Abstract
Background
Decompressive craniectomy (DC) is a last-tier treatment for managing refractory intracranial hypertension in patients with aneurysmal subarachnoid hemorrhage (aSAH), though concerns persist about whether it primarily prolongs survival in a state of severe disability. This study investigated patient characteristics, surgical indications, complications, and outcomes following DC in aSAH.
Methods
In this Swedish, retrospective multi-center study, 123 aSAH patients treated with DC between 2008–2022 were included. Data collection included demographic details, aSAH characteristics, injury severity, DC indication, complications, and outcome at roughly six months post-DC (modified Rankin scale [mRS]) dichotomized as survival vs. mortality (0–5 vs. 6) and favorable vs. unfavorable (0–3 vs. 4–6).
Results
The median age was 53 years and 66% were females. Two thirds presented with a WFNS grade 4–5 and 83% with a Fisher grade 4 hemorrhage. Most aneurysms were located at the middle cerebral artery (65%) and treated with clip ligation (59%). DC significantly reduced midline shift from 9 to 2 mm and obliteration rates of basal cisterns from 95 to 22% (p < 0.05). Reoperation for hematomas or extension of the DC were rare (< 5%). At follow-up, 20% were deceased, while 33% had recovered favorably. In univariate logistic regressions, younger age was associated with favorable outcome and reduced mortality. Other patient demographics, injury severity, and factors related to the DC surgery lacked association with outcome.
Conclusions
aSAH patients treated with DC presented with severe primary brain injuries and signs of intracranial hypertension. DC resulted in radiological improvements regarding mass effect and a low rate of postoperative complications. Although the results were based on a selected population of aSAH patients, an encouraging rate of favorable outcome was found, particularly among younger patients. However, the absence of additional outcome predictors underscores the ongoing challenges in improving patient selection for DC in aSAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) continues to carry a high mortality and morbidity rate [30]. Modern management focuses on early aneurysm occlusion to prevent rebleeding, cerebrospinal fluid (CSF) drainage to treat acute hydrocephalus, and vigilant efforts to prevent delayed cerebral ischemia (DCI) [9, 11]. Furthermore, neurocritical care (NCC) aims to optimize cerebral physiology, particularly by controlling intracranial pressure (ICP) to prevent brain herniation and ischemia [8, 9, 35, 38, 39]. Elevated ICP is common after aSAH, occurring both immediately after ictus and later during the acute phase due to rebleeding, hydrocephalus, and brain edema [24, 31, 36, 47]. Intracranial hypertension may be indicated by a deterioration in consciousness noted in neurological examinations and presence of mass effect on imaging. In addition, unconscious aSAH patients who are intubated are typically monitored for ICP using either an external ventricular drainage (EVD) or an intraparenchymal probe when repeated neurological examinations are less feasible. In these situations, maintaining ICP below 20 mmHg is generally targeted, although robust evidence supporting specific ICP thresholds in aSAH remain scarce [31, 34, 35, 38]. NCC strategies focus on both preventing and actively managing intracranial hypertension through interventions such as CSF drainage, hematoma evacuation, controlling pCO₂, optimized sedation, and hyperosmolar therapy [8, 31, 38]. Despite these first-line measures, approximately 10% of aSAH patients develop refractory intracranial hypertension, for whom last-tier interventions, including barbiturates and decompressive craniectomy (DC), may be considered [3, 4, 6, 10, 12, 15, 19, 25, 44]. The role of DC has been extensively studied in randomized controlled trials and meta-analyses for other acute brain injuries [21, 22, 29]. Specifically, DC has become a widespread treatment to alleviate intracranial hypertension in traumatic brain injury (TBI) [16, 22] and to prevent brain herniation in malignant middle cerebral artery (MCA) infarction, both lowering mortality and resulting in an increased chance of a favorable outcome [21, 29, 42]. In contrast, the role of DC in aSAH remains less well-established, having been primarily evaluated in a limited number of cohort studies [3, 10, 12, 15, 18, 44] that have demonstrated rather poor long-term results with only 10–15% reaching favorable outcome in two recent studies [6, 44]. Notably, aSAH patients treated with DC are in general older with more comorbidities than TBI patients, and often sustain both a global injury at ictus and secondary focal injuries from aneurysm treatments and DCI while DC-treated patients with MCA infarctions exhibit more focally restricted brain injuries. These factors may predispose to a reduced baseline capacity for functional recovery and result in a more severe and widespread cerebral insult compared to other acute brain injury subtypes. Consequently, significant concerns persist regarding whether DC can result in meaningful recovery in aSAH patients with refractory intracranial hypertension or merely prolong survival in a state of severe disability.
To address these questions, the primary aim of this Swedish multi-center study was to investigate the indications for DC and its ability to reduce mass effect and lead to favorable outcomes following aSAH. Based on these data, a secondary aim was to identify predictors of outcomes, including mortality. We hypothesized that DC was relatively rare in aSAH management and that the majority had an unfavorable outcome. It was further hypothesized that younger age, less severe primary brain injury, and the absence of DCI could be associated with more favorable outcomes.
Materials and methods
Patients and study design
This retrospective observational, multi-center study included all patients who underwent DC following aSAH during a 15-year period (2008–2022) at any of the neurosurgical departments in Gothenburg (2008–2018 only), Stockholm, Uppsala, and Umeå. These centers collectively provide neurosurgical care to a catchment area encompassing 7.5 million people, representing 72% of the Swedish population.
Treatment protocol
Patients with aSAH were admitted to the neurosurgical department within their respective catchment areas. All centers generally adhered to treatment principles outlined in international guidelines [17, 39], though variations between local management protocols existed [2]. Aneurysms were typically treated as early as possible, either by endovascular intervention or surgical clipping. Significant intracerebral hemorrhages (ICH) were surgically evacuated, and all patients started administration of nimodipine upon admission. Poor-grade aSAH patients (World Federation of Neurosurgical Societies [WFNS] grade 4–5) were typically intubated, mechanically ventilated, and sedated. Unconscious patients and awake patients with hydrocephalus underwent placement of an external ventricular drainage (EVD). DCI was defined as focal neurological deficits or a decline in consciousness occurring at a delayed time point from ictus, not attributable to complications such as rebleeding, hydrocephalus, or meningitis. In cases of suspect DCI, modified HHH (hypertension, hypervolemia, and hemodilution)-therapy, as well as endovascular interventions (intra-arterial vasodilation or balloon angioplasty), were considered [13]. The modified HHH approach primarily focused on induced hypertension, near-zero fluid balance (with strict avoidance of hypovolemia), and generous administration of colloid fluids, though with some variation among centers. At some institutions, vasospasm treatment was avoided at the manifestation of cerebral infarctions and/or difficulties to control ICP. Furthermore, ICP was typically maintained below 20 mmHg, and cerebral perfusion pressure (CPP) above 60–70 mmHg. Basal ICP-lowering treatments included head of bed elevation to 30° at most centers, avoiding hypercapnia, CSF drainage via an EVD, and sedation (primarily propofol or midazolam, with barbiturates employed as a second-tier treatment for refractory ICP).
Primary DC without ICP monitoring was performed early on in cases of severe mass effect or perioperative brain swelling, typically in conjunction with surgical clipping and evacuation of an ICH. Secondary DC was reserved as a last-tier intervention for refractory intracranial hypertension. Hemicraniectomy was the preferred approach for lateralized brain injury or swelling with midline shift, whereas bifrontal DC was employed for diffuse bilateral brain swelling without midline shift. The main objective in all DCs was maximal decompression, achieved through the removal of a large bone flap with duraplasty.
Data acquisition
Clinical, radiological, and outcome data were collected from medical records and radiological imaging. Demographic variables included age and sex. The severity of the primary injury was assessed using clinical (WFNS) and radiological (Fisher) grades. Pupillary reactivity at admission was classified as normal, one unreactive pupil, or two unreactive pupils. Data on aneurysm location and treatment modality were recorded, along with information on surgical evacuation of ICH, ICP monitoring, and barbiturate treatment.
The extent of mass effect, quantified by midline shift (mm) and the status of the basal cisterns (categorized as open, compressed, or obliterated), was assessed using head computed tomography (CT) scans obtained before and after DC. The type of DC (hemicraniectomy vs. bifrontal craniectomy) and the area of decompression (cm2) were also evaluated. The latter was determined by measuring the anteroposterior width (from tabula externa) and craniocaudal height of the decompression on CT imaging [32, 37]. Postoperative complications were systematically analyzed, including reoperations for postoperative hematomas, extensions of the cranial decompression, surgical site infections, and subdural hygromas. The presence of postoperative external brain herniation exceeding 1.5 cm [46] and CSF circulation disturbances requiring shunt placement were also recorded.
Lastly, the functional status was assessed by retrospective chart review and determined according to the modified Rankin Scale (mRS) [5] at a time point as close to six months post-DC as possible. Outcome was dichotomized into survival vs. mortality (mRS 0 to 5 vs. 6) and favorable vs. unfavorable (mRS 0 to 3 vs. 4 to 6). All collection of data, including mRS outcomes, was performed by neurosurgical trainees (1–3 per center) with previous experience of chart review and assessment of functional outcomes in similar retrospective study settings. All mRS assessments were based on thorough scrutiny of medical records, including notes on out-patient follow-up visits and telephone follow-ups, while also medical notes from additional in-patient care in close proximity to the six months post-DC endpoint. All information available, including nursing documentation, was used to determine the patient’s ability to live independently (or with community care), ambulate, including their working capacity, and in turn transformed into a functional outcome according to the mRS. In any case where there was insufficient information to determine functional status, the outcome was reported as missing. Also, to ensure consistency among centers, all personnel involved in the collection of data adhered to a pre-defined study protocol that included a list of variables and information on how these should be interpreted and reported (including mRS ascertainments) to make the data assessments as uniform as possible.
Statistical analysis
Statistical analyses were performed using RStudio software (version 2022.12.0). Continuous and ordinal variables were summarized as medians with interquartile ranges (IQR), while categorical variables were reported as counts and proportions. Changes in radiological mass effect, including midline shift and status of the basal cistern, before and after DC were evaluated using the Wilcoxon test or McNemar’s test, depending on the data type. With mortality and favorable outcome as dependent variables, demographic factors, injury severity, and surgical aspects of DC were analyzed using univariate logistic regression, described with odds ratios (OR) and 95% confidence intervals (CIs). A p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
In the cohort of 123 aSAH-patients treated with DC, the median age was 53 (IQR 45–59) years and 81 (66%) were females (Table 1). At admission, 67% presented with a WFNS grade 4 to 5, while 13% patients exhibited one or two unreactive pupils (missing data, n = 1). All patients had a grade 3 or 4 Fisher score and most aneurysms were located at the MCA (65%), followed by the internal carotid artery (ICA; 22%), anterior cerebral artery (ACA; 12%), and the posterior circulation (1%). Most aneurysms were treated with clip ligation (59%), 35% underwent endovascular embolization, 5% received both treatments, and aneurysm occlusion was not feasible in 1%. A majority (54%) of patients had an ICH that required evacuation. Nearly all patients (99%) underwent ICP monitoring, using either an EVD in 63%, an intraparenchymal monitor in 8%, or both in 28% of cases. DCI occurred in 20%, and 46% were treated with barbiturates.
DC – indication, timing, type, size, and impact on mass effect
A minority of cases (8%) underwent primary DC (Table 2), while the majority were secondary DCs performed due to refractory intracranial hypertension and/or radiological mass effect. The median time from ictus to DC was 3 days (IQR 2–5). Nearly all DCs (98%) were unilateral. The median DC area was 103 (IQR 95–112) cm2. The extent of midline shift significantly improved after DC for all patients in the cohort, from a median of 9 mm (IQR 5–12) preoperatively to 2 mm (IQR 0–4) postoperatively (p < 0.001) (Table 3). Furthermore, basal cisterns were compressed or obliterated in 95% of cases before DC, decreasing to 22% post-DC (p < 0.001).
DC – complication rate
A minority of DC cases required reoperation, including evacuation of postoperative hematomas (2%), enlargement of the cranial decompression (2%), evacuation of a subdural hygroma (1%), or revision due to a surgical site infection (1%). External brain herniation (> 1.5 cm) was observed in 89% of the patients, whereas 18% required a shunt at any time point after ictus due to a non-resolved CSF circulation disorder (24% when considering only non-deceased patients).
DC – functional outcome and prognostic factors
The median time point for outcome assessment was 6 (IQR 5–11) months post-ictus, of which 10 patients had outcome status reported as missing (8%). At follow-up, the median mRS was 4 (IQR 3–5), with one-third (33%) of patients demonstrating a favorable outcome, and 20% being deceased (Table 1 and Fig. 1). In both the primary and secondary DC sub-cohorts, mortality was 20% (p = 0.98), with the rate of favorable outcome slightly higher in the group treated with primary DC (50% vs. 31%, p = 0.22). As shown in Table 4, older age was associated with higher mortality (OR [95%CI] = 1.05 [1.01–1.11], p = 0.04) and a lower rate of favorable outcome (OR [95% CI] = 0.93 [0.90–0.97], p < 0.001). However, no significant associations were identified between mortality or functional outcomes and comorbidities, injury severity, DCI, or the timing and indication for DC. As shown in Fig. 2, mortality was relatively low, and the rate of favorable outcomes was 25% or higher in patients aged below 60 years. In contrast, the majority of patients aged over 60 either survived with a poor outcome or were deceased at follow-up.
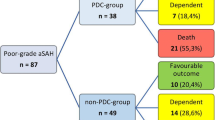
Six-months functional outcome after DC in aSAH. Illustrating the percentage of patients within each mRS category in the aSAH patients treated with DC. Ten patients had no available outcome data and they were excluded from this figure. aSAH = Aneurysmal subarachnoid hemorrhage. DC = Decompressive craniectomy. mRS = Modified Rankin Scale
Mortality- and favorable outcome rates for different age categories. Depicting the percentage of patients who were deceased or had reached favorable outcome at follow-up six months after DC with age stratified into decades. Young adolescents and patients in early to middle adulthood (up to 60 years) demonstrated relatively low mortality and high rates of favorable outcomes, whereas individuals above 60 years of age exhibited less favorable recovery. DC = Decompressive craniectomy
Discussion
In this observational multi-center study of 123 aSAH patients, treatment with DC resulted in immediate radiological improvements in mass effect and a low rate of complications requiring reoperation. Survival at six months was 80%, and 33% achieved favorable outcome. While younger age was associated with better outcomes, other patient- and injury-related predictors of recovery provided limited prognostic information. These results suggest that favorable outcome is possible for a significant proportion of aSAH patients with refractory intracranial hypertension who were treated with DC. However, it is important to stress the lack of a control group of patients with refractory intracranial hypertension who were managed conservatively and that the results must be interpreted in the light of this limitation. Furthermore, the lack of robust prognostic factors, aside from age, highlights the challenges in predicting which aSAH patients that may sustain a favorable outcome after DC.
Only 123 aSAH patients underwent DC over a 15-year period (10 years in Gothenburg) within a catchment area of 7.5 million people. The estimated annual incidence of spontaneous SAH in Sweden is approximately 10 per 100,000 individuals and year [26, 33], with aneurysmal etiology accounting for roughly 75–85% of these cases [26, 43]. Based on these estimates, only 1–2% of the aSAH population within our catchment area were treated with DC, a proportion notably lower than the 10% reported in a recent meta-analysis by Darkwah Oppong et al. [10]. These discrepancies may stem from an overestimated aSAH incidence in our calculations, selection bias in earlier studies (e.g., excluding patients with poor prognoses who were not admitted to tertiary centers), and differences in DC indications across centers. Regardless, it is evident that DC is reserved for a small and highly selected subgroup of aSAH patients.
Demographics of our DC patients, including age and sex, were consistent with general aSAH populations [6], with the majority being female at around 50 years of age, and only two patients older than 70 years, likely reflecting a tendency to withhold DC in older patients due to the anticipated poor prognosis for meaningful recovery. As expected, our data demonstrated that the majority of aSAH patients treated with DC presented with a severe primary brain injury, as reflected by high WFNS and Fisher grades. Consistent with previous studies, most DC patients had an MCA aneurysm with an ICH and underwent surgical clipping with hematoma evacuation. Notably, primary DCs in immediate conjunction with ICH evacuation or surgical clipping were rare (8%). Instead, the majority of DC procedures were performed later, after a median of 3 days from ictus (IQR 2–5). This time interval corresponds to the early effects of the primary brain injury, the progression of brain edema, and potential complications following aneurysm treatment. DCI typically happens later and occurred in approximately 20% of the cases, aligning with rates reported in broader aSAH cohorts [6, 8]. However, we cannot exclude that DCI may have contributed to cytotoxic edema and ICP elevation in some of the DC patients studied herein, not least considering that the presence of substantial early brain injury and associated neurological deficits may have masked the effects of DCI and hence led to underdiagnosis of this complication.
Before DC, radiological imaging demonstrated undoubtable signs of mass effect, including midline shifts and compression of the basal cisterns, which significantly improved postoperatively. DC was also associated with a low surgical complication rate, with reoperations due to postoperative hematomas, subdural hygromas, and surgical site infections found in less than 5% of patients. The median size of the DC was approximately 100 cm2, i.e., fairly large, while the need for craniectomy extension at a second stage was rare (Table 2). Most patients exhibited external brain herniation at the craniectomy site, potentially leading to cerebral venous congestion due to impingement against the bony edges of the craniectomy. However, the clinical relevance of this observation and exact mechanism remains unclear [46]. Chronic hydrocephalus requiring shunt placement was relatively frequent in this cohort. While this is a common phenomenon in aSAH in general, DC itself is also known to alter CSF dynamics, including reduced ICP pulse waveforms and impaired resorption, which may independently contribute to hydrocephalus [28]. In keeping with this theory, the 18–24% shunt-dependency rate observed in our cohort (with range depending on if the denominator was based on patients alive at follow-up exclusively or not) was slightly higher than rates reported in broader cohorts of aSAH patients, which include both DC-treated and non-DC-treated individuals, typically ranging from 6–18% [1, 23, 27]. Given the relatively low surgical complication rate of DC in this cohort, its use could potentially be considered earlier in selected cases, particularly in relation to high-dose barbiturate therapy that typically sustains several medical complications [6, 7].
Consistent with the short-term improvements in mass effect after DC, six-months outcomes were highly encouraging with a mortality limited to 20% and 33% of patients achieving favorable outcome. These figures are more favorable than most previous studies on DC in aSAH, with mortality rates ranging from 30–96% and favorable outcomes found in only 4–20% of cases [10, 14, 41, 44], although, some single-center studies have showed more promising results [20]. These differences in DC outcomes may reflect variations in surgical indications and timing. For instance, applying a lower threshold for DC would include patients with less severe injuries, who inherently have a better prognosis. On the other hand, extending the use of alternative ICP management strategies may result in performing DC too late, i.e., at a stage when potentially salvageable neurological injuries have already become irreversible. Additionally, a more conservative approach may influence outcomes after DC, as only patients with a better prognosis are likely to undergo the procedure. The role of DC in aSAH management must also be viewed in a broader context. To obtain successful outcomes, these patients require attentive NCC management throughout the acute phase [38] followed by vigorous neurorehabilitation, to both reduce the burden of secondary brain injury, and to promote early recovery. Variations in six-months outcomes could thus also in part be attributed to differences in these aspects between centers.
As mentioned previously, DC has become a last-tier surgical option for intracranial hypertension in a variety of different acute brain injuries. Of particular interest, the rate of favorable outcome seems to be slightly worse in aSAH patients treated with DC compared to in TBI [22, 45] or in malignant MCA infarctions [21, 29, 45]. Several factors may account for these inconsistencies. One explanation could be that aSAH patients tend to be older than those with TBI and therefore harbor lower functional reserves to recover. Brain atrophy that increases with age also implies that a relatively worse underlying brain injury with more additional edema/hemorrhage volume is required to elicit ICP elevations as when compared to younger individuals. Moreover, aSAH is characterized by both severe global and focal brain injury, of which the global injury typically arises at ictus of the initial bleeding – often accompanied by a transient global hypoperfusion. Focal injuries, on the other hand, commonly occur when aneurysms, particularly in the MCA territory, rupture into the parenchyma, causing an ICH and localized tissue destruction. Treatment of the ruptured aneurysm can also lead to further complications, such as infarctions in downstream vascular territories. Additionally, aSAH patients frequently experience vasospasm and DCI that can entail both focal and widespread infarctions. These focal injuries, especially those involving the MCA territory, often affect motor and language functions that can result in significant disabilities such as hemiparesis and aphasia. The edema in aSAH patients is usually cytotoxic and corresponds to irreversible brain damage, in contrast to TBI wherein vasogenic edema with potentially salvageable brain tissue is more common [40]. Malignant MCA infarction patients also exhibit cytotoxic brain edema, but lack the global brain injury characteristic of aSAH. Altogether, the fact that aSAH patients are generally older, exhibit significant widespread primary and secondary brain injuries, and that the underlying edema often represents cytotoxic swelling due to irreversible brain damage, may contribute to significant morbidity specific for aSAH patients, as compared to DC outcomes after other types of acute brain injuries. Again, nonetheless, results of the present study indicate that favorable results can be achieved with DC for aSAH patients with refractory intracranial hypertension.
Consequently, judicious patient selection seems to be of crucial importance when considering DC in aSAH patients, and in turn suggests identifying predictors of long-term outcome remains a key factor to aid these decisions. To aid these decisions, potential determinants of six-months outcomes among aSAH patients treated with DC were therefore analyzed herein. Regrettably, higher age emerged as the sole risk factor linked to higher mortality and a reduced likelihood of favorable outcomes, particularly for patients above 60 years. Again, this observation likely owes to factors mentioned previously: younger patients generally have greater functional reserves, enhanced capacity for neuroplasticity, and are more susceptible to ICP issues with smaller volumes of edema or bleeding. In contrast, variables related to injury severity (e.g., WFNS or Fisher grades), complications (e.g., DCI), or the timing of DC were not significantly associated with functional outcomes or survival. These findings suggest that the underlying cause and timing of ICP elevation may be less critical for predicting outcomes, as all patients in this cohort ultimately sustained severe brain injuries. This aligns with observations from two smaller studies [6, 44]. A plausible interpretation is that predicting recovery in individual patients remains inherently challenging. In our opinion, however, given the relatively favorable outcomes observed in the current study, it seems reasonable to consider DC in aSAH-patients even when prognostication is uncertain, particularly in those aged under 60.
Methodological considerations
The primary strength of this study lies in its multi-center design and relatively large cohort. The dataset was mostly near-complete, encompassing detailed information on demographics, aSAH characteristics, treatments, clinical course, and outcomes. Considering the rarity of DC in aSAH, the lack of consensus regarding treatment timing and indications, and ethical concerns around withholding potentially life-saving treatments, conducting large randomized controlled trials on this topic in the near future remains unlikely. Thus, this study provides valuable "real-world" data that contribute to the understanding of DC in aSAH management.
However, there are also several study limitations. The main drawback is the retrospective design and data collection based on historical information, in which selection bias and residual confounding are difficult to account for. In addition, the study included patients from four different centers with slightly different management protocols, e.g., in terms of strategies for aneurysm occlusion, escalation of ICP-lowering treatments, and indication for DC. Also, one of the principal findings – namely the encouraging degree of favorable outcome – was based on outcomes determined by means of retrospective chart review. While these assessments may be subject to inter-rater variability and were based on medical notes that were sometimes summary, several academic neurosurgeons with previous experience of chart review participated in the collection of data, for which patients who could not be categorized with regards to outcome were strictly reported as data missing. Furthermore, while imprecise mRS assessments could theoretically have resulted in minor inaccuracies, all analyses were conducted with mRS outcomes dichotomized between favorable (mRS 0–3) vs. unfavorable (mRS 4–6), i.e., essentially a cut-off between those ambulatory vs those not, and therefore helped to minimize the potential impact of outcome grading precision. While the exact time point for assessment of functional outcome also varied slightly within the cohort, it typically occurred at or later than six months post-DC, and thus allowed for recovery to take place, while otherwise in effect resulting in an underestimation of the chances to achieve a favorable outcome. In addition, reporting the number of patients treated with cranioplasty would have been of additional value to the study results and constitutes another drawback. However, as cranial reconstruction is a known important factor for functional recovery, the expected effect of outcome assessments possibly based on a few patients still harboring a cranial defect would be an underestimation of the overall results and chances of a favorable outcome after DC in aSAH. Thus, an outcome assessment beyond six-months post-ictus (probably at least twelve months) would for several reasons have been more appropriate to capture the actual long-term outcome in these patients. Moreover, as part of a broader study project on DCs, the study did not include a reference group of aSAH patients managed conservatively. This is important to bear in mind when interpreting the results, as some patients in poor condition (or for other various reasons) were probably disqualified for DC and, had they been treated, could theoretically have attenuated the somewhat favorable results demonstrated. While the analyses utilized detailed clinical and radiological data routinely employed in clinical practice, only age emerged as a significant predictor of six-months outcomes. A more nuanced radiological assessment of the extent and neuroanatomical location of each brain injury could have added further predictive information and should likely be considered in future research attempts aiming to understand outcomes after DC for aSAH. Although this study constitutes one of the largest cohorts to date on aSAH patients treated with DC, the treatment remains rare. Consequently, the statistical power of the analyses – particularly regarding mortality and favorable outcome predictors – was limited. To enable more robust analyses and develop firmer multivariable regression models, larger cohorts are required.
Conclusions
DC in patients with aSAH demonstrated immediate radiological reduction of mass effect and a low incidence of complications requiring reoperation. Despite the severe underlying brain injuries predisposing patients to intracranial hypertension, 80% survived at six months, with 33% achieving favorable outcome. Younger age was identified as the strongest predictor of favorable outcomes, while other patient- and injury-specific factors offered limited prognostic value. These results underscore the potential for meaningful recovery in a subset of aSAH patients treated with DC. However, the absence of robust predictors beyond age complicates the selection of candidates for DC in cases of refractory intracranial hypertension. Future prospective studies are needed to refine the indications and patient selection for DC and to identify factors associated with optimal outcomes.
Data availability
Data are available upon reasonable request.
References
Adams H, Ban VS, Leinonen V, Aoun SG, Huttunen J, Saavalainen T, Lindgren A, Frosen J, Fraunberg M, Koivisto T, Hernesniemi J, Welch BG, Jaaskelainen JE, Huttunen TJ (2016) Risk of shunting after aneurysmal subarachnoid hemorrhage: a collaborative study and initiation of a consortium. Stroke 47:2488–2496. https://doi.org/10.1161/strokeaha.116.013739
Aineskog H, Baldvinsdóttir B, Ronne Engström E, Eneling J, Enblad P, Svensson M, Alpkvist P, Fridriksson S, Klurfan P, Hillman J, Kronvall E, Nilsson OG, Lindvall P (2024) A national cohort with aneurysmal subarachnoid hemorrhage-patient characteristics, choice of treatment, clinical outcome, and factors of prognostic importance. World Neurosurg 190:e513–e524. https://doi.org/10.1016/j.wneu.2024.07.164
Alotaibi NM, Elkarim GA, Samuel N, Ayling OGS, Guha D, Fallah A, Aldakkan A, Jaja BNR, de Oliveira Manoel AL, Ibrahim GM, Macdonald RL (2017) Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 127:1315–1325. https://doi.org/10.3171/2016.9.Jns161383
Alotaibi NM, Wang JZ, Pasarikovski CR, Guha D, Al-Mufti F, Mamdani M, Saposnik G, Schweizer TA, Macdonald RL (2017) Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: time for a consensus? Neurosurg Focus 43:E13. https://doi.org/10.3171/2017.7.Focus17426
Banks JL, Marotta CA (2007) Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 38:1091–1096. https://doi.org/10.1161/01.STR.0000258355.23810.c6
Björk S, Hånell A, Ronne-Engström E, Stenwall A, Velle F, Lewén A, Enblad P, Svedung Wettervik T (2023) Thiopental and decompressive craniectomy as last-tier ICP-treatments in aneurysmal subarachnoid hemorrhage: is functional recovery within reach? Neurosurg Rev 46:231. https://doi.org/10.1007/s10143-023-02138-6
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J (2017) Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80:6–15. https://doi.org/10.1227/neu.0000000000001432
Claassen J, Park S (2022) Spontaneous subarachnoid haemorrhage. Lancet 400:846–862. https://doi.org/10.1016/s0140-6736(22)00938-2
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Darkwah Oppong M, Golubovic J, Hauck EF, Wrede KH, Sure U, Jabbarli R (2020) Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: who and when? - a systematic review and meta-analysis. Clin Neurol Neurosurg 199:106252. https://doi.org/10.1016/j.clineuro.2020.106252
Diringer MN, Bleck TP, Claude Hemphill J 3rd, Menon D, Shutter L, Vespa P, Bruder N, Connolly ES Jr, Citerio G, Gress D, Hänggi D, Hoh BL, Lanzino G, Le Roux P, Rabinstein A, Schmutzhard E, Stocchetti N, Suarez JI, Treggiari M, Tseng MY, Vergouwen MD, Wolf S, Zipfel G (2011) Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care 15:211–240. https://doi.org/10.1007/s12028-011-9605-9
Dorfer C, Frick A, Knosp E, Gruber A (2010) Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage. World Neurosurgery 74:465–471. https://doi.org/10.1016/j.wneu.2010.08.001
Engquist H, Rostami E, Ronne-Engström E, Nilsson P, Lewén A, Enblad P (2018) Effect of HHH-therapy on regional CBF after severe subarachnoid hemorrhage studied by bedside xenon-enhanced CT. Neurocrit Care 28:143–151. https://doi.org/10.1007/s12028-017-0439-y
Goedemans T, Verbaan D, Coert BA, Sprengers MES, van den Berg R, Vandertop WP, van den Munckhof P (2018) Decompressive craniectomy in aneurysmal subarachnoid haemorrhage for hematoma or oedema versus secondary infarction. Br J Neurosurg 32:149–156. https://doi.org/10.1080/02688697.2017.1406453
Güresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J (2009) Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus 26:E4. https://doi.org/10.3171/2009.3.Focus0954
Hawryluk GWJ, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J (2020) Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery 87:427–434. https://doi.org/10.1093/neuros/nyaa278
Hoh BL, Ko NU, Amin-Hanjani S, Chou S-Y, Cruz-Flores S, Dangayach NS, Derdeyn CP, Du R, Hänggi D, Hetts SW, Ifejika NL, Johnson R, Keigher KM, Leslie-Mazwi TM, Lucke-Wold B, Rabinstein AA, Robicsek SA, Stapleton CJ, Suarez JI, Tjoumakaris SI, Welch BG (2023) 2023 guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 54:e314–e370. https://doi.org/10.1161/str.0000000000000436
Hwang US, Shin HS, Lee SH, Koh JS (2014) Decompressive surgery in patients with poor-grade aneurysmal subarachnoid hemorrhage: clipping with simultaneous decompression versus coil embolization followed by decompression. Journal of cerebrovascular and endovascular neurosurgery 16:254–261. https://doi.org/10.7461/jcen.2014.16.3.254
Jabbarli R, Darkwah Oppong M, Roelz R, Pierscianek D, Shah M, Dammann P, Scheiwe C, Kaier K, Wrede KH, Beck J, Sure U (2020) The PRESSURE score to predict decompressive craniectomy after aneurysmal subarachnoid haemorrhage. Brain communications 2:fcaa134. https://doi.org/10.1093/braincomms/fcaa134
Jabbarli R, He SQ, Darkwah Oppong M, Herten A, Chihi M, Pierscianek D, Dammann P, Sure U, Wrede KH (2021) Size does matter: the role of decompressive craniectomy extent for outcome after aneurysmal subarachnoid hemorrhage. Eur J Neurol 28:2200–2207. https://doi.org/10.1111/ene.14835
Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, Witte S, Jenetzky E, Hacke W (2007) Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 38:2518–2525. https://doi.org/10.1161/strokeaha.107.485649
Kolias AG, Adams H, Timofeev IS, Corteen EA, Hossain I, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Posti JP, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, Hutchinson PJ (2022) Evaluation of outcomes among patients with traumatic intracranial hypertension treated with decompressive craniectomy vs standard medical care at 24 months: a secondary analysis of the RESCUEicp randomized clinical trial. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2022.1070
Lai L, Morgan MK (2013) Predictors of in-hospital shunt-dependent hydrocephalus following rupture of cerebral aneurysms. J Clin Neurosci 20:1134–1138. https://doi.org/10.1016/j.jocn.2012.09.033
Magni F, Pozzi M, Rota M, Vargiolu A, Citerio G (2015) High-resolution intracranial pressure burden and outcome in subarachnoid hemorrhage. Stroke 46:2464–2469. https://doi.org/10.1161/strokeaha.115.010219
Nagel A, Graetz D, Vajkoczy P, Sarrafzadeh AS (2009) Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: relation to cerebral perfusion pressure and metabolism. Neurocrit Care 11:384–394. https://doi.org/10.1007/s12028-009-9269-x
Nilsson OG, Lindgren A, Ståhl N, Brandt L, Säveland H (2000) Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden. J Neurol Neurosurg Psychiatry 69:601–607. https://doi.org/10.1136/jnnp.69.5.601
Paisan GM, Ding D, Starke RM, Crowley RW, Liu KC (2018) Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: predictors and long-term functional outcomes. Neurosurgery 83:393–402. https://doi.org/10.1093/neuros/nyx393
Papaioannou V, Czosnyka Z, Czosnyka M (2022) Hydrocephalus and the neuro-intensivist: CSF hydrodynamics at the bedside. Intensive Care Med Exp 10:20. https://doi.org/10.1186/s40635-022-00452-9
Reinink H, Jüttler E, Hacke W, Hofmeijer J, Vicaut E, Vahedi K, Slezins J, Su Y, Fan L, Kumral E, Greving JP, Algra A, Kappelle LJ, van der Worp HB, Neugebauer H (2021) Surgical decompression for space-occupying hemispheric infarction: a systematic review and individual patient meta-analysis of randomized clinical trials. JAMA Neurol 78:208–216. https://doi.org/10.1001/jamaneurol.2020.3745
Rinkel GJ, Algra A (2011) Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. The Lancet Neurology 10:349–356. https://doi.org/10.1016/s1474-4422(11)70017-5
Ryttlefors M, Howells T, Nilsson P, Ronne-Engström E, Enblad P (2007) Secondary insults in subarachnoid hemorrhage: occurrence and impact on outcome and clinical deterioration. Neurosurgery 61:704–714. https://doi.org/10.1227/01.Neu.0000298898.38979.E3
Skoglund TS, Eriksson-Ritzén C, Jensen C, Rydenhag B (2006) Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma 23:1502–1509. https://doi.org/10.1089/neu.2006.23.1502
Sundström J, Söderholm M, Söderberg S, Alfredsson L, Andersson M, Bellocco R, Björck M, Broberg P, Eriksson M, Eriksson M, Forsberg B, Fransson EI, Giedraitis V, Theorell-Haglöw J, Hallqvist J, Hansson PO, Heller S, Håkansson N, Ingelsson M, Janson C, Järvholm B, Khalili P, Knutsson A, Lager A, Lagerros YT, Larsson SC, Leander K, Leppert J, Lind L, Lindberg E, Magnusson C, Magnusson PKE, Malfert M, Michaëlsson K, Nilsson P, Olsson H, Pedersen NL, Pennlert J, Rosenblad A, Rosengren A, Torén K, Wanhainen A, Wolk A, Engström G, Svennblad B, Wiberg B (2019) Risk factors for subarachnoid haemorrhage: a nationwide cohort of 950 000 adults. Int J Epidemiol 48:2018–2025. https://doi.org/10.1093/ije/dyz163
Svedung Wettervik T, Hånell A, Howells T, Ronne-Engström E, Lewén A, Enblad P (2022) Intracranial pressure- and cerebral perfusion pressure threshold-insults in relation to cerebral energy metabolism in aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 164:1001–1014. https://doi.org/10.1007/s00701-022-05169-y
Svedung Wettervik T, Hånell A, Howells T, Ronne Engström E, Lewén A, Enblad P (2023) ICP, CPP, and PRx in traumatic brain injury and aneurysmal subarachnoid hemorrhage: association of insult intensity and duration with clinical outcome. J Neurosurg 138:446–453. https://doi.org/10.3171/2022.5.Jns22560
Svedung Wettervik T, Howells T, Lewén A, Ronne-Engström E, Enblad P (2021) Temporal dynamics of ICP, CPP, PRx, and CPPopt in high-grade aneurysmal subarachnoid hemorrhage and the relation to clinical outcome. Neurocrit Care. https://doi.org/10.1007/s12028-020-01162-4
Svedung Wettervik T, Lenell S, Enblad P, Lewén A (2021) Decompressive craniectomy in traumatic brain injury-craniectomy-related and cranioplasty-related complications in a single center. World Neurosurg 148:e508–e517. https://doi.org/10.1016/j.wneu.2021.01.013
Svedung Wettervik T, Lewén A, Enblad P (2023) Fine tuning of neurointensive care in aneurysmal subarachnoid hemorrhage: from one-size-fits-all towards individualized care. World Neurosurg X 18:100160. https://doi.org/10.1016/j.wnsx.2023.100160
Treggiari MM, Rabinstein AA, Busl KM, Caylor MM, Citerio G, Deem S, Diringer M, Fox E, Livesay S, Sheth KN, Suarez JI, Tjoumakaris S (2023) Guidelines for the neurocritical care management of aneurysmal subarachnoid hemorrhage. Neurocrit Care. https://doi.org/10.1007/s12028-023-01713-5
Unterberg AW, Stover J, Kress B, Kiening KL (2004) Edema and brain trauma. Neuroscience 129:1021–1029. https://doi.org/10.1016/j.neuroscience.2004.06.046
Uozumi Y, Sakowitz O, Orakcioglu B, Santos E, Kentar M, Haux D, Unterberg A (2014) Decompressive craniectomy in patients with aneurysmal subarachnoid hemorrhage: a single-center matched-pair analysis. Cerebrovascular diseases (Basel, Switzerland) 37:109–115. https://doi.org/10.1159/000356979
van der Worp HB, Hofmeijer J, Jüttler E, Lal A, Michel P, Santalucia P, Schönenberger S, Steiner T, Thomalla G (2021) European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J 6:Xc-cx. https://doi.org/10.1177/23969873211014112
van Gijn J, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124:249–278. https://doi.org/10.1093/brain/124.2.249
Veldeman M, Weiss M, Daleiden L, Albanna W, Schulze-Steinen H, Nikoubashman O, Clusmann H, Hoellig A, Schubert GA (2022) Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage-justifiable in light of long-term outcome? Acta Neurochir (Wien) 164:1815–1826. https://doi.org/10.1007/s00701-022-05250-6
Wettervik TS, Lenell S, Nyholm L, Howells T, Lewén A, Enblad P (2018) Decompressive craniectomy in traumatic brain injury: usage and clinical outcome in a single centre. Acta Neurochir (Wien) 160:229–237. https://doi.org/10.1007/s00701-017-3418-3
Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, Zhan RY (2008) Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir (Wien) 150:1241–1247. https://doi.org/10.1007/s00701-008-0145-9
Zoerle T, Lombardo A, Colombo A, Longhi L, Zanier ER, Rampini P, Stocchetti N (2015) Intracranial pressure after subarachnoid hemorrhage. Crit Care Med 43:168–176. https://doi.org/10.1097/ccm.0000000000000670
Acknowledgements
Not applicable.
Funding
Open access funding provided by Uppsala University. The study was supported by the Uppsala University Hospital and the Foundation for Stroke Research in Northern Sweden.
Author information
Authors and Affiliations
Contributions
TSW and KH conceptualized the study. TSW, AC, MS, BS, AFS, and KH contributed with data collection. TSW performed the formal analysis. TSW and KH wrote the original draft. All authors contributed with reviewing and editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Human ethics and consent to participate
The study was approved by the Swedish Ethical Review Authority (Dnr: 2023-02347-01, decision date: 2023-05-17). Informed consent was waived for this retrospective study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svedung Wettervik, T., Corell, A., Sunila, M. et al. Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: can favorable outcome be achieved?. Acta Neurochir 167, 68 (2025). https://doi.org/10.1007/s00701-025-06485-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-025-06485-9