org/10.1007/s10637-022-01304-9
Stanke-Labesque F, Gautier-Veyret E, Chhun S, Guilhaumou R (2020) Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther 215:107627. https://doi.org/10.1016/j.pharmthera.2020.107627
Coutant DE, Kulanthaivel P, Turner PK, Bell RL, Baldwin J, Wijayawardana SR, Pitou C, Hall SD (2015) Understanding disease-drug interactions in cancer patients: implications for dosing within the therapeutic window. Clin Pharmacol Ther 98:76–86. https://doi.org/10.1002/cpt.128
Robertson GR, Liddle C, Clarke SJ (2008) Inflammation and altered drug clearance in cancer: transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin Pharmacol Ther 83:894–897. https://doi.org/10.1038/clpt.2008.55
Sansone P, Bromberg J (2012) Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 30:1005–1014. https://doi.org/10.1200/jco.2010.31.8907
Guo Y, Xu F, Lu T, Duan Z, Zhang Z (2012) Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 38:904–910. https://doi.org/10.1016/j.ctrv.2012.04.007
Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ (2001) The interleukin-6 -174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J 22:2243–2252. https://doi.org/10.1053/euhj.2001.2678
Koh SJ, Jang Y, Hyun YJ, Park JY, Song YD, Shin KK, Chae JS, Kim BK, Ordovas JM, Lee JH (2009) Interleukin-6 (IL-6) -572C–>G promoter polymorphism is associated with type 2 diabetes risk in Koreans. Clin Endocrinol (Oxf) 70:238–244. https://doi.org/10.1111/j.1365-2265.2008.03315.x
Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao L, Wang J, Li B (2018) Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget 9:12351–12364. https://doi.org/10.18632/oncotarget.24033
Zhou L, Zheng Y, Tian T, Liu K, Wang M, Lin S, Deng Y, Dai C, Xu P, Hao Q, Kang H, Dai Z (2018) Associations of interleukin-6 gene polymorphisms with cancer risk: evidence based on 49,408 cancer cases and 61,790 controls. Gene 670:136–147. https://doi.org/10.1016/j.gene.2018.05.104
Buraczynska M, Jozwiak L, Ksiazek P, Borowicz E, Mierzicki P (2007) Interleukin-6 gene polymorphism and faster progression to end-stage renal failure in chronic glomerulonephritis. Transl Res 150:101–105. https://doi.org/10.1016/j.trsl.2007.03.003
Zhu RM, Lin W, Zhang W, Ren JT, Su Y, He JR, Lin Y, Su FX, Xie XM, Tang LY, Ren ZF (2017) Modification effects of genetic polymorphisms in FTO, IL-6, and HSPD1 on the associations of diabetes with breast cancer risk and survival. PLoS ONE 7:12:e0178850. https://doi.org/10.1371/journal.pone.0178850
Aitken AE, Morgan ET (2007) Gene-specific effects of inflammatory cytokines on cytochrome P450 2 C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 35:1687–1693. https://doi.org/10.1124/dmd.107.015511
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG (2011) Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos 39:1415–1422. https://doi.org/10.1124/dmd.111.038679
Mimura H, Kobayashi K, Xu L, Hashimoto M, Ejiri Y, Hosoda M, Chiba K (2015) Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet 30:105–110. https://doi.org/10.1016/j.dmpk.2014.09.004
Chen Y, Ma F, Jones N, Deng R, Li C, Li CC (2024) Assessment of CYP3A-mediated drug interaction via cytokine (IL-6) elevation for mosunetuzumab using physiologically-based Pharmacokinetic modeling. CPT Pharmacometrics Syst Pharmacol 13:234–246. https://doi.org/10.1002/psp4.13073
Kitamura A, Hasegawa G, Obayashi H, Kamiuchi K, Ishii M, Yano M, Tanaka T, Yamaguchi M, Shigeta H, Ogata M, Nakamura N, Yoshikawa T (2002) Interleukin-6 polymorphism (-634 C/G) in the promotor region and the progression of diabetic nephropathy in type 2 diabetes. Diabet Med 19:1000–1005. https://doi.org/10.1046/j.1464-5491.2002.00844.x
Gao SP, Liang S, Pan M, Sun RL, Chen C, Luan H, Jiang MH (2014) Interleukin-6 genotypes and serum levels in Chinese Hui population. Int J Clin Exp Med 7:2851–2857
Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, Longatto-Filho A (2017) High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE 12:e0181125. https://doi.org/10.1371/journal.pone.0181125
Li L, Li Z, Lu C, Li J, Zhang K, Lin C, Tang X, Liu Z, Zhang Y, Han R, Wang Y, Feng M, Zhuang Y, Hu C, He Y (2022) Ibrutinib reverses IL-6-induced osimertinib resistance through Inhibition of laminin Α5/FAK signaling. Commun Biol 5:155. https://doi.org/10.1038/s42003-022-03111-7
Patel SA, Nilsson MB, Yang Y, Le X, Tran HT, Elamin YY, Yu X, Zhang F, Poteete A, Ren X, Shen L, Wang J, Moghaddam SJ, Cascone T, Curran M, Gibbons DL, Heymach JV (2023) IL-6 mediates suppression of T- and NK-cell function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin Cancer Res 29:1292–1304. https://doi.org/10.1158/1078-0432.Ccr-22-3379
Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, Huang MS, Chen HL, Li YJ, Yang PC, Chen CJ, Hsiung CA, Su WC (2013) Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer 132:1977–1985. https://doi.org/10.1002/ijc.27892
van Veelen A, Veerman GDM, Verschueren MV, Gulikers JL, Steendam CMJ, Brouns A, Dursun S, Paats MS, Tjan-Heijnen VCG, van der Leest C, Dingemans AC, Mathijssen RHJ, van de Garde EMW, Souverein P, Driessen JHM, Hendriks LEL, van Geel R, Croes S (2024) Exploring the impact of patient-specific clinical features on osimertinib effectiveness in a real-world cohort of patients with EGFR mutated non-small cell lung cancer. Int J Cancer 154:332–342. https://doi.org/10.1002/ijc.34742
Brown K, Comisar C, Witjes H, Maringwa J, de Greef R, Vishwanathan K, Cantarini M, Cox E (2017) Population pharmacokinetics and exposure-response of osimertinib in patients with non-small cell lung cancer. Br J Clin Pharmacol 83:1216–1226. https://doi.org/10.1111/bcp.13223
Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, Chen S, Li C, Yue X, Liang X, Ma C, Xu L, Gao L (2020) IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-κB. Cell Prolif 53:e12776. https://doi.org/10.1111/cpr.12776
Hung CH, Wu SY, Yao CD, Yeh HH, Lin CC, Chu CY, Huang TY, Shen MR, Lin CH, Su WC (2024) Defective N-glycosylation of IL-6 induces metastasis and tyrosine kinase inhibitor resistance in lung cancer. Nat Commun 15:7885. https://doi.org/10.1038/s41467-024-51831-7
Tan J, Zhu L, Shi J, Zhang J, Kuang J, Guo Q, Zhu X, Chen Y, Zhou C, Gao X (2024) Evaluation of drug resistance for EGFR-TKIs in lung cancer via multicellular lung-on-a-chip. Eur J Pharm Sci 199:106805. https://doi.org/10.1016/j.ejps.2024.106805
Funding
Open Access funding provided by Akita University.
This research was funded by grants (no. 24H02625 and 24K09934) from the Japan Society for the Promotion of Science, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
H.Y. and M.M. contributed to the study conception and design. K.S., S.S., Y.O., M.T, and K.N. were responsible for patient enrollment and were involved in data acquisition. H.Y. performed the genotyping. M.M. analyzed the plasma concentrations of osimertinib. H.Y. and Y.A. analyzed IL-6 concentrations. H.Y. and M.M. were responsible for the statistical analysis. The first draft of the manuscript was written by H.Y., Y.A., and M.M. K.S., S.S., Y.O., M.T., and K.N. helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Akita University School of Medicine (approval number: 2826).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yokota, H., Sato, K., Sakamoto, S. et al. Influence of interleukin-6 on the pharmacokinetics and pharmacodynamics of osimertinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 95, 49 (2025). https://doi.org/10.1007/s00280-025-04772-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00280-025-04772-x