Abstract
Purpose
Cholesterol regulation is essential to maintain pulmonary homeostasis. Studies suggest that increased high-density lipoprotein cholesterol (HDL-C) levels correlate with better lung function. However, the longitudinal association of HDL-C with lung function remains unknown. We aimed to analyze the long-term correlation of HDL-C with lung function decline in a population-based cohort study.
Methods
We included 7,652 participants from a prospective community-based cohort study in South Korea. Participants were categorized into five trajectory groups based on repeated HDL-C measurements. Generalized linear mixed models with random intercepts and slopes were used to examine the longitudinal relationship between HDL-C levels and lung function decline within these groups.
Results
In the five HDL-C trajectory group analyses, the very low HDL-C trajectory group (Group 1) showed faster declines in forced vital capacity (FVC) (−3.1 mL/year) and forced expiratory volume in one second (FEV1) (−3.1 mL/year) than the middle HDL-C group (Group 3, the reference group) did. The low HDL-C trajectory group (Group 2) also exhibited faster FVC (−1.5 mL/year) and FEV1 (−1.7 mL/year) declines than the middle HDL-C group; however, the estimated difference was smaller than that in Group 1. Faster lung function decline in the low HDL-C trajectory group was consistently observed even when the population was analyzed using three- or four-HDL-C trajectory groups instead of five.
Conclusion
Participants in the low HDL-C trajectory groups experienced a more rapid lung function decline over time than the reference groups, suggesting a negative longitudinal association between HDL-C and lung function decline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Background
Lung function naturally declines with age due to several anatomical, physiological, and immunological changes within the respiratory system [1, 2]. Individuals undergo various lung function trajectories throughout their lifetimes [2].
Pulmonary surfactants are composed of various lipids and proteins that are crucial for maintaining lung homeostasis [3,4,5]. Cholesterol is a major lipid component of pulmonary surfactants and is tightly regulated through plasma lipoproteins and lipid efflux transporters [6,7,8]. However, dysregulation of pulmonary cholesterol results in deterioration of surfactant function, leading to dysfunctional immune responses, increased lung inflammation, and mechanical stress-induced lung injury risk [4, 9, 10].
High-density lipoprotein (HDL) is important for maintaining cholesterol levels because it performs reverse cholesterol transport and delivers it to the liver [11]. In the lung, cholesterol is mainly imported from plasma lipoproteins and cleared by plasma HDL, thereby maintaining healthy cholesterol levels [7, 8, 12].
However, the clinical significance of HDL-cholesterol (HDL-C) for lung function remains unclear in the general population. Several cross-sectional studies have shown that high HDL-C levels correlate with higher lung function in adult populations [13,14,15,16]. Since lung function typically peaks at 20–25 years of age and deteriorates thereafter, HDL might play a protective role in age-related lung function decline [2, 15]. However, to date, no study has proven this hypothesis.
To determine whether HDL-C levels show a long-term correlation with lung function deterioration over time, we conducted a comprehensive analysis of the data from a 12-year population-based cohort study.
Methods
Study Design and Population
We analyzed data from a prospective population-based cohort study conducted in South Korea, which is a component of the Korean Genome and Epidemiology Study (KoGES) supported by the National Genome Research Institute [17]. The participants, adults aged 40–69 years who had lived for more than 6 months in either Ansan (urban) or Ansung (rural) areas, were recruited from 2001 to 2002 [17, 18]. Follow-up evaluations were conducted biennially, with the longest follow-up period spanning 12 years, up to the sixth follow-up [17]. During both the baseline and biennial visits, comprehensive data were collected, including socio-demographic status, lifestyle characteristics, medical history, disease history, anthropometric and biochemical measurements, and pulmonary function test results [17].
Of the 10,030 participants at baseline, we excluded those who lacked initial spirometry data, height or weight measurements, smoking or drinking history, or lipid profiles. Individuals with a previous diagnosis of chronic lung disease or asthma were also excluded. Additionally, participants without valid spirometry results from the first to sixth follow-up examinations were excluded (Fig. 1).
High-Density Lipoprotein Cholesterol Measurements
During the study, participants provided fasting blood samples for biochemical measurement; these were collected in two ethylenediaminetetraacetic acid tubes and a serum separator tube [17]. A comprehensive metabolic panel was performed using a Hitachi 747 chemistry analyzer (Hitachi Ltd., Tokyo, Japan) to measure HDL-C [19].
Spirometry
Lung function was measured using a spirometer (Vmax-229, Sensor-Medics Corporation, Yorba Linda, CA, USA) during the baseline and follow-up visits [20]. Spirometry was performed by well-trained pulmonary technologists following the standards set by the American Thoracic Society [21]. The forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were measured using a standardized protocol. We used the Global Lung Function Initiative 2012 equations to calculate the predicted values for FVC and FEV1 [22].
Statistical Analyses
We conducted a one-way analysis of variance to compare continuous variables. Pearson’s chi-square or Fisher’s exact test was used to compare categorical variables among the different HDL-C groups.
To classify individuals based on the longitudinal change in HDL-C over time, we employed group-based trajectory modeling using a finite mixture modeling approach with the stepFlexmix function from the “flexmix” package in R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria) [23, 24]. Using group-based trajectory modeling, we categorized the participants into distinct groups based on similar patterns of HDL-C change over time [24]. To determine the optimal HDL-C trajectory groups, we conducted multiple iterations of the model with three, four, and five groups [24]. For each iteration, we compared the model fit using both the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to select a representative model (Table S1). The chosen model revealed distinct HDL-C trajectories in all the groups (Fig. 2, Figure S1). The five-group model demonstrated the best statistical fit and was, therefore, selected for further analysis.
HDL-C Trajectories Based on Age for the Model with Five Groups. Each line represents the estimated HDL-C trajectory for one of the five HDL-C trajectory groups across ages. Shaded areas indicate 95% confidence intervals. Single dots represent individual data points. HDL-C levels are measured in mg/dL. HDL-C high-density lipoprotein cholesterol
Generalized linear mixed models were used to test the longitudinal association of HDL-C with lung function decline [20, 25]. The models included random intercepts and slopes with unstructured covariance matrices to account for repeated measures within participants [26]. For our analyses, we constructed a series of progressively adjusted models. Model 1 was adjusted for essential physiological parameters (age, sex, and height) that determine lung function. Model 2 expanded on Model 1 by adding BMI, BMI2, residential area (rural/urban), and smoking exposure as factors that may affect lung function. Based on previous studies showing that both low and high BMI are associated with reduced lung volume, we included both the linear (BMI) and quadratic (BMI2) terms of BMI in the adjustment models [15, 27, 28]. Our main analysis used Model 3, which further expanded on Model 2 by adding factors related to lipid metabolism (self-reported history of dyslipidemia and alcohol consumption), representing our fully adjusted model. Model 4 was similar to Model 3 but excluded self-reported dyslipidemia as a covariate due to the limited number of participants with this condition at baseline [29].
Age was centered at 40 years for the analysis, as the cohort data were collected from individuals aged 40 years and older [27]. The estimates of the interaction terms between the centered age and HDL-C trajectory groups were interpreted as the differences in annual lung function changes [30, 31]. All statistical analyses except HDL-C trajectory modeling were performed using SAS software (version 9.4; SAS Institute Inc., Cary, North Carolina, USA). Statistical significance was set at P < 0.05.
Ethical Considerations
The study protocol followed the ethical guidelines outlined in the Declaration of Helsinki. All participants provided informed consent at recruitment and subsequent visits, with initial ethical approval from the Korea Centers for Disease Control and Prevention [17]. Our research team submitted our study protocol and Institutional Review Board approval (IRB number: 4-2023-0156) from Severance Hospital to the National Institute of Health, who reviewed these documents before providing us with the de-identified data for analysis.
Results
Baseline Characteristics
Table 1 presents the baseline characteristics of the study population, stratified by the five HDL-C trajectories. Of the participants, 48.2% were men, and the mean age was 51.6 ± 8.7 years at baseline. The mean baseline HDL-C levels for each trajectory group, ranging from lowest to highest, were 32.7 mg/dL, 38.9 mg/dL, 45.8 mg/dL, 53.6 mg/dL, and 65.3 mg/dL, respectively.
Association Between HDL-C and Lung Function by Cross-Sectional Analysis
Table 2 shows the association between HDL-C levels and lung function in the study cohort. For each standard deviation increase in HDL-C, FVC was 7.5 mL higher and FEV1 was 4.7 mL higher. The percentage predicted FVC and FEV1 increased by 0.19% and 0.13%, respectively. No statistically significant relationship was observed between HDL-C levels and the FEV1/FVC ratio.
Longitudinal Association Between HDL-C and Lung Function Decline
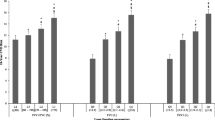
The adjusted differences in lung function decline based on the five HDL-C trajectory groups are shown in Fig. 3. The middle HDL-C group (Group 3) served as a reference group to compare the differences in lung function decline between the HDL-C trajectory groups. The very-low HDL-C group (Group 1) showed a faster decline in FVC (3.1 mL/year) and FEV1 (3.1 mL/year) than the reference group did. The low HDL-C group (Group 2) exhibited a faster decline rate of 1.5 mL/year in FVC and 1.7 mL/year in FEV1 relative to the reference group but a smaller estimated difference than the very-low HDL-C group did. The high and very-high HDL-C groups (Groups 4 and 5) did not demonstrate differences in annual lung function change rates compared to the reference group.
Estimated Differences in Annual Decline Rates of FVC and FEV1 According to Five HDL-C Trajectory Groups. Estimated differences in the annual decline rates of (A) FVC and (B) FEV1 changes in the five-group HDL-C trajectory model. Results from generalized linear mixed models adjusted for sex, centered age, height, BMI, BMI2, residential area, smoking exposure (pack-years), history of dyslipidemia, and alcohol consumption. Estimates represent the difference in annual changes compared to the reference group (Group 3). The interaction term (centered age × HDL-C trajectory group) was used to interpret the differences in the annual lung function changes. Negative values indicate a faster decline compared with the reference group. BMI body mass index, CI confidence interval, FEV1 forced expiratory volume in one second, FVC forced vital capacity, HDL-C high-density lipoprotein cholesterol, Ref Reference
Based on the observation that the HDL-C trajectories exhibited largely parallel patterns over time, we extended our analysis to address whether initial HDL-C levels are associated with subsequent lung function decline. Intersection points from normalized distribution curves of each trajectory group were identified and used as new cutoff values (36.0, 42.8, 50.5, 60.4 mg/dL) (Figure S2A). Using these cutoffs, participants were categorized into five new groups based on their baseline HDL-C. Approximately 73.1% of individuals whose baseline HDL-C levels were below 36.0 mg/dL belonged to the very low HDL-C trajectory group (Figure S2B) and showed faster decline in FVC and FEV1 compared to the reference group (Figure S3A, B).
Sensitivity Analyses
The three- and four-group HDL-C trajectories were analyzed further to explore the longitudinal association between HDL-C and lung function decline. The baseline characteristics of the three and four HDL-C trajectory groups are shown in Tables S2 and S3. In the three-group HDL-C trajectory model, the low HDL-C group (Group 1) showed a significantly faster decline in both FVC (−2.5 mL/year) and FEV1 (−1.9 mL/year) than the reference group did (Group 2) (Figure S4A and S4B). In the four-group HDL-C trajectories, the low HDL-C trajectory group (Group 1) demonstrated significantly faster decline rates in both FVC (−1.8 mL/year) and FEV1 (−1.8 mL/year) (Figures S4C and S4D) than the reference group (Group 2) did.
Other sensitivity analyses using differently adjusted models are shown in Table S4 (FVC) and Table S5 (FEV1). In Models 1, 2, and 4, we observed similar patterns to our main analysis (Model 3), with the very-low HDL-C group consistently showing significantly faster decline rates in both FVC and FEV1 compared to the reference group. Complete results for the three-, four-, and five-group HDL-C trajectory models are presented in Tables S4 and S5.
Discussion
To our knowledge, our study represents the first comprehensive analysis of the long-term correlation of HDL-C levels with lung function decline in a population-based cohort. To account for this association, we categorized the participants into different HDL-C trajectory groups based on their repeated HDL-C measurements. This approach revealed that individuals in the low HDL-C trajectory group exhibited a faster decline in lung function. Notably, these results were consistent across the three-, four-, and five-group trajectory models, thus strengthening the robustness of our findings. The consistency of our findings across different covariate combinations in sensitivity analyses further strengthens the robustness of the observed relationship between HDL-C trajectories and lung function decline. Since the mean HDL-C levels in each trajectory group seemed relatively stable, we explored whether baseline HDL-C could predict lung function decline. Individuals with low initial HDL-C (< 36.0 mg/dL) tended to belong to the very low HDL-C trajectory group and showed faster decline in FVC and FEV1 than the reference group, suggesting that low initial HDL-C levels are also associated with rapid lung function decline. Comprehensively, our analysis indicates that low HDL-C trajectories are associated with accelerated lung function decline, suggesting potential relevance of HDL-C levels to pulmonary health throughout aging.
Lung function naturally declines with aging, and individuals exhibit diverse lung function trajectories throughout their lifetimes [1]. Recent studies have highlighted various biological mechanisms contributing to lung function decline, including cellular senescence, oxidative stress, dysregulated inflammatory responses, and extracellular matrix remodeling [2, 32,33,34]. Despite these findings, there are currently no established parameters that represent lung function decline in clinical practice [2]. Given that lung function tests are not routinely performed in the general population, identifying clinically applicable markers for rapid lung function decline is crucial for early intervention and prevention strategies. Based on our analysis, HDL-C might serve as an indicator of lung function decline rate.
Previous cross-sectional studies have demonstrated that high HDL-C levels correlate with higher FVC and FEV1 in adults [13,14,15,16]. Most evidence, including our findings, supports a correlation between low HDL-C levels and reduced lung function, particularly in the context of aging.
Although there are limited known clinical roles of HDL-C in the lungs, potential molecular biological mechanisms can be hypothesized based on current evidence. Pulmonary surfactants play a crucial role in reducing surface tension in the alveolar space and modulating immune and inflammatory responses [3, 5]. Pulmonary cholesterol, which is generally derived from plasma lipoproteins, is an important component of this surfactant [35, 36]. However, dysregulated and abnormally elevated cholesterol levels could impair the self-assembly of pulmonary surfactant, leading to functional failure [37]. Pulmonary cholesterol is thought to be cleared back into the plasma through interactions between plasma HDL-C and the lipid efflux transporter [7, 8, 12, 38]. Therefore, from a biological mechanism perspective, HDL-C is believed to play a crucial role in regulating proper cholesterol levels and maintaining lung health.
The strength of our study lies in having used data from a long-term prospective cohort study conducted over 12 years, which provided a substantial sample size and follow-up duration. This dataset allowed us to effectively demonstrate the long-term correlation of HDL-C levels with lung function.
Given that an individual’s HDL-C levels could change over time, we employed group-based trajectory modeling to categorize participants into different groups reflecting distinct HDL-C trends. Notably, regardless of the number of trajectory groups independently categorized, participants in the low HDL-C trajectory groups consistently showed a faster decline in lung function than those in the other groups did. This consistency across categorizations strengthens the robustness of our conclusions.
Moreover, the observed longitudinal correlation of a low HDL-C trajectory with declining lung function offers new insights into the complex relationship between lipid metabolism and respiratory health. By employing generalized linear mixed models to analyze this relationship over time, we could advance beyond previous cross-sectional observations and demonstrate the long-term correlation of HDL-C trajectories with lung function decline.
Furthermore, the original cohort study applied strict quality control measures to the spirometry results. Participants who failed or refused to adequately perform spirometry were identified and recorded from the first follow-up and their data were excluded from the analysis, enhancing the reliability of the lung function data.
Our study has certain limitations. First, we could not establish a causal relationship. This limitation stems from the observational nature of our study, with the inherent possibility of unmeasured confounding factors. Nevertheless, our results provide important epidemiological insights into the longitudinal association between HDL-C levels and lung function.
Second, the data used in this study were derived from a Korean community-based cohort, which limits the ethnic diversity. This homogeneity may affect the generalizability of our findings to other ethnic groups. However, Lee et al. previously reported a positive association between HDL-C levels and lung function in both American and Korean populations, suggesting this relationship may extend across different ethnicities [15]. Further studies encompassing diverse ethnicities should be conducted to consolidate the longitudinal correlation of HDL-C levels with lung function decline.
Third, a potential weakness of our study is the inability to directly assess dyslipidemia diagnosis and treatment status, relying instead on questionnaire responses. While it is clinically appropriate to consider dyslipidemia as a confounding factor for serum HDL-C level, it is important to note that the baseline survey of the cohort was conducted in 2001–2002, when both the diagnosis and treatment rates of dyslipidemia were relatively low in Korea [29]. This could potentially introduce bias due to the small proportion of diagnosed individuals at baseline. However, additional analyses excluding self-reported dyslipidemia produced similar results, suggesting that this limitation does not affect our main findings.
Conclusion
Our longitudinal analysis revealed that individuals with low HDL-C levels experience faster decline in lung function over time. These findings suggest that the HDL-C trajectory may serve as a predictor of long-term respiratory health in the general population. Further mechanistic studies are required to elucidate the underlying biological processes linking HDL-C levels to a decline in lung function.
Data Availability
The datasets used and analysed during the current study are available from the Ansung-Ansan cohort database (https://nih.go.kr/ko/main/contents.do?menuNo=300577) on reasonable request.
References
Sharma G, Goodwin J (2006) Effect of aging on respiratory system physiology and immunology. Clin Interv Aging 1(3):253–260
Agusti A, Faner R (2019) Lung function trajectories in health and disease. Lancet Respir Med 7(4):358–364
Han S, Mallampalli RK (2015) The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc 12(5):765–774
Vockeroth D et al (2010) Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 298(1):L117–L125
Chroneos ZC, Sever-Chroneos Z, Shepherd VL (2010) Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem 25(1):13–26
Fessler MB, Summer RS (2016) Surfactant lipids at the host-environment interface. Metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol 54(5):624–635
Baldán A et al (2006) Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem 281(39):29401–29410
Bates SR et al (2005) Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol 289(6):L980–L989
Schmidt R et al (2004) Changes in pulmonary surfactant function and composition in bleomycin-induced pneumonitis and fibrosis. Toxicol Appl Pharmacol 195(2):218–231
Hiansen JQ et al (2015) Cholesterol-mediated surfactant dysfunction is mitigated by surfactant protein A. Biochim Biophys Acta 1848(3):813–820
Azzam KM, Fessler MB (2012) Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab 23(4):169–178
Baldán A et al (2008) Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol 180(5):3560–3568
Burkart KM et al (2014) APOM and high-density lipoprotein cholesterol are associated with lung function and per cent emphysema. Eur Respir J 43(4):1003–1017
Cirillo DJ, Agrawal Y, Cassano PA (2002) Lipids and pulmonary function in the third national health and nutrition examination survey. Am J Epidemiol 155(9):842–848
Lee C et al (2023) Association between serum high-density lipoprotein cholesterol and lung function in adults: three cross-sectional studies from US and Korea National Health and Nutrition Examination Survey. BMJ Open Respir Res. https://doi.org/10.1136/bmjresp-2023-001792
Barochia AV et al (2015) Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med 191(9):990–1000
Kim Y, Han BG (2017) Cohort profile: the korean genome and epidemiology study (KoGES) consortium. Int J Epidemiol 46(2):e20
Lee JH et al (2023) Association of non-high-density lipoprotein cholesterol trajectories with the development of non-alcoholic fatty liver disease: an epidemiological and genome-wide association study. J Transl Med 21(1):435
Kim BS et al (2019) The relationship between decreased pulmonary function and atrial fibrillation in general population: findings from Ansung-Ansan cohort of the Korean Genome and Epidemiology Study. J Cardiol 74(6):488–493
Leem AY et al (2019) Longitudinal decline in lung function: a community-based cohort study in Korea. Sci Rep 9(1):13614
(1995) Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 152(3): 1107–1136
Quanjer PH et al (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343
Grün B, Leisch F (2008) FlexMix version 2: finite mixtures with concomitant variables and varying and constant parameters. J Stat Softw 28(4):1–35
Nagin DS, Odgers CL (2010) Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109–138
Xia Y, Sun J (2023) Introduction to generalized linear mixed models. Bioinformatic and statistical analysis of microbiome data: from raw sequences to advanced modeling with QIIME 2 and R. Springer International Publishing, Cham, pp 587–613
Kim JS et al (2024) Associations of plasma omega-3 fatty acids with progression and survival in pulmonary fibrosis. Chest 165(3):621–631
Jones RL, Nzekwu MM (2006) The effects of body mass index on lung volumes. Chest 130(3):827–833
Do JG et al (2019) Association between underweight and pulmonary function in 282,135 healthy adults: a cross-sectional study in Korean population. Sci Rep 9(1):14308
Cho SMJ et al (2021) Dyslipidemia fact sheets in Korea 2020: an analysis of nationwide population-based data. J Lipid Atheroscler 10(2):202–209
Oelsner EC et al (2020) Lung function decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir Med 8(1):34–44
Oelsner EC et al (2019) Albuminuria, lung function decline, and risk of incident chronic obstructive pulmonary disease. The NHLBI pooled cohorts study. Am J Respir Crit Care Med 199(3):321–332
Mercado N, Ito K, Barnes PJ (2015) Accelerated ageing of the lung in COPD: new concepts. Thorax 70(5):482–489
Zhang Z et al (2018) Molecular pathogenesis in chronic obstructive pulmonary disease and therapeutic potential by targeting AMP-activated protein kinase. J Cell Physiol 233(3):1999–2006
Salvi SS, Barnes PJ (2009) Chronic obstructive pulmonary disease in non-smokers. Lancet 374(9691):733–743
Turley SD, Andersen JM, Dietschy JM (1981) Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res 22(4):551–569
Gowdy KM, Fessler MB (2013) Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther 26(4):430–437
Leonenko Z et al (2007) An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys J 93(2):674–683
Cruz D et al (2008) Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest 118(8):2917–2928
Acknowledgements
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 6635-302), National Institute of Health, Korea Disease Control and Prevention Agency, Republic of Korea. We thank Editage (www.editage.co.kr) for the English language editing.
Funding
This work was supported by the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number 2024-31-0439 to B. Yoo); the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: 2024-31-0644 to C. Lee); the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT) (grant number NRF-2022R1A2C2003438 to S. H. Bae); and the Yonsei University College of Medicine (grant number 6-2022-0171 to S. H. Bae). The sponsors had no role in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y. S. Kim and C. Lee Data curation: S. H. Jung, Y. S. Kim, and C. Lee Formal analysis: B. Yoo and S. H. Jung Funding acquisition: B. Yoo, S. H. Bae, and C. Lee Methodology: B. Yoo, Y. S. Kim, and C. Lee Supervision: S. H. Bae, Y. S. Kim, and C. Lee Validation: B. Yoo, S. H. Jung, S. H. Bae, Y. S. Kim, and C. Lee Visualization: B. Yoo Writing – original draft: B. Yoo and S. H. Jung Writing – review & editing: B. Yoo, S. H. Jung, S. H. Bae, Y. S. Kim, and C. Lee.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Severance Hospital (IRB number: 4-2023-0156).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
408_2025_809_MOESM1_ESM.tif
Supplementary file1 (TIF 1756 KB)—Figure S1. HDL-C Trajectories Based on Age for the Models with Three and Four Groups. Each line represents the estimated HDL-C trajectory for (A) three trajectory groups and (B) four trajectory groups across age groups, with the shaded areas indicating the 95% confidence intervals. Single dots represent individual data points. HDL-C levels were measured in mg/dL. HDL-C = high-density lipoprotein cholesterol.
408_2025_809_MOESM2_ESM.tif
Supplementary file2 (TIF 2433 KB)—Figure S2. HDL-C Trajectory Group-Based Reclassification of Baseline HDL-C and Its Distribution. (A) Normalized probability density distributions of baseline HDL-C levels (mg/dL) for five HDL-C trajectory groups. The black vertical dashed lines indicate the cutoff points determined by the intersections of adjacent trajectory group distributions, which were used to reclassify baseline HDL-C levels. (B) A heatmap showing the distribution of participants across the reclassified baseline HDL-C groups (<36.0, 36.0–42.8, 42.8–50.5, 50.5–60.4, and ≥60.4 mg/dL) within each HDL-C trajectory group. The color intensity represents the percentage of participants in each cell. HDL-C = high-density lipoprotein cholesterol.
408_2025_809_MOESM3_ESM.tif
Supplementary file3 (TIF 982 KB)—Figure S3. Estimated Differences in Annual Decline Rates of FVC and FEV1 Based on Baseline HDL-C Groups. Estimated differences in the annual decline rates of (A) FVC and (B) FEV1 changes in the baseline HDL-C groups. Results from generalized linear mixed models adjusted for sex, centered age, height, BMI, BMI², residential area, smoking exposure (pack-years), history of dyslipidemia, and alcohol consumption. Estimates represent the difference in annual changes compared to the reference group (Group 3). The interaction term (centered age ×baseline HDL-C group) was used to interpret the differences in the annual lung function changes. Negative values indicate a faster decline compared with the reference group. BMI = body mass index; CI = confidence interval; FEV1= forced expiratory volume in one second; FVC = forced vital capacity; HDL-C =high-density lipoprotein cholesterol; Ref = Reference.
408_2025_809_MOESM4_ESM.tif
Supplementary file4 (TIF 1574 KB)—Figure S4. Estimated Differences in Annual Decline Rates of FVC and FEV1 Based on Three and Four HDL-C Trajectory Groups. Estimated differences in annual decline rates of (A) FVC and (B) FEV1 change in the three-group trajectory model, (C) FVC and (D) FEV1 change in the four-group trajectory model. Results from generalized linear mixed models adjusted for sex, centered age, height, BMI, BMI2, residential area, smoking exposure (pack-years), history of dyslipidemia, and alcohol consumption. The estimates represent the difference in annual change compared to that in the reference group (Group 2). The interaction term (centered age * HDL-C trajectory group) was used to interpret the differences in annual lung function change. Negative values indicated a faster decline than that in the reference group. BMI = body mass index; CI = confidence interval; FEV1= forced expiratory volume in one second; FVC = forced vital capacity; HDL-C =high-density lipoprotein cholesterol; Ref = Reference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yoo, B., Jung, S.H., Bae, S.H. et al. High-Density Lipoprotein Cholesterol Trajectories and Lung Function Decline: A Prospective Cohort Study. Lung 203, 54 (2025). https://doi.org/10.1007/s00408-025-00809-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00408-025-00809-3