Abstract
Anti-vascular endothelial growth factor (VEGF) therapies have transformed the treatment of retinal diseases. However, VEGF signaling is only one component of the complex, multifactorial pathophysiology of retinal diseases, and many patients have residual disease activity despite ongoing anti-VEGF treatment. The angiopoietin/tyrosine kinase with immunoglobulin and epidermal growth factor receptor-2 (Ang/Tie2) signaling pathway is critical to endothelial cell homeostasis, survival, integrity, and vascular stability. Ang-2 can interfere with Ang-1/Tie2 signaling and is increased in several retinal diseases. Lack of Tie2 signaling due to elevated Ang-2 levels drives vascular instability through pericyte dropout, neovascularization, vascular leakage, inflammation, and fibrosis. Although Ang-2 and VEGF can synergistically promote vascular instability and neovascularization, Ang-2 may also mediate vascular instability independently of VEGF. Faricimab is a bispecific antibody designed for intraocular use that inhibits two distinct pathways via Ang-2 and VEGF-A blockade. Clinical biomarkers of vascular instability are important for evaluating disease control and subsequent treatment decisions. These biomarkers include measurement/evaluation with optical coherence tomography (OCT) of intraretinal fluid, subretinal fluid, central subfield thickness, and pigment epithelial detachments (PEDs), and fluorescein angiography imaging of macular leakage and PEDs. Hyperreflective foci (HRF), thought to be representative of activated microglia, indicating an inflammatory microenvironment, and epiretinal membranes (ERMs), a marker for retinal fibrotic proliferation in diabetic macular edema (DME), are both also identified using OCT. Here we summarize data (secondary endpoint and prespecified exploratory analyses as well as post hoc analyses) from six Phase III trials suggest that dual therapy Ang-2/VEGF-A inhibition with faricimab (6 mg) has a greater effect on reducing/resolving biomarkers of vascular instability than aflibercept (2 mg), by both controlling neovascularization and vascular leakage (with resultant resolution of exudation associated with DME, neovascular age-related macular degeneration, and retinal vein occlusion), as well as by targeting inflammation (reduction of HRF in DME) and retinal fibrotic proliferation (reducing the risk of ERMs in eyes with DME). Modulation of both the Ang-2 and VEGF-A pathways with faricimab may therefore provide greater disease control than anti-VEGF monotherapy, potentially leading to extended treatment durability and improved long-term outcomes.
-
VEGF signaling is only one component of the multifactorial pathophysiology of retinal diseases; angiopoietin-2 (Ang-2) levels are also elevated, and Ang-2 acts synergistically with or independently of VEGF to mediate vascular instability.
-
Faricimab (dual Ang-2/VEGF-A bispecific antibody) showed non-inferior visual gains vs. aflibercept 2 mg (anti-VEGF) with extended durability in six Phase III trials (in diabetic macular edema, neovascular age-related macular degeneration, and retinal vein occlusion) that all met their primary endpoints.
-
Subsequent secondary, exploratory or post hoc analyses suggest faricimab has a greater effect on reducing/resolving biomarkers of vascular leakage, inflammation, and fibrosis than aflibercept.
-
Modulating both the Ang-2 and VEGF-A pathways with faricimab provides greater disease control than anti-VEGF monotherapy alone, potentially leading to extended treatment durability and improved long-term outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Multifactorial pathophysiology of retinal disease: evidence from biology
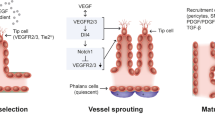
Anti-vascular endothelial growth factor (VEGF) therapies have revolutionized retinal disease treatment; however, VEGF signaling is only one component in the complex pathophysiology of retinal diseases (Fig. 1) [1,2,3]. VEGF signaling promotes angiogenesis and is modulated by multiple mechanisms. Under healthy, physiologic conditions, the vasculature is generally quiescent except during wound healing and the reproductive cycle [4]. Under pathologic conditions, elevated VEGF levels can occur in response to hypoxia, growth factors, and inflammatory cytokines [3, 5]. This results in neovascularization as well as vascular leakage in neovascular age-related macular degeneration (nAMD), diabetic retinopathy (DR), diabetic macular edema (DME), and retinal vein occlusion (RVO) [6, 7]. VEGF upregulation in nAMD promotes macular neovascularization; the fragility and permeability of these new vessels can result in retinal pigment epithelium detachment (PED), sub-retinal and intra-retinal edema, hemorrhage, and fibrosis [3, 6, 8].
Summary of potential factors involved in retinal diseases [2,3,4, 7, 9,10,11,12]. The roles of Ang-2 and VEGF-A are described in this manuscript. The complexity of retinal disease pathogenesis is further highlighted by the role of numerous other signaling pathways (indicated in grey). Ang-2, angiopoietin-2; CCL2, C–C motif ligand 2; EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; IL, interleukin; PDGF, platelet-derived growth factor; PIGF, placental growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor; TRAF6, tumor necrosis factor receptor-associated factor 6; VEGF-A, vascular endothelial growth factor-A
Despite anti-VEGF therapies, a substantial proportion of patients have residual disease activity following ongoing treatment, potentially due to the activity of other pathways and disease mechanisms. For example, the HAWK/HARRIER clinical trial data have shown that in patients with nAMD treated with brolucizumab or aflibercept, 24–39% of patients, respectively, had retinal fluid at 2 years [13]. In VIEW1/VIEW2, only half of patients treated with aflibercept or ranibizumab for nAMD were fluid-free at 96 weeks [14]. Similarly, data of patients with DME treated with aflibercept, bevacizumab or ranibizumab showed that 44–68% had persistent DME at 2 years [15]. Therefore, targeting additional pathways is of important clinical relevance.
The angiopoietin-1/tyrosine kinase with immunoglobulin and epidermal growth factor receptor-2 (Ang-1/Tie2) signaling pathway is critical to endothelial cell (EC) homeostasis, survival, integrity, and vascular stability [3, 7, 16]. Ang-2 can interfere with Ang-1/Tie2 signaling, with increased levels in nAMD, DR, proliferative diabetic retinopathy (PDR), and RVO [17]. Preclinical data have revealed elevated Ang-2 levels in response to stimuli including tumor necrosis factor, VEGF, fibroblast growth factor, shear stress, hyperglycemia, and hypoxia [9, 16, 18, 19].
Elevated Ang-2 levels drives vascular instability through pericyte dropout, neovascularization, and vascular leakage. This leads to inflammation and fibrosis [3, 10, 20, 21].
In mouse models, Ang-2 triggered blood–retinal barrier breakdown in a positive feedback loop while Ang-2 blockade prevented vascular destabilization [22]. Ang-2 can also potentiate VEGF effects by destabilizing vessels (rendering them more vulnerable to VEGF) and facilitating VEGF-mediated EC–EC junction destabilization, further driving neovascularization and vascular leakage [1, 9, 23]. Ang-2 regulates proinflammatory responses and can potentiate the effects of inflammatory cytokines [1, 20, 24]. Ang-2 signaling induces expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, promoting migration and adhesion of leucocytes into inflamed tissues [3]. This in turn results in the release of more inflammatory cytokines, growth cytokines, and vascular permeability factors, leading to altered EC junctions and a compromised blood–retinal barrier [25].
Subretinal fibrosis can be driven by several factors including inflammation, vascular leakage, neovascularization, and hemorrhage, which eventually lead to fibrovascular tissue formation and significant vision loss [26, 27]. Thus, modulating fibrosis development should be an important end goal for treatments that aim to optimize visual outcomes. Elevated Ang-2 levels in vitreous samples from patients with PDR have been shown to correlate with the degree of fibrosis as well as the presence of fibrovascular membranes [10]. Epiretinal membranes (ERMs) are fibrocellular proliferations on the internal limiting membrane of the macula likely caused by glial cell proliferation [28, 29]. ERMs can be either idiopathic, with cell proliferation occurring after posterior vitreous detachment [28], or secondary, as a result of existing retinal diseases such as DR, PDR, proliferative vitreoretinopathy, posterior uveitis, RVO as well as in the context of retinal breaks, retinal detachment surgery and inflammation [28, 29]. ERMs are a marker of retinal fibrotic proliferation in DME [30] and can result in anatomic disruption of the macula and vision loss [29]. Elevated Ang-2 levels have been identified in excised ERMs from eyes with ischemic retinal diseases [31], and Ang-2 expression in blood vessels was significantly correlated with the number of leucocytes in PDR, suggesting its capacity to modulate proinflammatory activities [32].
Although Ang-2 and VEGF signaling can act synergistically to promote vascular instability and neovascularization [16, 19, 20], Ang-2 may also act independently of VEGF to mediate vascular instability [19, 33]. In mouse models, Ang-2 overexpression was sufficient to mediate vascular leakage [34, 35], while Ang-2 inhibition alone stabilized retinal vessels, even under hypoxic or pericyte-deficient conditions [22]. Therefore, dual Ang-2/VEGF targeting may provide greater disease control (management of multiple pathways, allowing for the comprehensive management of factors including leakage, inflammation, and retinal fibrotic proliferation associated with the disease [3]) than anti-VEGF alone [24].
Clinical lessons and insights from trials
Six Phase III randomized clinical trials (RCTs) [36,37,38] have compared faricimab (6 mg), a dual Ang-2/VEGF-A bispecific antibody, with aflibercept (unless otherwise noted, all subsequent references to aflibercept are related to the 2 mg dose), a VEGF and placental growth factor inhibitor [39]. The YOSEMITE/RHINE trials evaluated faricimab every 8 weeks (Q8W), or with personalized treat & extend (T&E) regimens up to every 16 weeks (Q16W), vs. aflibercept Q8W in patients with DME [36], while the TENAYA/LUCERNE trials evaluated faricimab given up to Q16W vs. aflibercept Q8W in patients with nAMD [37]; the duration of the trials was 2 years [40, 41]. The BALATON/COMINO trials of patients with ME due to RVO assessed monthly injections of faricimab vs. aflibercept 2 mg (up to Week 24; with all patients receiving faricimab thereafter, up to Week 72) [38]. In this paper, we translate the preclinical evidence regarding dual Ang-2/VEGF-A pathway inhibition to clinical biomarkers from large RCTs in order to better understand the improved disease control in nAMD, DME, and RVO.
Dual Ang-2/VEGF-A inhibition: clinical biomarkers in retinal diseases
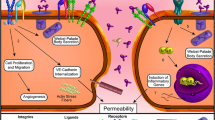
Clinical biomarkers of vascular instability are important for establishing disease control and subsequent treatment decisions (Fig. 2).
Role of VEGF and Ang-2 in the pathogenesis of retinal diseases and control of clinical biomarkers with dual VEGF/Ang-2 targeting [36, 42,43,44,45,46,47,48,49]. DME: YOSEMITE/RHINE trials; nAMD: TENAYA/LUCERNE trials; RVO: BALATON/COMINO trials. * Post hoc analyses not adjusted for multiplicity; no formal statistical conclusion should be made based on nominal p values. Ang-2, angiopoietin-2; DME, diabetic macular oedema; ERM, epiretinal membrane; FA, fluorescein angiography; HRF, hyperreflective foci; IRF, intraretinal fluid; nAMD, neovascular age-related macular degeneration; OCT, optical coherence tomography; PED, pigment epithelial detachment; Q12W, every 12 weeks; RVO, retinal vein occlusion; VEGF-A, vascular endothelial growth factor-A
Biomarkers of neovascularization and vascular leakage
Optical coherence tomography (OCT) is used to measure/evaluate intraretinal fluid (IRF), subretinal fluid (SRF), PED fluid and central subfield thickness (CST) [50]. In DME, YOSEMITE/RHINE trial data showed that dual Ang-2/VEGF-A inhibition with faricimab Q8W/T&E resulted in greater mean CST reduction (secondary endpoint) compared with aflibercept after the head-to-head dosing period (Week 16; –169.9 μm /–174.5 μm vs. –151.7 μm), at 1 year (–206.6 μm/–196.5 μm vs. –170.3 μm), and this was maintained through 2 years (–209.4 μm/201.0 μm vs. –190.9 μm) [36, 42]. Similar results were observed in the Phase II RUBY trial in DME, which showed greater CST reduction with the combination of nesvacumab (Ang-2 inhibitor) and aflibercept vs. aflibercept alone [51]. A post hoc analysis of YOSEMITE/RHINE showed that faricimab achieved median time to first absence of IRF and all fluid (IRF and SRF) more than 9 months faster and with fewer injections vs. aflibercept [43]. In nAMD, post hoc analyses of TENAYA/LUCERNE showed that treatment with faricimab resulted in greater CST reductions vs. aflibercept after the head-to-head dosing period (Week 12; –145.4 μm vs. –133.0 μm) [44]. Furthermore, patients treated with faricimab experienced faster absence of IRF and SRF (by 4 weeks in the median time) with fewer injections, compared with aflibercept [42, 44]. Faricimab also reduced the presence (4% of patients with serous PEDs at baseline still had serous PEDs, vs. 12% with aflibercept) and thickness (PED mean thickness was 27.9 μm thinner with faricimab vs. aflibercept) of serous PEDs to a greater extent than aflibercept at Week 12 [45, 46]. Retinal pigment epithelium tears were associated with larger baseline PED height in TENAYA/LUCERNE; incidence (2–3%) was similar between treatment arms [45, 46] and comparable to findings from other trials of anti-VEGF-A monotherapy (2–3%) [52].
Reduction in macular leakage area on FA is an important biomarker that has been correlated with improved anatomical (reduced IRF volume and microaneurysm count) and best corrected visual acuity outcomes in patients with DME [53]. In YOSEMITE/RHINE, a post hoc analysis showed resolution of angiographic macular leakage in almost twice as many patients with faricimab vs. aflibercept (28% vs. 15%) after the head-to-head dosing period [47]. In BALATON/COMINO, a prespecified exploratory analysis showed that more patients achieved absence of angiographic macular leakage with faricimab than aflibercept (34%/44% vs. 21%/30%) at Week 24 (after the head-to-head dosing period) [47].
Biomarker of inflammation
Hyperreflective foci (HRF) are detected with OCT and are defined as discrete, well-circumscribed, dot-shaped lesions up to 50 µm in diameter with equal or higher reflectivity compared with the retinal pigment epithelial band [54]. HRF are believed to be representative of activated microglia and may be a biomarker of an inflammatory microenvironment in retinal diseases [48, 54]; HRF reduction may be consistent with suppression of inflammatory pathways [48]. In a post hoc analysis of YOSEMITE/RHINE, in both the inner and outer retina, faricimab (Q8W and T&E regimens) reduced HRF number and volume more than aflibercept at Year 1 (p < 0.05 in all comparisons of faricimab vs. aflibercept) [48, 55].
Biomarker of fibrotic proliferation
ERMs, identified using OCT, are a marker of retinal fibrotic proliferation [56] and have been observed in patients with DME [30]. In YOSEMITE/RHINE, presence of ERMs was assessed in a masked fashion by a reading center and defined using OCT as the presence of a membrane overlying the internal limiting membrane, causing significant macular architecture distortion in the central subfield [49]. In patients with no ERMs at baseline, per study entry criteria, faricimab Q8W treatment showed a greater reduction in the risk of development of ERMs over 2 years by more than 50% compared with aflibercept Q8W (proportion of patients with ERMs at Week 100: 4% vs. 8%) [49]. However, longer follow-up and additional studies are needed to determine the clinical implications of ERM development.
Summary and clinical implications
Together, the data from six Phase III trials of the three disease states suggest that dual therapy Ang-2/VEGF-A inhibition with faricimab provides greater disease control than aflibercept 2 mg, by controlling neovascularization and vascular leakage (resolution of retinal anatomy, PEDs, and macular leakage; DME, nAMD, RVO) as well as by targeting inflammation (reduction of HRF; DME) and retinal fibrotic proliferation (reducing the risk of ERMs; DME) [42, 47,48,49].
We hypothesize that the observed biomarker benefits associated with faricimab compared with aflibercept 2 mg are likely attributable in part to the Ang-2 inhibition rather than solely the additional VEGF inhibition provided by faricimab’s higher molar anti-VEGF binding capacity. Indeed, numerous trials have explored higher molar doses of approved anti-VEGF therapies, revealing no or limited further reduction of CST with higher concentrations. For instance, in the HARBOR and READ-3 studies, ranibizumab doses of 2 mg and 0.5 mg achieved comparable CST reductions [57, 58]. In the PULSAR and PHOTON studies, aflibercept 8 mg achieved similar CST reductions as aflibercept 2 mg [59, 60]. Notably, the PHOTON study also demonstrated similar effects on macular leakage between aflibercept 8 mg and 2 mg during the head-to-head dosing period [61].
Conversely, faricimab reduced CST to a greater extent than aflibercept 2 mg in DME and nAMD [36, 42, 44], and also reduced macular leakage vs. aflibercept 2 mg in both DME and RVO [47]. Findings from the RUBY trial in DME conducted by Regeneron also support the role of Ang-2 in the anatomical response to faricimab. This trial revealed a greater reduction in CST with the addition of nesvacumab (an Ang-2 inhibitor) alongside aflibercept compared to aflibercept 2 mg alone [51], indicating potential additional benefits with dual Ang-2/VEGF inhibition. However, cross-trial comparisons between these higher-dose anti-VEGF trials and those involving faricimab must be interpreted with caution due to the differences in patient populations and study designs. Furthermore, as with any treatment, the risks with intravitreal injections of these agents – including potential endophthalmitis and intraocular inflammation – must be carefully considered alongside the clinical benefits associated with the control of the disease.
The biomarker evidence for faricimab, as outlined in the current manuscript, underscores the potential of faricimab to achieve greater control over neovascularization, vascular leakage, inflammation, and preretinal proliferation compared with aflibercept across various disease states. We hypothesize that these clinical benefits are a result of faricimab targeting two distinct pathways via Ang-2 and VEGF-A. The potential clinical significance for this differential biomarker effect, as it pertains to important patient outcomes, will need further exploration in future trials and real-world studies.
Up to 40% of patients with DME treated with anti-VEGF agents have persistent IRF and SRF accumulation after 3 years [23]. While this observation may be partly due to undertreatment, we hypothesize that pathways beyond VEGF are contributing to the exudation to some extent. Treatments aimed at achieving improved disease control with faster and more effective fluid resolution are important for improving patient outcomes. This includes improving patients' quality of life by preserving vision and potentially reducing clinic visits through increased treatment durability. Moreover, such advancements may alleviate treatment-related stress, minimize the use of transportation resources, decrease work absenteeism, and contribute to reducing carbon footprints [62]. Longer dosing intervals have been investigated in T&E regimens, which involve an initial monthly loading phase to maximize disease control and minimize anatomic signs of disease. Injection intervals are then modified based on the presence or absence of disease activity biomarkers such as retinal fluid, hemorrhage, or loss of vision [63,64,65]. In YOSEMITE/RHINE and TENAYA/LUCERNE, greater disease control with faricimab may have allowed for extended durability beyond every 12 weeks (Q12W). For example, in these trials, ~ 80% of patients with DME or nAMD achieved ≥ Q12W dosing at Year 2 with faricimab (Q16W, 62% and 63%, respectively) [44, 66]. Furthermore, over half of patients (56%) met the criteria for potential Q20W dosing intervals [67, 68].
In summary, modulation of both the Ang-2 and VEGF-A pathways with faricimab may provide greater disease control than with anti-VEGF monotherapy [69], leading to the potential for extended durability of Q12W or longer. Biomarker analyses revealed that faricimab not only leads to improvements in resolution of retinal fluid and macular leakage vs. aflibercept 2 mg [42, 44, 47], but also reduces HRF and risk of ERMs [48, 49]. Further research will continue to elucidate the importance of these clinical imaging biomarkers for evaluating retinal disease pathology/activity, their relationship with proteomic markers, and the impact of different treatment paradigms (fixed intervals/pro re nata/T&E) on disease control. Based on the evidence presented regarding disease control with a dual Ang-2/VEGF-A inhibitor, we hypothesize that earlier treatment with a dual pathway inhibitor has the potential to improve long-term patient outcomes; future evidence generation in terms of robust clinical trials is required to quantify the potential clinical benefit.
References
Nguyen QD, Heier JS, Do DV, Mirando AC, Pandey NB, Sheng H, Heah T (2020) The Tie2 signaling pathway in retinal vascular diseases: a novel therapeutic target in the eye. Int J Retina Vitreous 6:48. https://doi.org/10.1186/s40942-020-00250-z
Cabral T, Mello LGM, Lima LH, Polido J, Regatieri CV, Belfort R, Mahajan VB (2017) Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous 3:31. https://doi.org/10.1186/s40942-017-0084-9
Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M (2021) Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond) 35:1305–1316. https://doi.org/10.1038/s41433-020-01377-x
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29. https://doi.org/10.1016/s1350-9462(02)00043-5
Uemura A, Fruttiger M, D'Amore PA, De Falco S, Joussen AM, Sennlaub F, Brunck LR, Johnson KT, Lambrou GN, Rittenhouse KD, Langmann T (2021) VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res 84:100954. https://doi.org/10.1016/j.preteyeres.2021.100954
Bates DO (2010) Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87:262–271. https://doi.org/10.1093/cvr/cvq105
Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TYY, Loewenstein A, Schlottmann PG, Paris LP, Westenskow PD, Quezada-Ruiz C (2021) The angiopoietin/tie pathway in retinal vascular diseases: a review. Retina 41:1–19. https://doi.org/10.1097/IAE.0000000000003003
Rastoin O, Pagès G, Dufies M (2020) Experimental models in neovascular age related macular degeneration. Int J Mol Sci 21:4627. https://doi.org/10.3390/ijms21134627
Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol 10:165–177. https://doi.org/10.1038/nrm2639
Klaassen I, de Vries EW, Vogels IMC, van Kampen AHC, Bosscha MI, Steel DHW, Van Noorden CJF, Lesnik-Oberstein SY, Schlingemann RO (2017) Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS One 12:e0187304. https://doi.org/10.1371/journal.pone.0187304
Chauhan MZ, Rather PA, Samarah SM, Elhusseiny AM, Sallam AB (2022) Current and novel therapeutic approaches for treatment of diabetic macular edema. Cells 11:1950. https://doi.org/10.3390/cells11121950
Panos GD, Lakshmanan A, Dadoukis P, Ripa M, Motta L, Amoaku WM (2023) Faricimab: transforming the future of macular diseases treatment - a comprehensive review of clinical studies. Drug design, development and therapy 17:2861–2873. https://doi.org/10.2147/dddt.S427416
Hussain RM, Neal A, Yannuzzi NA, Patel KH, Huo S, Hariprasad SM, Bhatia SP (2021) Brolucizumab for persistent macular fluid in neovascular age-related macular degeneration after prior anti-VEGF treatments. Ther Adv Ophthalmol 13:25158414211055964. https://doi.org/10.1177/25158414211055964
Anguita R, Tasiopoulou A, Shahid S, Roth J, Sim SY, Patel PJ (2021) A review of aflibercept treatment for macular disease. Ophthalmol Ther 10:413–428. https://doi.org/10.1007/s40123-021-00354-1
Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, Melia M, Wells JA 3rd (2018) Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 136:257–269. https://doi.org/10.1001/jamaophthalmol.2017.6565
Saharinen P, Eklund L, Alitalo K (2017) Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov 16:635–661. https://doi.org/10.1038/nrd.2016.278
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G (2016) Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO molecular medicine 8:1265–1288. https://doi.org/10.15252/emmm.201505889
Mandriota SJ, Pepper MS (1998) Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83:852–859. https://doi.org/10.1161/01.res.83.8.852
Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A (2011) A potential role for angiopoietin 2 in the regulation of the blood–retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci 52:3784–3791. https://doi.org/10.1167/iovs.10-6386
Collazos-Alemán JD, Gnecco-González S, Jaramillo-Zarama B, Jiménez-Mora MA, Mendivil CO (2022) The role of angiopoietins in neovascular diabetes-related retinal diseases. Diabetes Ther 13:1811–1821. https://doi.org/10.1007/s13300-022-01326-9
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110. https://doi.org/10.2337/diabetes.53.4.1104
Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, Koh GY (2017) Plastic roles of pericytes in the blood–retinal barrier. Nat Commun 8:15296. https://doi.org/10.1038/ncomms15296
Wong HL, Fung NSK, Kwok AKH (2022) Brolucizumab and faricimab as new treatment options for diabetic macular edema: perspective. Hong Kong J Ophthalmol 26:53–57
Larsen HO, Grauslund J, Vergmann AS (2023) Efficacy, durability and safety of faricimab in neovascular age-related macular degeneration and diabetic macular oedema: lessons learned from registration trials. Ophthalmol Ther 12:2253–2264. https://doi.org/10.1007/s40123-023-00753-6
Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C (2015) Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res 2015:582060. https://doi.org/10.1155/2015/582060
Roy S, Amin S, Roy S (2016) Retinal fibrosis in diabetic retinopathy. Exp Eye Res 142:71–75. https://doi.org/10.1016/j.exer.2015.04.004
Tenbrock L, Wolf J, Boneva S, Schlecht A, Agostini H, Wieghofer P, Schlunck G, Lange C (2022) Subretinal fibrosis in neovascular age-related macular degeneration: current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res 387:361–375. https://doi.org/10.1007/s00441-021-03514-8
Tsotridou E, Loukovitis E, Zapsalis K, Pentara I, Asteriadis S, Tranos P, Zachariadis Z, Anogeianakis G (2020) A review of last decade developments on epiretinal membrane pathogenesis. Med Hypothesis Discov Innov Ophthalmol 9:91–110
Lee GW, Lee SE, Han SH, Kim SJ, Kang SW (2020) Characteristics of secondary epiretinal membrane due to peripheral break. Sci Rep 10:20881. https://doi.org/10.1038/s41598-020-78093-9
Akbar Khan I, Mohamed MD, Mann SS, Hysi PG, Laidlaw DA (2015) Prevalence of vitreomacular interface abnormalities on spectral domain optical coherence tomography of patients undergoing macular photocoagulation for Centre involving diabetic macular oedema. Br J Ophthalmol 99:1078–1081. https://doi.org/10.1136/bjophthalmol-2014-305966
Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y (2003) Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci 44:393–402. https://doi.org/10.1167/iovs.02-0276
Abu El-Asrar AM, Missotten L, Geboes K (2007) Expression of hypoxia-inducible factor-1alpha and the protein products of its target genes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol 91:822–826. https://doi.org/10.1136/bjo.2006.109876
Canonica J, Foxton R, Garrido MG, Lin CM, Uhles S, Shanmugam S, Antonetti DA, Abcouwer SF, Westenskow PD (2023) Delineating effects of angiopoietin-2 inhibition on vascular permeability and inflammation in models of retinal neovascularization and ischemia/reperfusion. Front Cell Neurosci 17:1192464. https://doi.org/10.3389/fncel.2023.1192464
Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A (2005) Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744. https://doi.org/10.1124/jpet.105.086553
Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP (2006) Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3:e46. https://doi.org/10.1371/journal.pmed.0030046
Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, Lin H, Loewenstein A, Mohan S, Pearce IA, Sakamoto T, Schlottmann PG, Silverman D, Sun JK, Wells JA, Willis JR, Tadayoni R, YOSEMITE and RHINE Investigators (2022) Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 399:741–755. https://doi.org/10.1016/s0140-6736(22)00018-6
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, Lai TYY, Silverman D, Regillo C, Swaminathan B, Viola F, Cheung CMG, Wong TY, TENAYA and LUCERNE Investigators (2022) Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 399:729–740. https://doi.org/10.1016/s0140-6736(22)00010-1
Hattenbach LO, Abreu F, Arrisi P, Basu K, Danzig CJ, Guymer R, Haskova Z, Heier JS, Kotecha A, Liu Y, Loewenstein A, Seres A, Willis JR, Wykoff CC, Paris LP (2023) BALATON and COMINO: phase III randomized clinical trials of faricimab for retinal vein occlusion: study design and rationale. Ophthalmol Sci 3:100302. https://doi.org/10.1016/j.xops.2023.100302
Regeneron Pharmaceuticals Inc. (2023) EYLEA® (aflibercept). Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125387s084lbl.pdf. Accessed 20 May 2024
Eter N, Singh RP, Abreu F, Asik K, Basu K, Baumal C, Chang A, Csaky KG, Haskova Z, Lin H, Ruiz CQ, Ruamviboonsuk P, Silverman D, Wykoff CC, Willis JR (2022) YOSEMITE and RHINE: phase 3 randomized clinical trials of faricimab for diabetic macular edema: study design and rationale. Ophthalmol Sci 2:100111. https://doi.org/10.1016/j.xops.2021.100111
Khanani AM, Guymer RH, Basu K, Boston H, Heier JS, Korobelnik J-F, Kotecha A, Lin H, Silverman D, Swaminathan B, Willis JR, Yoon YH, Quezada-Ruiz C (2021) TENAYA and LUCERNE: rationale and design for the phase 3 clinical trials of faricimab for neovascular age-related macular degeneration. Ophthalmol Sci 1:100076. https://doi.org/10.1016/j.xops.2021.100076
Dhoot D, Haskova Z, Ives J, Kotecha A, Margaron P, Souverain A, Tang Y, Willis JR (2023) YOSEMITE and RHINE Investigators. Improved retina fluid control with faricimab vs aflibercept in phase 3 trials in nAMD and DME. Presented at the macula society 46th annual meeting, Miami, FL
Ferrone PJ, Gibson K, Mar F, Wang T, Willis JR, YOSEMITE and RHINE Investigators (2023) New findings of Faricimab for diabetic macular edema. Presented at the 11th FLORetina - ICOOR meeting, Rome
Chaudhary V, Kotecha A, Willis JR, Souverain A, Shildkrot YE, Swaminathan B, Margaron P (2023) Individualized faricimab dosing up to every 16 weeks maintains robust anatomic and vision outcomes through 2 years in nAMD. Presented at the Association for Research in Vision and Ophthalmology annual meeting, New Orleans, LA
Lim JI, Margaron P, Souverain A, Yang M, Shildkrot YE, Kotecha A, Willis JR, Patel S, Khanani AM, Bakri SJ (2023) Greater reduction in pigment epithelial detachment size with Faricimab vs Aflibercept during head-to-head dosing in patients with nAMD. Presented at the 56th retina society annual scientific meeting, New York, NY
Lai TYY, Khanani AM, Souverain A, Yang M, Patel S, Margaron P (2023) Greater reduction in pigment epithelial detachment size with Faricimab vs Aflibercept during head-to-head dosing in patients with Neovascular age-related macular degeneration. Presented at the 16th congress of the Asia-Pacific Vitreo-retina society, Hong Kong
Goldberg R, Kolomeyer AM, Nudleman E, Csaky K, Willis J, Gibson K, Wang T, Haskova Z, Shildkrot YE, Amador M, Mar F (2023) Faricimab reduces macular leakage vs aflibercept in patients with DME. Presented at the Association for Research in Vision and Ophthalmology annual meeting, New Orleans, LA
Maunz A, von Schulthess E, Patel K, Chakravarthy U, Bachmeier I, Lloyd Jones I, Cohen Y, Gibson K, Willis JR, Glittenberg C, Fauser S (2023) Automated segmentation of hyperreflective foci in diabetic macular edema shows greater volume reduction by faricimab vs aflibercept in phase 3 YOSEMITE and RHINE. Presented at the Association for Research in Vision and Ophthalmology imaging in the eye conference, New Orleans, LA
Jaffe GJ, Ogura Y, Deák G, Gibson K, Schmidt-Erfurth U, Wang T, Westenskow P, Willis J (2023) Impact of Faricimab vs Aflibercept on Epiretinal membrane formation over 2 years in eyes with DME in the YOSEMITE/RHINE phase 3 trials. Presented at the American society of retina specialists annual meeting, Seattle, WA
Bhende M, Shetty S, Parthasarathy MK, Ramya S (2018) Optical coherence tomography: a guide to interpretation of common macular diseases. Indian J Ophthalmol 66:20–35. https://doi.org/10.4103/ijo.IJO_902_17
Brown DM, Boyer DS, Csaky K, Vitti R, Perlee L, Chu KW, Asmus F, Leal S, Zeitz O, Cheng Y, Schmelter T, Heier JS, RUBY Investigators (2022) Intravitreal nesvacumab (Antiangiopoietin 2) plus aflibercept in diabetic macular edema: phase 2 ruby randomized trial. Retina 42:1111–1120. https://doi.org/10.1097/iae.0000000000003441
Cunningham ET Jr, Feiner L, Chung C, Tuomi L, Ehrlich JS (2011) Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology 118:2447–2452. https://doi.org/10.1016/j.ophtha.2011.05.026
Sarici K, Yordi S, Martin A, Lunasco L, Mugnaini C, Chu K, Moini H, Vitti R, Srivastava SK, Ehlers JP (2023) Longitudinal quantitative ultrawide-field fluorescein angiography dynamics in the RUBY diabetic macular edema study. Ophthalmol Retina 7:543–552. https://doi.org/10.1016/j.oret.2023.01.018
Wu J, Zhang C, Yang Q, Xie H, Zhang J, Qiu Q, Liu K, Luo D, Liu F, Zhang J (2021) Imaging hyperreflective foci as an inflammatory biomarker after anti-VEGF treatment in neovascular age-related macular degeneration patients with optical coherence tomography angiography. Biomed Res Int 2021:6648191. https://doi.org/10.1155/2021/6648191
Chakravarthy U, Maunz A, von Schulthess E, Patel K, Bachmeier I, Lloyd Jones I, Cohen Y, Gibson K, Willis JR, Glittenberg C, Fauser S (2023) Automated segmentation of hyperreflective foci in DME shows greater volume reduction in eyes treated with faricimab compared to aflibercept in the phase 3 YOSEMITE and RHINE clinical trials. Presented at the 23rd EURETINA congress, Amsterdam
Fung AT, Galvin J, Tran T (2021) Epiretinal membrane: a review. Clin Experiment Ophthalmol 49:289–308. https://doi.org/10.1111/ceo.13914
Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, Rubio RG, Lai P, HARBOR Study Group (2013) Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 120:1046–1056. https://doi.org/10.1016/j.ophtha.2012.10.014
Do DV SYJ, Boyer D, Callanan D, Gallemore R, Bennett M, Marcus DM, Halperin L, Sadiq MA, Rajagopalan N, Campochiaro PA, Nguyen QD, READ-3 Study Group (2015) Month-6 primary outcomes of the READ-3 study (Ranibizumab for edema of the mAcula in diabetes-protocol 3 with high dose). Eye (Lond) 29:1538–1544. https://doi.org/10.1038/eye.2015.142
Boyer D, PHOTON study investigators (2023) Intravitreal Aflibercept injection 8mg for DME: 48-week results from the phase 2/3 PHOTON Trial. Presented at angiogenesis, exudation, and degeneration virtual meeting
Brown DM, PULSAR study investigators (2023) Aflibercept 8 mg in patients with nAMD: 48-week results from the phase 3 PULSAR trial. Presented at angiogenesis, exudation, and degeneration virtual meeting
Brown DM, PHOTON study investigators (2022) Intravitreal Aflibercept injection 8 mg for DME: 48-week results from the phase 2/3 PHOTON trial. Presented at the 55th annual scientific session of the retina society, Pasadena
Balaskas K, Amoaku WM, Cudrnak T, Downey LM, Groppe M, Mahmood S, Mehta H, Mohamed Q, Mushtaq B, Severn P, Vardarinos A, Yang YC (2021) Importance of anatomical efficacy for disease control in neovascular AMD: an expert opinion. Ophthalmol Ther 10:231–243. https://doi.org/10.1007/s40123-021-00342-5
Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, Hoerauf H, Lanzetta P, Michels S, Mitchell P, Monés J, Regillo C, Tadayoni R, Talks J, Wolf S (2015) Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina 35:1489–1506. https://doi.org/10.1097/iae.0000000000000627
Kertes PJ, Galic IJ, Greve M, Williams G, Baker J, Lahaie M, Sheidow T (2020) Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol 138:244–250. https://doi.org/10.1001/jamaophthalmol.2019.5540
Patel PJ, Villavicencio P, Hanumunthadu D (2023) Systematic review of neovascular age-related macular degeneration disease activity criteria use to shorten, maintain or extend treatment intervals with anti-VEGF in clinical trials: implications for clinical practice. Ophthalmol Ther 12:2323–2346. https://doi.org/10.1007/s40123-023-00768-z
Lim JI, Chen S-J, Steinle NC, Jaffe GJ, Gerendas BS, Abreu F, Camino A, Gibson K, Jain N, Shildkrot YE, Souverain A, Tang Y, Willis JR, Haskova Z (2023) Durable vision gains and greater fluid control with extended Faricimab dosing vs Aflibercept in patients with DME. Presented at the association for research in vision and ophthalmology annual meeting, New Orleans, LA
Rachitskaya A, Gibson K, Mar F, Tang Y, Willis J (2023) Time to retinal fluid control with Faricimab vs Aflibercept in patients with DME in the phase 3 YOSEMITE/RHINE trials. Presented at the American society of retina specialists annual meeting, Seattle, WA
London N, Querques G, Kotecha A, Willis JR, Souverain A, Shildkrot YE, Margaron P (2023) Faricimab rapidly improves fluid parameters in patients with nAMD. Presented at the American society of retina specialists annual meeting, Seattle, WA
Ferro Desideri L, Traverso CE, Nicolò M, Munk MR (2023) Faricimab for the treatment of diabetic macular edema and neovascular age-related macular degeneration. Pharmaceutics 15:1413. https://doi.org/10.3390/pharmaceutics15051413
Acknowledgements
Research funding in the form of medical writing support for this manuscript, furnished by Brian Law, PhD, of Nucleus Global, an Inizio company, was provided by F. Hoffmann-La Roche Ltd.
Funding
No funding was received for this research. All authors received support in the form of third-party writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work, drafted or critically revised the work, approved the final version for publication, and agree to be accountable for all aspects of the work.
Srinivas Sadda and Antonia M. Joussen are editors of Graefe's Archive for Clinical and Experimental Ophthalmology but had no involvement in the peer review process for this article.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of interest
Varun Chaudhary has received advisory board fees from Alcon, Roche, Bayer, Novartis, Appelis, Boehringer Ingelheim, and grants from Bayer, Novartis, Roche. Florie Mar and Manuel J. Amador are employees of Genentech, Inc., and own stocks/shares with Genentech, Inc./F. Hoffmann-La Roche Ltd. Andrew Chang has received consulting role fees from Novartis, Bayer, Allergan, Roche, Alcon, Apellis, Zeiss, Astellas Pharma, Opthea, and research grants from Roche, Bayer, Novartis. Kara Gibson, Philippe Margaron, and Insaf Saffar are employed by F. Hoffmann-La Roche Ltd and own stocks/shares with F. Hoffmann-La Roche Ltd. Antonia M. Joussen has received consulting role fees from Novartis, Roche, Bayer, Genentech, Inc., AbbVie, Boehringer Ingelheim, and grants from Novartis, Roche, Bayer, Genentech, Inc., AbbVie, Boehringer Ingelheim, Johnson & Johnson. Judy E. Kim has received advisory board fees from AbbVie, Alimera Sciences, Amgen, Apellis, Bausch & Lomb, Clearside Biomedical, Dutch Ophthalmic Research Center, Eyepoint, Genentech/Roche, Neurotech, Notal Vision, Ophthalmic Therapeutics, Regeneron. Junyeop Lee has received consulting role fees from AbbVie/Allergan, AMC sciences, Bayer, Curacle, Novartis, Roche; and grants/research support from Bayer, Novartis. David Wong has received grants/research support from Bayer, Novartis, Roche, and consulting role fees from AbbVie/Allergan, Alcon, Apellis, Bausch Health, Biogen, Boehringer Ingelheim, Bayer, Novartis, Ripple Therapeutics, Roche, Topcon, Zeiss. Charles Wykoff has received consulting fees/honoraria for ongoing services for 4DMT, AbbVie, Adverum, Aerie, AGTC, Alcon, Alimera, Alkeus, Allgenesis, Alnylam, AMC Sciences, Annexon, Apellis, Arrowhead, Ascidian, Aviceda, Bausch + Lomb, Bayer, Biocryst, Bionic Vision, Boehringer Ingelheim, Cholgene, Clearside, Curacle, Emmecell, EyeBiotech, EyePoint, Foresite, Frontera, Genentech, Gyroscope, IACTA, InGel, IVERIC Bio, Janssen, Kato, Kiora, Kodiak, Kriya, Merck, Merit, Nanoscope, Neurotech, NGM, Notal Vision, Novartis, OccuRx, Ocular Therapeutix, Ocuphire, Ocuterra, OliX, ONL, Opthea, Osanni, Oxular, Palatin, Perceive Bio, Perfuse, Ray, RecensMedical, Regeneron, RegenXBio, Resonance, Roche, Sandoz, Sanofi, Santen, SciNeuro, Stealth, Surrozen, Suzhou Raymon, Sylentis, THEA, Therini, TissueGen, Valo, Verana, Visgenx, Zeiss, grants paid to institution for ongoing research support as a Principal Investigator for trials sponsored by 4DMT, Adverum, AffaMed, Alexion, Alimera, Alkahest, Allgenesis, Amgen, Annexin, Annexon, Apellis, Ascidian, Asclepix, Aviceda, Bayer, Boehringer Ingelheim, Chengdu Kanghong, Chengdu Origen, Clearside, Curacle, EyeBiotech, EyePoint, Gemini, Genentech, GlaxoSmithKline, Gyroscope, IONIS, iRENIX, IVERIC bio, Janssen, Kodiak, Kyoto DDD, Kyowa Kirin, Nanoscope, Neurotech, NGM, Novartis, Ocugen, Ocular Therapeutix, Ocuphire, OcuTerra, OliX, Opthea, Outlook Therapeutics, Oxular, Oxurion, Oyster Point, Perceive Bio, Pykus, RecensMedical, Regeneron, RegenXBio, Rezolute, Roche, SamChunDang Pharm, Sandoz, Shanghai Henlius, Skyline, Stealth, UNITY, Valo, Verily, Xbrane, and stock options (not owner) ongoing from private for-profit entities: InGel, ONL, Osanni, Panther, PolyPhotonix, RecensMedical, TissueGen, Visgenx, Vitranu. Srinivas Sadda has received consulting role fees from Roche/Genentech, Apellis, Amgen, AbbVie/Allergan, Alexion, Samsung Bioepis, Biogen, Boehringer Ingelheim, Iveric Bio, Novartis, Bayer, Pfizer, Astellas, Nanoscope, Jannsen, CenterVue, Optos, Heidelberg, Notal Vision, Topcon, Eyepoint, Character, Ocular Therapeutics, Neurotech, and honoraria/speaker fees from Novartis, Roche, Optos, Heidelberg, NIDEK, is a member of data safety monitoring boards for Regeneron, Regenxbio, and received Research Instruments from iCare, Carl Zeiss Meditec, Optos, NIDEK, Topcon, Heidelberg. Dr Srinivas Sadda and Dr Antonia Joussen are co-editors-in-chief of Graefe's Archive for Clinical and Experimental Ophthalmology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaudhary, V., Mar, F., Amador, M.J. et al. Emerging clinical evidence of a dual role for Ang-2 and VEGF-A blockade with faricimab in retinal diseases. Graefes Arch Clin Exp Ophthalmol 263, 1239–1247 (2025). https://doi.org/10.1007/s00417-024-06695-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-024-06695-4