Abstract
Background
Different methods for induction and monitoring of urethral sphincter deficiency were explored in a large animal model.
Methods
Sphincter deficiency was established in female pigs by dilatation and cauterization, and amount and frequencies of voiding were monitored and explored by pad test. Sphincteric closure pressures were recorded prior to and immediately after treatment of each animal, and on day 21 by two techniques: standard urethral pressure profilometry (s-UPP) and high-definition urethral pressure profilometry (HD-UPP). Tissue samples of the urethrae were analyzed by histochemistry (AZAN- and Sirius Red staining) and by immunohistochemistry detecting desmin and fast-myosin to depict muscular tissues.
Results
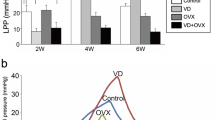
After 3 weeks of observation animals treated by dilatation plus electrocautery presented with sphincter deficiency: measurements by both, s-UPP and HD-UPP demonstrated the maximal closure pressure reduced to baseline levels and a diminished area under the curve. Histological analyses documented, that dilatation yielded a pitted connective tissue and cauterization lead to muscle damage. Animals treated by either dilatation only or proximal injury only recovered within 3 weeks. By pad test no significant differences between untreated and treated animals or between the differently treated groups were recorded.
Conclusion
Significant urethral sphincter deficiency can be induced in female pigs by a combination of urethral dilatation and distal electrocautery. Sphincter deficiency can be measured by standard and high-definition urethral pressure profilometry. It was maintained over 21 days after induction and correlated with visible changes in the tissue structure of the distal urethra.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P (2011) Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 108:1132–1138
Luber KM (2004) The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol 6:S3–S9
Loughlin KR, Prasad MM (2010) Post-prostatectomy urinary incontinence: a confluence of 3 factors. J Urol 183:871–877
Sangsawang B, Sangsawang N (2013) Stress urinary incontinence in pregnant women: a review of prevalence, pathophysiology, and treatment. Int Urogynecol J 24:901–912
Sievert KD, Selent-Stier C, Wiedemann J, Greiner TO, Amend B, Stenzl A, Feil G, Seibold J (2012) Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol 187:1101–1109
Amend B, Kelp A, Vaegler M, Klünder M, Frajs V, Klein G, Sievert KD, Sawodny O, Stenzl A, Aicher WK (2017) Precise injection of human mesenchymal stromal cells in the urethral sphincter complex of Göttingen minipigs without unspecific bulking effects. Neurourol Urodyn. doi:10.1002/nau.23182
Aufderklamm S, Vaegler M, Kelp A, Maurer S, Gustafsson L, Mundhenk J, Busch S, Daum L, Stenzl A, Amend B, Sievert K-D (2017) Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: first results in a xenograft minipig model. World J Urol 35(7):1125–1132. doi:10.1007/s00345-016-1959-3
Köhn F, Sharafi AR, Simianer H (2007) Modeling the growth of the Göttingen minipig. J Anim Sci 85:84–92
McAnulty PA, Dayan AD, Ganderup NC, Hastings KL (2012) The minipig in biomedical research. CRC Press, Boca Raton
Zini L, Lecoeur C, Swieb S, Combrisson H, Delmas V, Gherardi R, Abbou C, Chopin D, Yiou R (2006) The striated urethral sphincter of the pig shows morphological and functional characteristics essential for the evaluation of treatments for sphincter insufficiency. J Urol 176:2729–2735
Burdzinska A, Crayton R, Dybowski B, Koperski L, Idziak M, Fabisiak M, Paczek L, Radziszewski P (2012) Urethral distension as a novel method to simulate sphincter insufficiency in the porcine animal model. Int J Urol 19:676–682
Lecoeur C, Swieb S, Zini L, Riviere C, Combrisson HLN, Gherardi R, Abbou C, Yiou R (2007) Intraurethral transfer of satellite cells by myofiber implants results in the formation of innervated myotubes exerting tonic contractions. J Urol 178:332–337
Rivière C, Lecoeur C, Wilhelm C, Péchoux C, Combrisson H, Yiou R, Gazeau F (2009) The MRI assessment of intraurethrally delivered muscle precursor cells using anionic magnetic nanoparticles. Biomaterials 30:6920–6928
Burdzinska A, Crayton R, Dybowski B, Idziak M, Gala K, Radziszewski P, Paczek L (2013) The effect of endoscopic administration of autologous porcine muscle-derived cells into the urethral sphincter. Urology 82(743):e741–e748
Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Plattner R, Klima GN, Strasser H (2008) Corrigendum to: functional and histological changes after myoblast injections in the porcine rhabdosphincter [Eur Urol 2007;52:1736–43]. Eur Urol 54:1208
Klunder M, Amend B, Vaegler M, Kelp A, Feuer R, Sievert KD, Stenzl A, Sawodny O, Ederer M (2016) High definition urethral pressure profilometry: evaluating a novel microtip catheter. Neurourol Urodyn 35:888–894
Krhut J, Zachoval R, Smith PP, Rosier PFWM, Valansky L, Martan A, Zvara P (2014) Pad weight testing in the evaluation of urinary incontinence. Neurourol Urodyn 33:507–510
Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, Chancellor MB (2004) A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn 23:166–171
Klunder M, Feuer R, Amend B, Kelp A, Stenzl A, Sievert KD, Sawodny O, Ederer M (2015) Eliminating pulse-induced artifacts in urethral pressure data. Conf Proc IEEE Eng Med Biol Soc 2015:2779–2783
Klunder M, Sawodny O, Amend B, Ederer M, Kelp A, Sievert KD, Stenzl A, Feuer R (2016) Signal processing in urodynamics: towards high definition urethral pressure profilometry. Biomed Eng Online 15:31
Herrera-Imbroda B, Lara MF, Izeta A, Sievert KD, Hart ML (2015) Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv Drug Deliv Rev 82–83:106–116
Phull HS, Pan HQ, Butler RS, Hansel DE, Damaser MS (2011) Vulnerability of continence structures to injury by simulated childbirth. Am J Physiol Renal Physiol 301:F641–F649
Boncher N, Vricella G, Kavran M, Xiao N, Hijaz A (2012) Setting a new standard: updating the vaginal distention translational model for stress urinary incontinence. Neurourol Urodyn 31:190–194
Eberli D, Andersson KE, Yoo JJ, Atala A (2009) A canine model of irreversible urethral sphincter insufficiency. BJU Int 103:248–253
Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL (2006) External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn 25:388–396
Badra S, Andersson KE, Dean A, Mourad S, Williams JK (2013) A nonhuman primate model of stable urinary sphincter deficiency. J Urol 189:1967–1974
Yiou R, Yoo JJ, Atala A (2003) Restoration of functional motor units in a rat model of sphincter injury by muscle precursor cell autografts. Transplantation 76:1053–1060
Lim JJ, Jang JB, Kim JY, Moon SH, Lee CN, Lee KJ (2010) Human umbilical cord blood mononuclear cell transplantation in rats with intrinsic sphincter deficiency. J Korean Med Sci 25:663–670
Hong SH, Piao S, Kim IG, Lee JY, Cho HJ, Kim SW, Hwang TK, Lee JY (2013) Comparison of three types of stress urinary incontinence rat models: electrocauterization, pudendal denervation, and vaginal distension. Urology 81(465):e461–e466
Acknowledgements
Funding was provided by DFG (Grant no. KFO273), BMBF (Grant no. Multimorb-INKO) and Prostatastiftung (Grant no. ø).
Author information
Authors and Affiliations
Contributions
AK: protocol development, data collection/management/analysis, manuscript writing/editing. AA: data collection, manuscript writing. BA: protocol development, data collection, manuscript editing. MK: data collection/analysis, manuscript writing. PR: data management, manuscript editing. OS: project development, data management, manuscript editing. AS: project development, protocol development, manuscript editing. WKA: project development, data collection/management/analysis, manuscript writing/editing
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to be reported for any of the authors for this animal study.
Ethical approval
The pre-clinical animal study was conducted after approval by the State of Baden-Württemberg Animal Welfare Authorities (Regierungspräsidium Tübingen, file # CU1/15). Human participants or samples from human donors were not included in this study at all.
Rights and permissions
About this article
Cite this article
Kelp, A., Albrecht, A., Amend, B. et al. Establishing and monitoring of urethral sphincter deficiency in a large animal model. World J Urol 35, 1977–1986 (2017). https://doi.org/10.1007/s00345-017-2088-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2088-3