Abstract
Background
Treatment options for intracranial atherosclerotic stenosis (ICAS) are limited, but endovascular intervention has gained increasing attention in recent years.
Aim
To evaluate the safety and efficacy of the Neuroform EZ stent for treating ICAS in the posterior circulation.
Material & methods
Patients’ (n = 50) eligibility depended on ICAS with severe stenosis (≥ 70%) in the intracranial segment of the vertebral artery or basilar artery shown by cerebral angiography. General information of the participants were recorded, Adverse events during the perioperative period were observed, including in-stent thrombosis, postoperative hyperperfusion, stroke, and mortality. Before the procedure, neurological deficits (NIHSS score) and neurological recovery (mRS score) were recorded at 12 months postoperatively. The degree of vascular stenosis was evaluated prior to and following the procedure, and in-stent restenosis (ISR) was recorded at 12 months post-operation.
Results
Fifty-two stents were successfully placed in 50 patients, followed by standardized medication. Angiographic follow-up was completed 12-months postoperatively, and there was only one case of ISR (4.35%) was observed. Postoperative stenosis of responsible vessel was significantly relieved (77.98 ± 7.69 vs. 33.85 ± 9.11), with statistically significant differences (P < 0.01). The extent of neurological deficits and effects on daily living activities at 12 months postoperatively exhibited significant improvements, as evidenced by NIHSS scores (2.40 ± 1.37 vs. 0.82 ± 0.77) and mRS scores ≤ 2 (82.0% vs. 98.0%) (P < 0.01). Cerebral perfusion improved, with no significant perioperative complications.
Conclusions
The Neuroform EZ stent is a safe and effective treatment approach for ICAS in the posterior circulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke represents the most frequent form of stroke and accounts for 69.6–70.8% of the stroke incidence in China [1, 2]. This is frequently caused by intracranial atherosclerotic stenosis (ICAS), alternatively termed intracranial atherosclerotic disease (ICAD), with its incidence differing significantly among races, occurring mainly in Asian, black, and Hispanic populations; the worldwide burden of ICAD-associated stroke is increasing [3, 4]. The incidence of stroke due to atherosclerosis in China is as high as 46.6% [5]. Ischemic stroke resulting from atherosclerotic stenosis of the posterior intercranial circulation often leads to a high rate of disability and mortality [6, 7]. Therefore, effective treatment and preventive measures are needed for addressing posterior circulation intracranial atherosclerotic disease, particularly requiring the attention from frontline clinicians.
Currently, the primary treatment options for ICAS include standardized medication and endovascular intervention (such as percutaneous transluminal angioplasty and stenting). Endovascular intervention has gained increasing attention in the present advances in the development of new techniques and devices. Nonetheless, it still carries a higher risk of perioperative complications and surgical risks. The effectiveness and safety of the Neuroform EZ stent (Stryker Neurovascular, Freemont, CA, USA) in the intervention treatment of ICAS has been confirmed by several studies [8,9,10,11], demonstrating lower perioperative complications and surgical risks. However, with the primary focus of current research being mainly on treating middle cerebral artery stenosis (MCAS), there are no reports on the safety and feasibility of the Neuroform EZ stent in treating symptomatic posterior circulation ICAS. In this retrospective study, we analyzed patients from our center who underwent Neuroform EZ stent treatment for symptomatic posterior circulation ICAS via the radial artery approach to provide relevant evidence-based support for the treatment using endovascular Neuroform EZ stent in the posterior circulation intracranial vessels.
Materials and methods
We enrolled patients treated from January 2021 to June 2023, the Neurology Department at the Affiliated Minzu Hospital of Guangxi medical university, the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University and Ganxian County Branch, Medical Group of the Ganzhou People’s Hospital. Severe stenosis in the intercranial segment of the vertebral or basilar arteries was diagnosed by cerebral angiography, with indications for stent placement. The included patients had arterial stenosis due to atherosclerosis. General anesthesia was administered during the procedures. This research protocol was approved by Ethics Committee of The Affiliated Minzu Hospital of Guangxi Medical University. Prior to the surgery, informed consent forms were signed by all patients or their family members. The extent of arterial stenosis was determined as described by Samuels et al. [12], who calculated the stenosis percentage as [1 - D (stenosis)/D(normal)] × 100%, where D (stenosis) is the diameter of the most severe stenotic segment and D (normal) is the diameter of the proximal normal artery. In the case of pathology in the proximal segment, D (normal) was measured at emergency sites: distal artery (second choice) or supplying artery (third choice).
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria were: (1) patients with severe stenosis (≥ 70%) in the intracranial segments of the vertebral or basilar arteries confirmed by cerebral angiography with clinical symptoms, according to Samuels et al. [12]; (2) presence of hypoperfusion in the responsible vascular supply area based on computed tomography perfusion (CTP) or perfusion-weighted imaging (PWI), according to Suzuki et al. [13]; (3) strokes or transient ischemic attacks in the responsible vascular supply area or progressive stenosis of the target vessel regardless under standardized medication (Anti-plate therapy, lipid-regulating therapy or lifestyle modifications as recommended in trials such as SAMPPRIS and WEAVE); (4) The patients were given dual antiplatelet therapy which was continued for at least three months prior to surgery, as well as routine blood pressure and blood glucose management, but the results were unsatisfactory.
Exclusion criteria
The criteria for exclusion were: (1) history of hemorrhagic diseases within the past 3 months; (2) patients with severe irreversible neurological impairment (mRS ≥ 4) associated with with severe stenosis of the intracranial segments of the vertebral or basilar arteries; (3) non-atherosclerotic ICAS, including suspected vasculitis or arterial dissection; (4) presence of intracranial tumors, arteriovenous malformations, or other intracranial vascular diseases; (5) patients with severe hepatic or renal dysfunction, cardiac or pulmonary insufficiency, or those unable to tolerate general anesthesia.
Preoperative preparation
Patients were given 100 mg of aspirin orally, 75 mg of clopidogrel tablets each day, and 40 mg of atorvastatin calcium tablets at night. Dual antiplatelet therapy was continued for a minimum of three months prior to surgery, as well as routine blood pressure and blood glucose management, but the results were unsatisfactory. Thromboelastogram examination were all performed before the endovascular procedures(AAi > 50%,ADPi > 30%).
Choice of surgical approach
The radial artery approach was chosen as the standard procedure. If the lesion involved the basilar artery, the choice of entry route (left or right radial approach) was determined according to the location of the vertebral foramen on both sides and the dominant vertebral artery. If the lesion involved the V4 segment of the vertebral artery, we opted for the same-side radial artery approach. In cases where the radial artery approach was difficult or contraindicated, the femoral artery approach was utilized.
Conventional radial artery approach interventional procedure
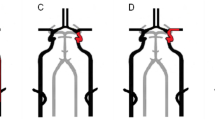
A 6 F radial sheath was implanted using the Seldinger technique with modifications, and systemic heparinization was performed. The contrast agent was manually injected to visualize the radial artery. Throughout the procedure, continuous intravenous infusion of nimodipine (2–3 mL/h) was administered. In cases of a large angle between the vertebral artery ostium and the distal end subclavian artery (Fig. 1a) and low position of the ostium, a 6 F intermediate catheter with a 180 cm ultra smooth guidewire was directly advanced into the vertebral artery V3 and V4 segments or basilar artery. When the angle between the vertebral artery ostium and the subclavian artery was small (Fig. 1b), a 5 F vertebral artery catheter with a guidewire of 180 cm length was first advanced into the V1 segment of the vertebral artery. Then, this guidewire was removed and replaced with a 260 cm guidewire. The tip of the 260 cm guidewire was allowed to reach the distal end of the V1 segment, followed by fixation of the vertebral angiographic catheter in place. The intermediate catheter tip was then guided by the 260 cm guidewire to the V3-4 segments of the vertebral artery or to the basilar artery.
A microcatheter with a 2 M microwire (Synchro2, 0.014’’, 200 cm, Stryker) was employed to pass through the narrow lesion, and the stent delivery catheter was allowed to advance slowly through the lesion under the guidance of the microwire. After securing the catheter, we removed the 2 M microwire and replaced with a 3 M microwire (Synchro2, 0.014’’, 300 cm, Stryker). The 3 M microwire was fixed to the distal end of the lesion, and the stent delivery catheter was then removed. Then, an appropriate-sized GATEWAY balloon catheter was allowed to rapidly reach the site of stenosis. The balloon diameter is sized to match the reference vessel diameter, typically using a 0.8:1 balloon-to-artery ratio. The length should fully span the stenotic lesion. Once positioned, the balloon was slowly inflated, and repeated inflation was limited to no more than three times. After balloon dilation, the appropriate-sized stent delivery catheter was guided to its original position along the 3 M microwire. The stent diameter is selected to match the corresponding arterial lumen dimensions, and its length should be sufficient to fully cover the stenotic segment while preserving critical vasculature at both proximal and distal landing zones. The 3 M microwire was removed and the chosen Neuroform EZ stent was brought to the vicinity of the lesion; the stent catheter and stent were adjusted to maintain slow release. When the stent shape was unsatisfactory, post-dilation was performed with a balloon. This was observed for at least 10 min and when there was no thrombosis in the stent, all systems were slowly removed, and the arterial sheath was removed. Radial artery was compressed using a pressure device for 6 h. Oxygen inhalation and central monitoring were conducted for 24 h postoperatively, and the blood pressure was controlled at < 130/80 mmHg. Standardized dual antiplatelet therapy was maintained, and after 12 months, cerebral angiography was repeated.
Clinical evaluation
General data of the participants, such as sex, age, hypertension, hyperlipidemia, type 2 diabetes, smoking, alcohol consumption history, and medication history were recorded. Adverse events during the perioperative period were observed, including in-stent thrombosis, postoperative hyperperfusion, stroke, and mortality. Before the procedure, neurological deficits (NIHSS score) and neurological recovery (mRS score) were recorded at 12 months postoperatively. The extent of vascular stenosis was observed prior to and following the procedure, and in-stent restenosis (ISR) was recorded at 12 months post-operation. ISR represented a stenosis of > 50% in any location within or adjacent to the stent compared to baseline [14]. This was followed with clinical assessment and follow-up by dedicated personnel.
Statistical analysis
Continuous data are presented as mean ± standard deviation, and between-group differences were analyzed with t-tests. The Chi-square test was used for categorical variables and mortality analysis. For statistical analysis, SPSS 23.0 was used. A p-value < 0.05 was considered statistically significant. Statisticians were blinded to these characteristics of the subjects.
Results
Baseline characteristics of participants
Fifty patients were included after strict screening. Of these, 28 were male and 22 were female, with an average age of 65.86 ± 5.41 years. Among the enrolled patients, there were 40 cases of hypertension, 16 cases of diabetes, 30 cases of hyperlipidemia, 18 cases of smoking history, and 16 cases of alcohol consumption. Surgery for all patients was successful through the radial artery route, with 31 cases through the right radial artery and 19 cases through the left radial artery. Among them, 10 cases received stent implantation in the simple basilar artery, 23 cases received stent implantation in the right V4 segment of the vertebral artery, and 17 cases received stent implantation in the left V4 segment of the vertebral artery. One patient had stent implantation in both a basilar artery and a right V4 segment of the vertebral artery, while another had two stents implanted in the V4 segment. In total, 52 stents were placed in fifty patients, The follow-up duration was 365.24 ± 16.06 days. (Table 1).
Data collection and follow-up
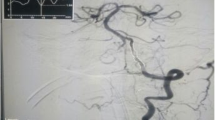
Surgery through the radial artery route for all 50 enrolled patients was successful, with a 100% success rate. Typical cases of basilar artery treatment and the V4 segment of the vertebral artery treatment are shown in Figs. 2 respectively. The main preoperative symptoms of the patients were recurrent dizziness and gait instability, with an average stenosis rate of 77.98 ± 7.69%. One patient had blurred vision, and the ophthalmologist ruled out ophthalmic diseases such as cataracts after consultation, considering it to be caused by occipital lobe ischemia. There were no significant complications during the perioperative period, such as in-stent thrombosis, postoperative hyperperfusion, stroke, or death. The average postoperative stenosis rate was 33.85 ± 9.11%. All patients received standardized medication treatment, and the 12-months follow-up showed significant improvement in their symptoms. At the 12-months follow-up, cerebral angiography was performed and only a case of restenosis was observed in the V4 segment of the vertebral artery (Fig. 3). Although restenosis was observed in this patient during the follow-up, he remained asymptomatic with no complications or clinical manifestations of posterior circulation ischemia. Consequently, he continued to receive standardized secondary preventive treatment for ischemic stroke, alongside regular clinical monitoring. When subsequently develop symptoms of posterior circulation ischemia confirmed by perfusion imaging, endovascular intervention will be reconsidered during later follow-up assessments [15]. The differences in NIHSS scores, mRS scores, and vascular stenosis relative to preoperative values showed statistical sigificance (P < 0.01) (Table 2).
Typical cases (1) A typical case of basilar artery treatment; (2) A typical case of V4 segment of the vertebral artery treatment. a: Re-confirmation of the stenotic segment and its degree; b: Roadmap with the balloon in place (balloon size of 80% of vessel diameter was selected); c: Post-balloon dilation vascular condition; d: Neuroform EZ in place, roadmap image after the slow withdrawal and release of the delivery catheter
Discussion
Currently, ICAS is widely recognized as the main cause of cerebral ischemic events [16]. The CICAS study conducted in China revealed as high as 46.6% incidence of ICAS in individuals diagnosed with ischemic stroke or transient ischemic attacks, which is much higher than in Western populations [5, 17]. Even with standardized medication, the likelihood of recurrent symptomatic ICAS stroke remains elevated [18,19,20]. Therefore, interventional treatment for symptomatic ICAS is a promising approach. The benefits of endovascular treatment have been reported, with rapid developments in interventional technology and improvements in interventional devices. Studies by WEAVE [21], CASSISS [22], and Wang et al. [23] confirmed the safety and feasibility of endovascular treatment. Through strict patient selection and by following standardized medication treatment, endovascular treatment can provide long term benefit to patients, effectively preventing the occurrence of cerebral ischemic events with low perioperative complications.
Initially, Neuroform EZ stent was designed for adjunctive embolization treatment of intracranial aneurysms [22,23,24,25,26]. In the present study, Neuroform EZ stents were successfully implanted in all 50 patients, followed by standardized medication treatment. Angiographic follow-up was completed 12-months postoperatively for all patients, and there was only one case of restenosis within the stent, resulting in 1.9% restenosis rate, lower than rates reported in other studies [27,28,29]. The lower restenosis rate may have resulted from the reduced radial support force of the Neuroform EZ stent. Relevant researchers have suggested that repeat endovascular intervention is unnecessary in the absence of symptoms indicative of posterior circulation ischemia, advocating instead for continuation of standardized secondary preventive measures, This recommendation is grounded in the understanding that microcirculatory compensation at the ischemic site often develops gradually over time [30]. Endovascular therapy will be performed again if necessary during subsequent follow-up, refer to symptom recurrence or the results of cerebral perfusion assessment. Postoperative stenosis of responsible vessel was significantly relieved ((77.98 ± 7.69 vs. 33.85 ± 9.11)), with statistically significant differences (P < 0.01). The extent of neurological deficit and daily living restrictions at 12 months postoperatively exhibited significant improvements, as evidenced by NIHSS scores (2.40 ± 1.37 vs. 0.82 ± 0.77)) and mRS scores ≤ 2 (82.0% vs. 98.0%) (P < 0.01). Cerebral perfusion imaging revealed a significant improvement in low perfusion, and no significant complications occurred during the perioperative period. Thus, these observations showed that the endovascular treatment of symptomatic ICAS using Neuroform EZ stents via the radial artery route was safe and effective.
In this study, surgery for all patients was through the radial artery route. The primary challenges and risks were that the fairly small diameter of the radial artery caused difficulties in puncture, particularly when the distal radial artery approach was used [31]. Also, intervention may be difficult for severe stenotic lesions in the right vertebral artery for patients with severe tortuosity of the right subclavian artery or right brachiocephalic trunk artery or with type II or III aortic arch. In such cases, accessing the vessel through the right radial artery route for vessel access can completely avoid these difficulties, concomitantly reducing costs, shortening operation time, and lowering the difficulty of the procedure, thus ensuring patient safety [32]. This could be one of the reasons for the absence of significant complications during the perioperative period in our study, and further large-sample studies are warranted.
Neuroform EZ stent is delivered through a microcatheter, which enables access to small arteries located at a distance, and it has some self-expanding properties. Neuroform EZ stent is less costly than the Neuroform Atlas, and its deployment method is quite flexible and simple. It has a moderate radial support force, which can reduce the incidence of perforation events. After deployment, it adheres more closely to the vessel wall, which can effectively prevent thrombus formation within the stent. The Neuroform EZ stent was chosen for its mature and comprehensive clinical validation compared to the Neuroform Atlas. Supported by extensive validated safety and efficacy data from long-term use, It also demonstrates superior navigability and deployment stability in complex anatomies. While the Atlas features advanced designs, direct comparative and long-term efficacy data remain limited. Additionally, the Neuroform EZ stent features a larger mesh size, which reduces the likelihood of damage to small branches. Its slow-release delivery minimizes injury to the vascular intima compared to balloon-expandable stents, resulting in a lower rate of restenosis within the stent. Several stroke centers have confirmed the efficacy of this stent in treating atherosclerotic stenosis in the anterior circulation [8,9,10,11]. The Wingspan stent, characterized by its relatively rigid structure, is predominantly utilized in the treatment of intracranial stenosis within the anterior circulation. However, it demonstrates limited advantages when applied to tortuous posterior circulation vessels. Furthermore, its deployment process presents notable technical complexity, necessitating higher procedural expertise from the operator [33].
This study had certain limitations. First, the participant selection was stringent, comprising posterior circulation symptomatic ICAS cases. Considering the limitations of the lumen size of the radial artery, the Neuroform EZ stent was selected, which has relatively fewer requirements for the catheter size in the distal access. Second, performing surgery via the radial artery route is difficult for patients with a low opening of the vertebral artery, closer to the proximal subclavian artery; however, this situation was not encountered in this study. Although the 12-month follow-up results of this study demonstrate the safety and efficacy of the Neuroform EZ stent in treating posterior circulation ICAS, it is imperative to acknowledge the critical importance of long-term follow-up for a comprehensive assessment of the stent’s safety and efficacy profile. Delayed ISR, stroke recurrence, and long-term vessel patency are key factors that influence patient outcomes and may only manifest after an extended period post-operation. Therefore, while this study provides preliminary evidence supporting the use of the Neuroform EZ stent, we emphasize the necessity of extended follow-up studies to fully understand the long-term effects of the treatment. Furthermore, this study design was pre-procedure vs. post-procedure comparative, which may introduce selection bias. Although the rate of restenosis within the stent was lower compared to that in other centers, the sample size was relatively small, necessitating more research for confirmation. Therefore, more large-scale randomized, double-blind, and prospective studies are needed to provide more evidence-based medical support regarding the safety and efficacy of Neuroform EZ stent application in posterior circulation ICAS.
Data availability
No datasets were generated or analysed during the current study.
References
Wang W, Jiang B, Sun H et al (2017) Prevalence, incidence, and mortality of stroke in china: results from a nationwide Population-Based survey of 480 687 Adults[J]. Circulation 135(8):759–771. https://doi.org/10.1161/CIRCULATIONAHA.116.025250
Wang D, Liu J, Liu M et al (2017) Patterns of stroke between Universitv hospitals and nonuniversity hospitals in Mainland china: prospective muhicenter Hospital-Based registry Study[J]. World Neurosurg 98:258–265. https://doi.org/10.1016/j.wneu.2016.11.006
Qureshi AI, Caplan LR (2014) Intracranial atherosclerosis. Lancet 383(9921):984–998. https://doi.org/10.1016/S0140-6736(13)61088-0
Flusty B, Havenon Ad, Prabhakaran S et al (2020) Intracranial atherosclerosis treatment: past, present, and future. Stroke 51(3):e49–e53. https://doi.org/10.1161/STROKEAHA.119.028528
Wang YJ, Zhao XQ, Liu LP et al (2014) Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in china: the Chinese intracranial atherosclerosis(CICAS)study. Stroke 45(3):663–669
Mattle HP, Arnold M, Lindsberg PJ et al (2011) Basilar artery occlusion[J]. Lancet Neurol 10(11):1002–1014. https://doi.org/10.1016/S1474-4422(11)70229-0
Tao CR, Li R, Zhu YY et al (2022) Endovascular treatment for acute Basilar artery occlusion: a multicenter randomized controlled trial (ATTENTION). Int J Stroke 17(7):815–819. https://doi.org/10.1177/17474930221077164
Du ZH, Mang J, Yu SY et al (2018) Weighing in on the off-label use: initial experience of neuroform EZ stenting for intracranial arterial stenosis in 45 patients. Front Neurol 9: 852.https://doi.org/10.3389/fneur.2018.00852
Li H, Zhang L, Wang P et al (2022) The safety and efficacy of the neuroform ez stent for the treatment of symptomatic atherosclerotic stenosis in the middle cerebral artery[J]. Clin Imaging 82:210–215. https://doi.org/10.1016/j.clinimag.2021.11.024
Xu H, Quan T, Zaidat OO et al (2019) Neuroform EZ stenting for symptomatic intracranial artery stenosis: 30 days outcomes in a high-volume stroke center[J]. Front Neurol 10:428. https://doi.org/10.3389/fneur.2019.00428
Zhou K, Cao Y, He XH et al (2021) A comparison of safety and effectiveness between wingspan and neuroform stents in patients with middle cerebral artery stenosis. Front Neurol 20(12):527541. https://doi.org/10.3389/fneur.2021.527541
Samuels OB, Joseph GJ, Lynn MJ et al (2000) A standardized method for measuring intracranial arterial stenosis[J]. Am J Neuroradiol 21(4):643–664
Suzuki Y, Nakajima M, Ikeda H et al (2005) Evaluation of hyperacute stroke using perfusion computed tomography[J]. Neurol Med Chir (Tokyo) 45(7):333–343. https://doi.org/10.2176/nmc.45.333
Zheng D, Mingyue Z, Wei S et al (2018) The incidence and risk factors of In-Stent restenosis for vertebrobasilar artery stenting. World Neurosurg 110:e937–e941. https://doi.org/10.1016/j.wneu.2017.11.112
(2022) Chinese Experts Consensus on Endovascular Treatment for Symptomatic Intracranial Atherosclerotic Stenosis 2022. Zhongguo Cuzhong Zzazhi 17(8): 863–888. https://doi.org/10.3969/j.issn.1673-5765.2022.08.013
Wong KS, Gao S, Chan YL et al (2002) Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study[J]. Ann Neurol 52(1):74–81. https://doi.org/10.1002/ana.10250
Wong LK (2006) Global burden of intracranial atherosclerosis[J]. Int J Stroke 1(3):158–159. https://doi.org/10.1111/j.1747-4949.2006.00045.x
Chimowitz MI, Lynn MJ, Derdeyn CP et al (2011) Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 365(11):993–1003. https://doi.org/10.1056/NEJMoa1105335
Zaidat OO, Fitzsimmons BF, Woodward BK et al (2015) Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial[J]. JAMA 313(12):1240–1248. https://doi.org/10.1001/jama.2015.1693
Elhfnawy AM, Heuschmann PU, Pham M et al (2019) Stenosis length and degree interact with the risk of cerebrovascular events related to internal carotid artery Stenosis[J]. Front Neurol 10:317. https://doi.org/10.3389/fneur.2019.00317
Lexander MJ, Zauner A, Chaloupka JC et al (2019) WEAVE trial: final results in 152 on-label patients[J]. Stroke 50(4):889–894. https://doi.org/10.1161/STROKEAHA.118.023996
Gao P, Zhao ZW, Wang DM et al (2015) China angioplasty and stentin gforsymptomatic intracranial severe stenosis (CASSISS):a new prospective, multicenter,randomized controlled trial in China[J]. Interv Neuroradiol 21(2):196–204. https://doi.org/10.1177/1591019915581778
Wang T, Yang K, Luo J et al (2020) Outcomes after stenting for symptomatic intracranial arterial stenosis: a systematic review and meta-analysis[J]. J Neurol 267(3):581–590. https://doi.org/10.1007/s00415-018-09176-x
(2012) Neuroform EZ Stent System. Directions for use. Fremont (CA): Boston Corporation
Mangubat EZ, Johnson AK, Keigher KM et al (2012) Initial experience with neuroform EZ in the treatment of Wide-neck cerebral Aneurysms[J]. Neurointervention 7(1):34–39. https://doi.org/10.5469/neuroint.2012.7.1.34
Liu XL, Wang B, Zhao LB et al (2022) Overlapping stents-Assisted coiling for vertebral artery dissecting aneurysm: LVIS stent within neuroform EZ stent[J]. J Korean Neurosurg Soc 65(4):523–530. https://doi.org/10.3340/jkns.2021.0275
Meyer L, Leischner H, Thomalla G et al (2020) Stenting with Acclino (flex) for symptomatic intracranial stenosis as secondary stroke prevention[J]. J Neurointerv Surg 12(11):1127–1131. https://doi.org/10.1136/neurintsurg-2019-015744
Mohammaden MH, Nogueira RG, Tekle W et al (2022) Safety and efficacy of balloon-mounted stent in the treatment of symptomatic intracranial atherosclerotic disease: a multicenter experience[J]. J Neurointerv Surg 14(8):756–761. https://doi.org/10.1136/neurintsurg-2021-017818
Vajda Z, Schmid E, Güthe T et al (2012) The modified Bose method for the endovascular treatment of intracranial atherosclerotic arterial stenoses using the enterprise stent. Neurosurgery 70(1):91–101. https://doi.org/10.1227/NEU.0b013e31822dff0f
Han X, Duan H, Zhang M, Wang L, Kong L (2022) Cerebral vascular collateral circulation assessment for restenosis after cerebrovascular intervention. Asian J Surg 45(1):554–555. https://doi.org/10.1016/j.asjsur.2021.09.027
Brunet M, Chen S, Sur S et al (2019) Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography[J]. J Neurointerv Surg 11(7):710–713. https://doi.org/10.1136/neurintsurg-2019-014718
Khanna O, Sweid A, Mouchtouris N et al (2019) Radial artery catheterization for neuroendovascular procedures. Stroke 50(9):2587–2590. https://doi.org/10.1161/STROKEAHA.119.025811
Zhu Z et al (2024) Evaluation of safety and efficacy of intracranial self-expanding drug-eluting stents for symptomatic intracranial atherosclerotic stenosis: a prospective, multicentre, randomised controlled, superiority clinical trial protocol. BMJ open 14,11 e091152. 27 Nov. https://doi.org/10.1136/bmjopen-2024-091152
Funding
This research was supported by grants from the project from Science and Technology Department of Sichuan Province, China (No. 2022YFS0618), Major Science and Technology Special Project of Sichuan Provincial Department of Science and Technology, China (No.2022ZDZX0022).
Author information
Authors and Affiliations
Contributions
Li Chen, Yu Lan and Kaimin Xiao designed and conceptualized the study. Cai Zhong and Liang Zhong acquired data. Kaimin Xiao, Rongxin He and Hongmei Chen drafted the manuscript. Ping Liu and Jiao Wu participated in the data analysis. Heling Chu and Yuping Tang revised the manuscript. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Declaration of Helsinki and its later amendments or comparable ethical standards. This research protocol was approved by Ethics Committee of The Affiliated Minzu Hospital of Guangxi Medical University, the review number is: Gui Min Yi Lun Shen Tong Zi [2021] No.103.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, K., He, R., Chen, H. et al. Safety and efficacy of the neuroform EZ stent for treating intracranial atherosclerotic stenosis in the posterior circulation: a comparative study of pre-procedure and post-procedure outcomes. Langenbecks Arch Surg 410, 186 (2025). https://doi.org/10.1007/s00423-025-03773-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-025-03773-x