Abstract
Antimicrobial peptides (AMPs) have broad-spectrum antimicrobial activity, enabling them to rapidly detect and eliminate targets. In addition, many AMPs are natural peptides, making them promising candidates for therapeutic drugs. This review discusses the basic properties and mechanisms of action of AMPs, highlighting their ability to disrupt microbial membranes and modulate host immune responses. It also reviews the current state of research into using AMPs against various viral infections, focusing on their therapeutic potential against viruses that contribute to the global health crisis. Despite promising developments, therapies based on AMPs still face challenges such as stability, toxicity, and production costs. In this text, we will discuss these challenges and the latest technological advances aimed at overcoming them. The combination of nanotechnology and bioengineering approaches offers new ways to enhance the delivery, efficacy, and safety of AMPs. We emphasize the importance of further research to fully exploit the potential of AMPs in antiviral therapy, advocating a multifaceted approach that includes optimizing clinical use and exploring synergies with existing antiviral drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial peptides (AMPs) are naturally occurring molecules that are part of the innate immunity of virtually all organisms. Their broad-spectrum activity against bacteria, fungi and viruses plays a critical role in defense against invading microorganisms (Ageitos et al. 2017). The main mechanisms of action of AMPs for bacterial inhibition are divided into four main categories (Lei et al. 2019; Li et al. 2022; Luo Y et al. 2021; Patel et al. 2017) (Fig. 1). Compared to conventional antibiotics, AMPs are small, amphiphilic and cationic, and exert their antimicrobial effects by disrupting microbial membranes, making microorganisms less likely to develop resistance, unlike antibiotics that target cellular activity (e.g., synthesis of proteins, DNA, or cell walls) (Pimchan et al. 2023; Saini et al. 2022). Currently, the main focus of AMPs research is on antimicrobial activity against a wide range of bacteria, fungi, and viruses, exploring their potential as alternatives to antibiotics in response to rising antibiotic resistance (Arasu et al. 2023).
Main mechanism of action of AMPs for bacterial inhibition Once bound to the membrane, AMPs can form pores or translocate across the membrane to release or translocate major intracellular components, leading to bacterial cell death, modes of action include concave cylinder, ring pore, and carpet modes. Some antimicrobial peptides exert antimicrobial effects by affecting the synthesis of cell wall components and disrupting cell wall structure. Some antimicrobial peptides enter cells by direct penetration or endocytosis, and exert antimicrobial effects by targeting the nucleus, organelles, proteins present in fungi or intracellular proteins. Alternatively, they block nucleic acid synthesis, protein synthesis or enzyme activity to exert antimicrobial effects
The exploration of AMPs in antiviral therapy is urgent due to the global challenges posed by infections with multiple viral variants. Viruses such as Human immunodeficiency virus (HIV), influenza and coronaviruses have had a significant impact on global public health, economy and society. In particular, the COVID-19 pandemic has highlighted the urgent need for multifunctional antiviral drugs capable of prevention and treatment of emerging viral threats (Kolanthai et al. 2022). Although vaccination is the most effective method of prevention of viral infections, the evolution of epidemics and differences between available vaccines can limit its effectiveness (Wang et al. 2022). AMPs offer a promising alternative due to their unique mechanism of action, which includes direct neutralization of viruses and modulation of host immune responses. Exploring AMPs in an antiviral context is not only critical to address current treatment gaps, but also to prevent future viral outbreaks.
Based on recent advances in AMPs research, this review aims to elucidate the potential of antimicrobial peptide antivirals, with a particular focus on their use against HIV, influenza viruses and coronaviruses, and to highlight the exploitation of the direct antiviral effects of AMPs and their immunomodulatory properties. Through this focused exploration, we contribute to ongoing innovative antiviral strategies and advocate for the integration of AMPs into the wider antiviral disease repertoire.
Basic properties and mechanism of action of AMPs
AMPs are short chains of amino acids, typically 10 to 50 units, with a net positive charge of at least + 2 (usually between + 3 and + 5) and are amphiphilic. AMPs exhibit diverse functions on host cells, resulting in a wide range of antimicrobial activities (Liu et al. 2023). As of January 2024, the Antimicrobial Peptide Database (APD) contains 3940 peptides (Fig. 2). AMPs have been classified in a variety of ways, based on structure, including (i) α-helical, (ii) β-sheets (at least two), (iii) αβ, and (iv) non-αβ (Bin Hafeez et al. 2021). Based on amino acid composition and structural features, they can be classified into four subclasses, including (i) linear peptides forming an α -helix and lacking cysteine residues segments (e.g., aspergillus and housewife AMPs, etc.), (ii) peptides containing cysteine residues with internal disulfide bridges (e.g., defensin and drosomycin), (iii) peptides with an overrepresentation of proline residues (e.g., apidaecin, drosocin and lebocin), (iv) peptides with an overrepresentation of glycine residues (e.g., attacin and gloverin) (Li et al. 2024). The existence of AMPs and their role in innate immunity, the host’s first line of defense against pathogens, provides an opportunity to use them as a class of antibiotics (Talapko et al. 2022).
The antimicrobial effects of AMPs are mainly due to their capacity to disrupt bacterial cell membranes. This interaction is facilitated by the electrostatic attraction between positively charged AMPs and negatively charged components of the bacterial membrane, such as lipopolysaccharides in Gram-negative bacteria and lipoteichoic acid in Gram-positive bacteria (Chakraborty et al. 2022). Several models have been proposed to explain the membrane cleavage mechanism of the antimicrobial action of AMPs. The barrel-stave model emphasizes the ability of the peptide to insert and diffuse laterally through the lipid bilayer, arranging itself into a helix and forming a barrel-like channel across the membrane. The toroidal-pore model reveals that peptide molecules rotate and insert into the membrane bilayer, causing rapid changes in membrane conformation and generating ring pores. However, the carpet model elaborates that peptides parallel to the membrane surface form a “carpet” that, at certain peptide concentrations, disrupts the membrane bilayer structure in a detergent-like manner, leading to micelle formation (Wu et al. 2018). These modes of action distinguish AMPs from conventional antibiotics and offer a potential solution to the growing problem of antibiotic resistance by reducing the likelihood of resistance developing due to physical disruption of cell membranes (Zhang et al. 2022).
Recent studies have shown that AMPs have the ability to link the innate and adaptive immune systems and regulate the magnitude of the immune response to ward off infection, modulate inflammation, and influence immune homeostasis, including leukocyte recruitment, chemotactic stimulation, pro- and anti-inflammatory cytokine induction, endotoxin neutralization, and activation and differentiation of immune cell lineages (Luo Y et al. 2021; Pinkenburg et al. 2016). Cathelicidins are potent microbicidal molecules for the control of bacterial infections, and exert different degrees of immunomodulatory functions by stimulating neutrophil chemotaxis, inducing reactive oxygen species production, and promoting the formation of extracellular traps in neutrophils (Dlozi et al. 2022; Xie et al. 2020). In addition, it can also increase the expression of TLR4 in LAD2 mast cells to increase the ability of mast cells to detect pathogens, or promote the differentiation of monocytes to macrophages with a pro-inflammatory phenotype, decrease the secretion of interleukin-10 (IL-10) and increase the secretion of interleukin-12 (IL-12) in macrophages with an anti-inflammatory phenotype (Duarte-Mata et al. 2023). IDR-1002 limits the release of pro-inflammatory cytokines and has a better preventive effect against Pseudomonas aeruginosa-induced toxicity, but does not affect the number of bacteria in the alginate model (Wuerth et al. 2017). Similarly, BCCY-1 does not have direct antibacterial effects in vitro, but promotes monocyte/macrophage recruitment to the site of infection and protects mice from pathogen-induced lethal infections (Cai et al. 2021). These immunomodulatory effects prompt the host immune system to respond to microbial infections, thereby limiting the potential development of antimicrobial resistance and the negative impact of antibiotics on the host microbiota.
The mechanism of action by which AMPs exert antiviral activity may be (i) blocking of the early steps of viral entry through surface carbohydrate interactions; (ii) blocking of viral adsorption or penetration of host cells through interactions with specific cellular receptors (Fig. 3); (iii) interaction and inactivation of viral envelope glycoproteins; (iv) modulation of host cell antiviral responses; (v) blocking of the intracellular expression of viral genes and/or production of viral proteins (Feng et al. 2020; Gudima et al. 2023; Lee et al. 2022; Luo et al. 2021). It was found that disruption of the viral vesicle membrane by AMPs such as LL-37, MXB-5, and MXB-9 can directly inactivate HSV-1 extracellularly, preventing binding to and infection of host cells (Diamond et al. 2021). StPIP1 triggers the plant defense response against potato Y virus (PVY) by inducing the production of ROS, and the expression of defense-related genes (Combest et al. 2021). Magainin exhibits an effective virocidal effect against viruses belonging to the Herpesviridae family all showed effective virucidal effects, probably through interaction with viral capsid components and subsequent disruption of capsid integrity (Dean et al. 2010). This multifaceted approach to antiviral activity makes AMPs promising candidates for the development of broad-spectrum antiviral therapeutics capable of addressing existing and emerging viral threats (Urmi et al. 2024).
Main mechanism of action of antimicrobial peptides (AMPs) to inhibit viruses The mechanism of action of AMPs covers almost all stages of the entire viral life cycle: viral particle inhibition; adsorption; viral entry; endosomal escape; viral capsid deconjugation; transcription and translation of the viral genome, and release of mature viral particles
AMPs in anti-HIV therapy
Human immunodeficiency virus (HIV) remains a major global health challenge, with the pandemic concentrated in sub-Saharan Africa, where approximately 26 million people are infected (Okano et al. 2020). The pathogenic process involves rapid viral replication and weakening of the host immune system, primarily through destruction of host CD4 + immune cells. The compromised immune system, due to depletion of key immune cells, leads to increased morbidity and may increase the risk of death in affected hosts (Dlozi et al. 2022). Despite significant advances in antiretroviral therapy (ART), issues of resistance, side effects, and accessibility continue to complicate treatment efforts (Parikh et al. 2017). To address these challenges, AMPs have emerged as potential drug candidates due to novel mechanisms of action and low drug resistance (Brakel et al. 2023), aiming to complement existing treatments and address unmet medical needs in HIV management.
AMPs exhibit a range of inhibitory mechanisms against HIV that span the viral life cycle, from entry to replication to eventual release from the host cell (Lee et al. 2022). A cationic 18-amino acid peptide, which crosses cell membranes into the cytoplasm and nucleus, acts and affects the production and maturation phases of HIV-1 virus, inhibiting the production of both HIV-1 strains in human cell lines (Samuels et al. 2017). GRFT blocks the binding of CD4-dependent glycoprotein (gp) 120 to receptor-expressing cells and binds to viral capsid glycoproteins (e.g., gp120, gp41, and gp160) in a glycosylation-dependent manner. (e.g., gp120, gp41, and gp160) to prevent HIV entry into the cell, blocking its interaction with CD4 receptors, CCR5-tropic, and CXCR4 on the host cell surface (Lee 2019; Pimchan et al. 2023). Alternatively, by interfering with the fusion process between the virus and the host cell membrane, which is a key step in antiviral resistance. Kalata B1 was found to inhibit HIV infection by rapidly covering a small surface area of the viral membrane (Nawae et al. 2017). Certain AMPs can also inhibit HIV replication by targeting viral reverse transcriptases or integrases, which are required for the integration of viral DNA into the host genome. For example, α-defensins, in addition to this, can inactivate the virus by direct inactivation, emphasizing the existence of at least a dual mechanism for this peptide in its anti-HIV activity (Madanchi et al. 2020). Surprisingly, LL-37 delivers specifically bound cGAMP to target cells, and the transferred cGAMP activates a robust interferon response and host antiviral immunity in a STING-dependent manner (Wei et al. 2022). These mechanisms highlight the potential for AMPs to act at multiple stages of the HIV life cycle, providing a multifaceted therapeutic approach that could reduce the likelihood of drug resistance development. Stellettapeptines antiviral activity, assessed by cell viability assays, demonstrated significant anti-HIV-1 activity, however, further mechanisms for inhibiting viral entry have not been well characterized (Sukmarini 2022).
Recent studies and case reports have highlighted the therapeutic potential of anti-HIV antibodies, showing efficacy in both in vitro and in vivo models. PAC-113 has completed Phase II clinical trials to determine the optimal dose of PAC-113 for oral mouthwash for oral candidiasis in HIV carriers (Greber et al. 2017). Sifuvirtide is currently undergoing Phase III clinical trials. Two clinical studies are evaluating the efficacy of the drug when administered at 20 mg once daily, compared to 90 mg of enfuvirtide administered twice daily (Cao et al. 2017; Freitas et al. 2022). Clinical trials and experimental therapies are currently investigating the safety, effectiveness, and optimal administration of AMP-based treatments. Preliminary findings indicate that they hold promise as a component of a combination therapy regimen (Madanchi et al. 2020). These advances confirm the role of AMPs in the fight against HIV and pave the way for future research to harness their therapeutic potential, offering hope for more effective and comprehensive HIV treatment strategies. However, the therapeutic potential of AMPs in the treatment of HIV still needs to be further confirmed by clinical trials.
AMPs in anti-influenza virus therapy
Influenza viruses are a leading cause of respiratory infections globally, resulting in significant morbidity and mortality annually. These RNA viruses have high mutation rates and can evade the immune system, leading to seasonal epidemics and occasional pandemics with severe public health consequences (Hutchinson 2018). Vaccination has reduced the number of people with the disease and slowed the spread of the virus. However, the genetic instability of the virus complicates vaccine development and antiviral therapy (Ye et al. 2020).
Efforts have been made to develop new antiviral drugs against influenza A virus (IAV), as many of the current drugs used for treatment, including neuraminidase inhibitors and adamantanes, do not effectively minimize the risk of adverse effects (De Clercq 2013). AMPs have shown considerable promise as novel antiviral drugs. The mechanisms of action of antiviral drugs involve direct interaction with the virus, which can disrupt the viral envelope or capsid. Additionally, these drugs can modulate the host immune response to enhance antiviral defense. For instance, Flufirvitide interferes with viral invasion and modulates the immune system by activating the production of anti-inflammatory cytokines and chemokines, increasing neutrophil activity, and enhancing phagocytosis by macrophages (Skalickova et al. 2015). Esculentin-1GN and urumins, which bind to hemagglutinins on the surface of influenza viruses and inhibit the ability of the virus to attach and enter host cells (Sukmarini 2022; Vineeth Kumar et al. 2018; Yang et al. 2021). In addition, when combined with existing antiviral agents, they can induce a protective immune response, produce antiviral cytokines, and inhibit the production of inflammatory mediators, which can help to control viral transmission and infection, e.g., Temporin G and alloferon (Appiah et al. 2024; De Angelis et al. 2021). It was demonstrated that LL-37 interacts with the viral capsid to form oligomers, leading to the production of a fibrillar super molecular structure that exhibits a circular pore model, where the peptide further assembles into a transmembrane pore and leads to destabilization of the viral membrane or improves the therapeutic efficacy in IAV infected mice by inhibiting viral replication and decreasing the production of inflammatory cytokines (Mousavi Maleki et al. 2021). Preliminary evidence suggests that caerin 1 inhibits HIV transmission in vitro and inhibits the transfer of viral particles from dendritic cells to T-cells with low toxicity to T-cells and cervical epithelial cell lines (Rollins-Smith et al. 2020). These multifaceted effects make AMPs attractive candidates for the development of new influenza therapies that may be less susceptible to drug resistance and more effective against different viral subtypes (Hsieh et al. 2016).
Research on the use of AMPs in anti-influenza virus therapy has been advancing, with several case studies and clinical trials highlighting their potential. A 27 aa peptide from the N-terminal part of human bactericidal/permeability-increasing protein (BPI) interferes with the viral envelope and inhibits the infectivity of several IAV strains (H1N1, H3N2, and H5N1), in contrast to the homologous mouse BPI peptide, which showed no activity against IAV (Pinkenburg et al. 2016). TL was screened as the best candidate peptide, and TL derivatives and their analogs exhibited significant inhibitory activity against herpesviruses, paramyxoviruses, influenza viruses, and coronaviruses, including SARS-CoV-2. significant inhibitory activity. In addition, the lower cytotoxicity and better antiviral effects were further demonstrated by lipidation modifications that promoted the insertion of the peptide in lipid membranes and/or induced self-organizing micelles (Zannella et al. 2022). In animal models infected with influenza strains, specific AMPs were efficacious in reducing viral load and improving survival. Lactoferrin was found to be anti-inflammatory and attenuate intestinal damage, thereby modulating the immune response induced by influenza infection, as demonstrated by studies in H5N1-infected mice (Huang et al. 2023). While the results of these studies are encouraging, progress in clinical trials is still in the early stages and, therefore, in-depth research is needed as a supplement or alternative to conventional antiviral therapy.
AMPs in anti-coronaviral therapy
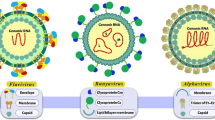
Coronaviruses are encapsidated positive-sense RNA viruses that have been identified as important pathogens in humans and animals, causing diseases ranging from the common cold to severe respiratory syndrome (Wang et al. 2020). Notably, three major outbreaks caused by coronaviruses have occurred in the 21st century: SARS (Severe Acute Respiratory Syndrome) in 2003, MERS (Middle East Respiratory Syndrome) in 2012, and the ongoing global pandemic caused by SARS-CoV-2 (COVID-19) that began in 2019. These viruses bind their S-glycoprotein (S1 & S2) to the cellular receptor, which leads to cell penetration and disassembly of the viral capsid, releasing the viral RNA genome (Loffredo et al. 2024; Millet et al. 2016; Solanki et al. 2021). Studies have shown that mutations altering the SARS-CoV-2 antigenic phenotype are circulating and affecting immune recognition to some extent, which, together with their potential for cross-species transmission, poses a major challenge to the development of effective vaccines and therapies, highlighting the urgent need for innovative therapeutic strategies (Harvey et al. 2021).
Currently, there is no specific antiviral drug or universal vaccine against coronaviruses internationally (Tang et al. 2020), and AMPs are potential therapies against coronaviruses due to their broad-spectrum antiviral properties and mechanisms of action that are different from those of traditional antiviral drugs. It has been shown that AMPs can disrupt the viral envelope and interfere with the entry of coronaviruses into host cells (Huan et al. 2020). A lipopeptide, EK1C4, was the most potent fusion inhibitor against SARS-CoV-2 S-protein-mediated membrane fusion and pseudoviral infection, with greater therapeutic efficacy than the original EK1 peptide, a finding that was validated in a mouse model (Rani et al. 2022; Xia et al. 2020). Alternatively, some peptides have been found to have a dual function of directly targeting the virus while also modulating the immune response to reduce inflammation and lung injury. Defensins, in addition to inhibiting viral infection during SARS-CoV-2 entry into the cell, also exhibit anti-inflammatory activity, recruiting and activating T cells and myeloid lineage cells such as monocytes and dendritic cells (Xu et al. 2021). HD5 is associated with angiotensin Converting enzyme 2 (ACE2) ligand-binding domain (LBD), thereby reducing viral load into the cell, activating adaptive immune antigen-presenting phagocytes, and interfering with the nuclear enzymes that prevent viral cell replication (Solanki et al. 2021). In addition to this, lactoferrin also prevents viral entry into host cells via ACE2 (Kell et al. 2020). Surprisingly, the antiviral activity of plitidepsin was achieved by inhibiting a known target, eEF1A, which is an important host factor for viral replication, thus the peptide shows great potential for drug repurposing in the fight against COVID-19 (Vishvakarma et al. 2022; White et al. 2021). AMPs may synergize with other drugs to fight viral infection and transmission more efficiently. Bioactive forms of vitamin D and many other compounds induce the expression of LL-37, which directly binds to the S1 structural domain of SARS-CoV-2 and masks the angiotensin-converting enzyme 2 (ACE2) receptor, thereby limiting SARS-CoV-2 infection (Aloul et al. 2022). The versatility and potency of AMPs against various components of the coronavirus life cycle demonstrates a novel approach to the development of screened antiviral drugs, highlights their potential as part of a broader antiviral strategy, and will be important in realizing the goal of approving broad-spectrum anti-coronaviral drugs for human use (Kilianski et al. 2014).
In response to the COVID-19 pandemic, research on the application of AMPs against coronaviruses (especially SARS-CoV-2) has been intensified. Early studies and in vitro experiments have identified several AMPs with anti-SARS-CoV-2 activity that inhibit viral replication and reduce viral load (Heydari et al. 2021; Souza et al. 2020). These findings provide the basis for further studies and clinical trials to evaluate the efficacy, safety, and optimal delivery of AMPs-based therapies against SARS-CoV-2 and other coronaviruses, which not only contribute to our understanding of AMPs, but also open new avenues for the development of effective therapeutic approaches against current and future coronavirus outbreaks.
Limitations and challenges of AMPs in antiviral therapy
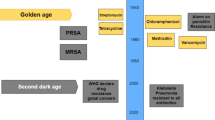
Current antiviral research, usually based on biochemical principles, focuses primarily on targeting one virus at a time, with very limited coverage of target viruses. At the same time, single-virus and single-target strategies are hampered by the ability of many viruses to mutate rapidly, and altering viral antigenic specificity can easily produce escape mutants against single-target antiviruses, making broad-spectrum antiviral strategies more suitable for dealing with the increasing diversity of highly pathogenic viruses (Zannella et al. 2022). AMPs, on the other hand, have been progressively studied as part of innate immunity, with unique mechanisms of action and relatively low rates of resistance induction (Table 1). However, facing the drawbacks of low in vivo stability, toxicity to host cells, high production cost and low potency in biological systems, thus limiting their accessibility and feasibility as therapeutic agents, only a few candidate peptides have shown efficacy in clinical trials so far (Sultana et al. 2021; You et al. 2023). These challenges require continued research to optimize the stability, safety, and cost-effectiveness of AMPs to ensure that they can be a viable option for antiviral therapy.
The application of nanotechnology shows great promise in enhancing the delivery and stability of AMPs (Guerra et al. 2024). Nano-formulations of AMPs may include, but are not limited to, liposomes, micelles, polymeric nanoparticles or lipid nanoparticles or lipid-polymer nanoparticles, which can be used to minimize toxicity and improve therapeutic efficacy by packaging peptides, protecting AMPs from enzymatic degradation, improving their bioavailability, and facilitating targeted delivery to the site of infection (Faya et al. 2020; Tang et al. 2021). A small number of studies have examined the nanoparticle encapsulation and therapeutic activity of AMPs. Nisin: CMC nanoparticles prolonged the antimicrobial activity of nisin and inhibited S. aureus proliferation. In addition, this inhibition was achieved with only a tenfold lower concentration of nisin than currently used (Çelen et al. 2023). Formulating liposomes by thin film hydration has the potential to carry cargo for intracellular delivery, which could be used to enhance the activity and permeability of AMPs, thereby improving the treatment of bacterial infections. Covalent attachment of AMPs to polyethylene glycol (PEG) or hyperbranched polyglycerol (HPG) polymers increased the antimicrobial activity of 73-derived peptides 2-8-fold, and all derivatives eradicated preformed S. aureus biofilms (Kumar et al. 2019). DJK-5 peptide loaded into hyaluronic acid (HA)-based octenylsuccinic anhydride-crosslinked nanogels (OSA-HA) retained DJK-5 antimicrobial activity when the peptide-loaded nanogels were tested against Pseudomonas aeruginosa (PA) infection-induced abscesses in mice by subcutaneous administration. This resulted in a 4-fold reduction in cytotoxicity compared to the commercially available peptide, a finding that supports the use of nanogels as a delivery system to improve the safety of AMPs (Cesaro et al. 2023). Using nanomaterials not only prevents degradation of AMPs, but also improves their therapeutic and pharmacokinetic properties, resulting in inhibiting bacterial growth and treating bacterial infections. It also opens up new avenues for the integration of existing antiviral therapeutic regimens.
Looking ahead, research on AMPs is likely to focus on improving their clinical activity and fully exploiting their antiviral therapeutic potential, which is challenging to develop effective and safe antiviral drugs that do not damage host cells (Mousavi Maleki et al. 2023). This includes efforts to better understand the mechanism of action of AMPs, to identify synergistic effects with other antiviral agents, and to further investigate their immunomodulatory effects. As research continues, AMPs are expected to become an integral part of the global strategy to combat viral diseases, offering the promise of more effective and broader-spectrum antiviral therapy.
Conclusion
The development of AMPs as antiviral drugs is a promising frontier in the fight against viral diseases. This review highlights significant advances in the understanding of the mechanism of action of AMPs, their application against a range of viruses including HIV, influenza viruses and coronaviruses, and technological advances aimed at overcoming existing challenges in their clinical application. The potential value of AMPs in the antiviral field lies in their broad-spectrum activity, their ability to modulate the immune system and the innovative strategies being developed to improve their efficacy and safety. Therefore, the urgency of further research cannot be overemphasized. As viral pathogens continue to pose a significant threat to global health, detailed investigation of the antiviral capabilities of AMPs, optimization of their clinical use and integration into existing therapeutic paradigms is critical. Continued research into AMPs is expected to yield novel antiviral therapeutic approaches that may have a significant impact on our ability to more effectively manage and control viral infections in the future.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AMPs:
-
Antimicrobial peptides
- HIV:
-
Human immunodeficiency virus
- HSV-1:
-
Herpes simplex virus type 1
- PVY:
-
Potato virus Y
- ROS:
-
Reactive oxygen species
- ART:
-
Antiretroviral therapy
- MD:
-
Molecular dynamics
- SARS-Cov-2:
-
Severe Acute Respiratory Syndrome Coronavirus-2
- ACE2:
-
Angiotensin-converting enzyme 2
- PA:
-
Pseudomonas aeruginosa
- GRFT:
-
Griffithsin
- Gp:
-
Glycoprotein
- CCR5:
-
C-C motif chemokine receptor 5
- CXCR4:
-
C-X-C chemokine receptor type 4
- kB1:
-
Kalata B1
- cGAMP:
-
Cyclic 2’,30’ -GMP-AMP
- BPI:
-
Bactericidal/permeability-increasing protein
- TL:
-
Temporin L
- CoVs:
-
Coronaviruses
- HD5:
-
Human defensin 5
- PEG:
-
Polyethylene glycol
- HPG:
-
Hyperbranched polyglycerol
References
Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG (2017) Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol 133:117–138. https://doi.org/10.1016/j.bcp.2016.09.018
Alahyaribeik S, Nazarpour M (2024) Peptide recovery from chicken feather keratin and their anti-biofilm properties against methicillin-resistant Staphylococcus aureus (MRSA). World J Microbiol Biotechnol 40(4):123. https://doi.org/10.1007/s11274-024-03921-3
Aloul KM, Nielsen JE, Defensor EB, Lin JS, Fortkort JA, Shamloo M, Cirillo JD, Gombart AF, Barron AE (2022) Upregulating Human Cathelicidin Antimicrobial peptide LL-37 expression may prevent severe COVID-19 inflammatory responses and reduce microthrombosis. Front Immunol 13:880961. https://doi.org/10.3389/fimmu.2022.880961
Amiss AS, von Pein JB, Webb JR, Condon ND, Harvey PJ, Phan MD, Schembri MA, Currie BJ, Sweet MJ, Craik DJ et al (2021) Modified horseshoe crab peptides target and kill bacteria inside host cells. Cell Mol Life Sci 79(1):38. https://doi.org/10.1007/s00018-021-04041-z
Appiah C, Chen S, Pori AI, Retyunskiy V, Tzeng C, Zhao Y (2024) Study of alloferon, a novel immunomodulatory antimicrobial peptide (AMP), and its analogues. Front Pharmacol 15:1359261. https://doi.org/10.3389/fphar.2024.1359261
Arasu MV, Al-Dhabi NA (2023) Antibacterial activity of peptides and bio-safety evaluation: in vitro and in vivo studies against bacterial and fungal pathogens. J Infect Public Health 16(12):2031–2037. https://doi.org/10.1016/j.jiph.2023.09.006
Bin Hafeez A, Jiang X, Bergen PJ, Zhu Y (2021) Antimicrobial peptides: An update on classifications and databases. Int J Mol Sci 22(21). https://doi.org/10.3390/ijms222111691
Brakel A, Grochow T, Fritsche S, Knappe D, Krizsan A, Fietz SA, Alber G, Hoffmann R, Müller U (2023) Evaluation of proline-rich antimicrobial peptides as potential lead structures for novel antimycotics against Cryptococcus neoformans. Front Microbiol 14:1328890. https://doi.org/10.3389/fmicb.2023.1328890
Cai J, Cui X, Wang X, You L, Ji C, Cao Y (2021) A novel anti-infective peptide BCCY-1 with immunomodulatory activities. Front Immunol 12:713960. https://doi.org/10.3389/fimmu.2021.713960
Cao P, Dou G, Cheng Y, Che J (2017) The improved efficacy of Sifuvirtide compared with enfuvirtide might be related to its selectivity for the rigid biomembrane, as determined through surface plasmon resonance. PLoS ONE 12(2):e0171567. https://doi.org/10.1371/journal.pone.0171567
Çelen T, Anumudu C, Miri T, Onyeaka H, Fernandez-Trillo P (2023) Nisin:Carboxymethylcellulose polyion complex (PIC) nanoparticles. Preparation and antimicrobial activity. Carbohydr Polym 317:121032. https://doi.org/10.1016/j.carbpol.2023.121032
Cesaro A, Lin S, Pardi N, de la Fuente-Nunez C (2023) Advanced delivery systems for peptide antibiotics. Adv Drug Deliv Rev 196:114733. https://doi.org/10.1016/j.addr.2023.114733
Chakraborty S, Chatterjee R, Chakravortty D (2022) Evolving and assembling to pierce through: evolutionary and structural aspects of antimicrobial peptides. Comput Struct Biotechnol J 20:2247–2258. https://doi.org/10.1016/j.csbj.2022.05.002
Chianese A, Zannella C, Monti A, De Filippis A, Doti N, Franci G, Galdiero M (2022) The broad-spectrum antiviral potential of the Amphibian peptide AR-23. Int J Mol Sci 23(2). https://doi.org/10.3390/ijms23020883
Chianese A, Zannella C, Monti A, Doti N, Sanna G, Manzin A, De Filippis A, Galdiero M (2023) Hylin-a1: a Pan-inhibitor against Emerging and re-emerging respiratory viruses. Int J Mol Sci 24(18). https://doi.org/10.3390/ijms241813888
Combest MM, Moroz N, Tanaka K, Rogan CJ, Anderson JC, Thura L, Rakotondrafara AM, Goyer A (2021) StPIP1, a PAMP-induced peptide in potato, elicits plant defenses and is associated with disease symptom severity in a compatible interaction with Potato virus Y. J Exp Bot 72(12):4472–4488. https://doi.org/10.1093/jxb/erab078
De Angelis M, Casciaro B, Genovese A, Brancaccio D, Marcocci ME, Novellino E, Carotenuto A, Palamara AT, Mangoni ML, Nencioni L (2021) Temporin G, an amphibian antimicrobial peptide against influenza and parainfluenza respiratory viruses: insights into biological activity and mechanism of action. Faseb j 35(2):e21358. https://doi.org/10.1096/fj.202001885RR
De Clercq E (2013) Antivirals: past, present and future. Biochem Pharmacol 85(6):727–744. https://doi.org/10.1016/j.bcp.2012.12.011
Dean RE, O’Brien LM, Thwaite JE, Fox MA, Atkins H, Ulaeto DO (2010) A carpet-based mechanism for direct antimicrobial peptide activity against Vaccinia virus membranes. Peptides 31(11):1966–1972. https://doi.org/10.1016/j.peptides.2010.07.028
Diamond G, Molchanova N, Herlan C, Fortkort JA, Lin JS, Figgins E, Bopp N, Ryan LK, Chung D, Adcock RS et al (2021) Potent antiviral activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals (Basel) 14(4). https://doi.org/10.3390/ph14040304
Dlozi PN, Gladchuk A, Crutchley RD, Keuler N, Coetzee R, Dube A (2022) Cathelicidins and defensins antimicrobial host defense peptides in the treatment of TB and HIV: pharmacogenomic and nanomedicine approaches towards improved therapeutic outcomes. Biomed Pharmacother 151:113189. https://doi.org/10.1016/j.biopha.2022.113189
Duarte-Mata DI, Salinas-Carmona MC (2023) Antimicrobial peptides´ immune modulation role in intracellular bacterial infection. Front Immunol 14:1119574. https://doi.org/10.3389/fimmu.2023.1119574
Faya M, Hazzah HA, Omolo CA, Agrawal N, Maji R, Walvekar P, Mocktar C, Nkambule B, Rambharose S, Albericio F et al (2020) Novel formulation of antimicrobial peptides enhances antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA). Amino Acids 52(10):1439–1457. https://doi.org/10.1007/s00726-020-02903-7
Feng M, Fei S, Xia J, Labropoulou V, Swevers L, Sun J (2020) Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front Immunol 11: 2030. https://doi.org/10.3389/fimmu.2020.02030
Freitas ED, Bataglioli RA, Oshodi J, Beppu MM (2022) Antimicrobial peptides and their potential application in antiviral coating agents. Colloids Surf B Biointerfaces 217:112693. https://doi.org/10.1016/j.colsurfb.2022.112693
Greber KE, Dawgul M (2017) Antimicrobial peptides under clinical trials. Curr Top Med Chem 17(5):620–628. https://doi.org/10.2174/1568026616666160713143331
Gudima G, Kofiadi I, Shilovskiy I, Kudlay D, Khaitov M (2023) Antiviral therapy of COVID-19. Int J Mol Sci 24(10). https://doi.org/10.3390/ijms24108867
Guerra MES, Vieira B, Calazans A, Destro GV, Melo K, Rodrigues E, Waz NT, Girardello R, Darrieux M, Converso TR (2024) Recent advances in the therapeutic potential of cathelicidins. Front Microbiol 15:1405760. https://doi.org/10.3389/fmicb.2024.1405760
Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ et al (2021) SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19(7):409–424. https://doi.org/10.1038/s41579-021-00573-0
Heydari H, Golmohammadi R, Mirnejad R, Tebyanian H, Fasihi-Ramandi M, Moosazadeh Moghaddam M (2021) Antiviral peptides against Coronaviridae family: a review. Peptides 139:170526. https://doi.org/10.1016/j.peptides.2021.170526
Hsieh IN, Hartshorn KL (2016) The role of antimicrobial peptides in Influenza Virus infection and their potential as antiviral and immunomodulatory therapy. Pharmaceuticals (Basel) 9(3). https://doi.org/10.3390/ph9030053
Hu J, Li S, Miao M, Li F (2024) Characterization of the antibacterial and opsonic functions of the antimicrobial peptide LvCrustinVI from Litopenaeus vannamei. Dev Comp Immunol 154:105146. https://doi.org/10.1016/j.dci.2024.105146
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:582779. https://doi.org/10.3389/fmicb.2020.582779
Huang Y, Zhang P, Han S, He H (2023) Lactoferrin alleviates inflammation and regulates gut microbiota composition in H5N1-Infected mice. Nutrients 15(15). https://doi.org/10.3390/nu15153362
Hutchinson EC (2018) Influenza virus. Trends Microbiol 26(9):809–810. https://doi.org/10.1016/j.tim.2018.05.013
Kell DB, Heyden EL, Pretorius E (2020) The Biology of Lactoferrin, an Iron-binding protein that can help defend against viruses and Bacteria. Front Immunol 11:1221. https://doi.org/10.3389/fimmu.2020.01221
Kilianski A, Baker SC (2014) Cell-based antiviral screening against coronaviruses: developing virus-specific and broad-spectrum inhibitors. Antiviral Res 101:105–112. https://doi.org/10.1016/j.antiviral.2013.11.004
Koksharova O, Safronova N, Dunina-Barkovskaya A (2022) New Antimicrobial peptide with two CRAC motifs: activity against Escherichia coli and Bacillus subtilis. Microorganisms 10(8). https://doi.org/10.3390/microorganisms10081538
Kolanthai E, Neal CJ, Kumar U, Fu Y, Seal S (2022) Antiviral nanopharmaceuticals: Engineered surface interactions and virus-selective activity. Wiley Interdiscip Rev Nanomed Nanobiotechnol 14(5):e1823. https://doi.org/10.1002/wnan.1823
Kumar P, Pletzer D, Haney EF, Rahanjam N, Cheng JTJ, Yue M, Aljehani W, Hancock REW, Kizhakkedathu JN, Straus SK (2019) Aurein-derived antimicrobial peptides formulated with Pegylated Phospholipid micelles to target Methicillin-resistant Staphylococcus aureus skin infections. ACS Infect Dis 5(3):443–453. https://doi.org/10.1021/acsinfecdis.8b00319
Lee C (2019) Griffithsin, a highly potent broad-spectrum antiviral lectin from Red Algae: from Discovery to Clinical Application. Mar Drugs 17(10). https://doi.org/10.3390/md17100567
Lee YJ, Shirkey JD, Park J, Bisht K, Cowan AJ (2022) An overview of antiviral peptides and rational Biodesign considerations. Biodes Res 2022(9898241). https://doi.org/10.34133/2022/9898241
Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11(7):3919–3931
Li X, Zuo S, Wang B, Zhang K, Wang Y (2022) Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 27(9). https://doi.org/10.3390/molecules27092675
Li Z, Ouyang L, Lu Y, Peng Q, Qiao X, Wu Q, Zhang B, Liu B, Wan F, Qian W (2024) Antibiotics suppress the expression of antimicrobial peptides and increase sensitivity of Cydia pomonella to granulosis virus. Sci Total Environ 947:174612. https://doi.org/10.1016/j.scitotenv.2024.174612
Liu Y, Ma A, Han P, Chen Z, Jia Y (2020) Antibacterial mechanism of brevilaterin B: an amphiphilic lipopeptide targeting the membrane of Listeria monocytogenes. Appl Microbiol Biotechnol 104(24):10531–10539. https://doi.org/10.1007/s00253-020-10993-2
Liu Q, Wang L, He D, Wu Y, Liu X, Yang Y, Chen Z, Dong Z, Luo Y, Song Y (2023) Application value of antimicrobial peptides in gastrointestinal tumors. Int J Mol Sci 24(23). https://doi.org/10.3390/ijms242316718
Loffredo MR, Nencioni L, Mangoni ML, Casciaro B (2024) Antimicrobial peptides for novel antiviral strategies in the current post-COVID-19 pandemic. J Pept Sci 30(1):e3534. https://doi.org/10.1002/psc.3534
Luo Y, Song Y (2021) Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and Antibiofilm activities. Int J Mol Sci 22(21). https://doi.org/10.3390/ijms222111401
Luo X, Wu W, Feng L, Treves H, Ren M (2021) Short peptides make a big difference: the role of Botany-Derived AMPs in Disease Control and Protection of Human Health. Int J Mol Sci 22(21). https://doi.org/10.3390/ijms222111363
Madanchi H, Shoushtari M, Kashani HH, Sardari S (2020) Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infect 34:100627. https://doi.org/10.1016/j.nmni.2019.100627
Memariani H, Memariani M, Moravvej H, Shahidi-Dadras M (2020) Melittin: a venom-derived peptide with promising anti-viral properties. Eur J Clin Microbiol Infect Dis 39(1):5–17. https://doi.org/10.1007/s10096-019-03674-0
Millet JK, Séron K, Labitt RN, Danneels A, Palmer KE, Whittaker GR, Dubuisson J, Belouzard S (2016) Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res 133:1–8. https://doi.org/10.1016/j.antiviral.2016.07.011
Mousavi Maleki MS, Rostamian M, Madanchi H (2021) Antimicrobial peptides and other peptide-like therapeutics as promising candidates to combat SARS-CoV-2. Expert Rev Anti Infect Ther 19(10):1205–1217. https://doi.org/10.1080/14787210.2021.1912593
Mousavi Maleki MS, Sardari S, Ghandehari Alavijeh A, Madanchi H (2023) Recent patents and FDA-Approved drugs based on antiviral peptides and other peptide-related antivirals. Int J Pept Res Ther 29(1):5. https://doi.org/10.1007/s10989-022-10477-z
Nastri BM, Chianese A, Giugliano R, Di Clemente L, Capasso C, Monti A, Doti N, Iovane V, Montagnaro S, Pagnini U et al (2024) Oreoch-1: a broad-spectrum virus and host-targeting peptide against animal infections. J Pept Sci e3593. https://doi.org/10.1002/psc.3593
Nawae W, Hannongbua S, Ruengjitchatchawalya M (2017) Molecular dynamics exploration of poration and leaking caused by Kalata B1 in HIV-infected cell membrane compared to host and HIV membranes. Sci Rep 7(1):3638. https://doi.org/10.1038/s41598-017-03745-2
Okano JT, Sharp K, Valdano E, Palk L, Blower S (2020) HIV transmission and source-sink dynamics in sub-saharan Africa. Lancet HIV 7(3):e209–e214. https://doi.org/10.1016/s2352-3018(19)30407-2
Omer AAM, Hinkula J, Tran PT, Melik W, Zattarin E, Aili D, Selegård R, Bengtsson T, Khalaf H (2022) Plantaricin NC8 αβ rapidly and efficiently inhibits flaviviruses and SARS-CoV-2 by disrupting their envelopes. PLoS ONE 17(11):e0278419. https://doi.org/10.1371/journal.pone.0278419
Parikh UM, McCormick K, van Zyl G, Mellors JW (2017) Future technologies for monitoring HIV drug resistance and cure. Curr Opin HIV AIDS 12(2):182–189. https://doi.org/10.1097/coh.0000000000000344
Patel S, Akhtar N (2017) Antimicrobial peptides (AMPs): the quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed Pharmacother 95:1276–1283. https://doi.org/10.1016/j.biopha.2017.09.042
Pimchan T, Tian F, Thumanu K, Rodtong S, Yongsawatdigul J (2023) Isolation, identification, and mode of action of antibacterial peptides derived from egg yolk hydrolysate. Poult Sci 102(7):102695. https://doi.org/10.1016/j.psj.2023.102695
Pinkenburg O, Meyer T, Bannert N, Norley S, Bolte K, Czudai-Matwich V, Herold S, Gessner A, Schnare M (2016) The human antimicrobial protein Bactericidal/Permeability-Increasing protein (BPI) inhibits the infectivity of Influenza A Virus. PLoS ONE 11(6):e0156929. https://doi.org/10.1371/journal.pone.0156929
Rani P, Kapoor B, Gulati M, Atanasov AG, Alzahrani Q, Gupta R (2022) Antimicrobial peptides: a plausible approach for COVID-19 treatment. Expert Opin Drug Discov 17(5):473–487. https://doi.org/10.1080/17460441.2022.2050693
Rollins-Smith LA, Smith PB, Ledeczi AM, Rowe JM, Reinert LK (2020) Caerin 1 antimicrobial peptides that inhibit HIV and Neisseria May spare protective lactobacilli. Antibiot (Basel) 9(10). https://doi.org/10.3390/antibiotics9100661
Saini J, Kaur P, Malik N, Lakhawat SS, Sharma PK (2022) Antimicrobial peptides: a promising tool to combat multidrug resistance in SARS CoV2 era. Microbiol Res 265:127206. https://doi.org/10.1016/j.micres.2022.127206
Samuels S, Alwan Z, Egnin M, Jaynes J, Connell TD, Bernard GC, Nashar T (2017) Novel Therapeutic Approach for Inhibition of HIV-1 using cell-penetrating peptide and bacterial toxins. J AIDS Clin Res 8(10). https://doi.org/10.4172/2155-6113.1000737
Shcherbak N, Prochaska H, Lystvan K, Prokhorova Y, Giritch A, Kuchuk M (2023) Accumulation of colicin M protein and its biological activity in transgenic lettuce and mizuna plants. Front Plant Sci 14:1271757. https://doi.org/10.3389/fpls.2023.1271757
Skalickova S, Heger Z, Krejcova L, Pekarik V, Bastl K, Janda J, Kostolansky F, Vareckova E, Zitka O, Adam V et al (2015) Perspective of Use of antiviral peptides against Influenza Virus. Viruses 7(10):5428–5442. https://doi.org/10.3390/v7102883
Solanki SS, Singh P, Kashyap P, Sansi MS, Ali SA (2021) Promising role of defensins peptides as therapeutics to combat against viral infection. Microb Pathog 155:104930. https://doi.org/10.1016/j.micpath.2021.104930
Souza PFN, Lopes FES, Amaral JL, Freitas CDT, Oliveira JTA (2020) A molecular docking study revealed that synthetic peptides induced conformational changes in the structure of SARS-CoV-2 spike glycoprotein, disrupting the interaction with human ACE2 receptor. Int J Biol Macromol 164:66–76. https://doi.org/10.1016/j.ijbiomac.2020.07.174
Sukmarini L (2022) Antiviral peptides (AVPs) of Marine Origin as propitious therapeutic drug candidates for the treatment of human viruses. Molecules 27(9). https://doi.org/10.3390/molecules27092619
Sultana A, Luo H, Ramakrishna S (2021) Antimicrobial peptides and their applications in Biomedical Sector. Antibiot (Basel) 10(9). https://doi.org/10.3390/antibiotics10091094
Talapko J, Meštrović T, Juzbašić M, Tomas M, Erić S, Horvat Aleksijević L, Bekić S, Schwarz D, Matić S, Neuberg M et al (2022) Antimicrobial peptides-mechanisms of Action, Antimicrobial effects and clinical applications. Antibiot (Basel) 11(10). https://doi.org/10.3390/antibiotics11101417
Tang B, Bragazzi NL, Li Q, Tang S, Xiao Y, Wu J (2020) An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect Dis Model 5:248–255. https://doi.org/10.1016/j.idm.2020.02.001
Tang Z, Ma Q, Chen X, Chen T, Ying Y, Xi X, Wang L, Ma C, Shaw C, Zhou M (2021) Recent advances and challenges in Nanodelivery Systems for antimicrobial peptides (AMPs). Antibiot (Basel) 10(8). https://doi.org/10.3390/antibiotics10080990
Urmi UL, Vijay AK, Willcox MDP, Attard S, Enninful G, Kumar N, Islam S, Kuppusamy R (2024) Exploring the efficacy of peptides and mimics against Influenza A Virus, Adenovirus, and murine norovirus. Int J Mol Sci 25(13). https://doi.org/10.3390/ijms25137030
Vineeth Kumar T, Asha R, Shyla G, George S (2018) Identification and characterization of novel host defense peptides from the skin secretion of the fungoid frog, Hydrophylax bahuvistara (Anura: Ranidae). Chem Biol Drug Des 92(2):1409–1418. https://doi.org/10.1111/cbdd.12937
Vishvakarma VK, Singh MB, Jain P, Kumari K, Singh P (2022) Hunting the main protease of SARS-CoV-2 by plitidepsin: molecular docking and temperature-dependent molecular dynamics simulations. Amino Acids 54(2):205–213. https://doi.org/10.1007/s00726-021-03098-1
Wan X, Wang W, Zhu J, Xiao Y (2024) Antibacterial peptide Reg4 ameliorates Pseudomonas aeruginosa-induced pulmonary inflammation and fibrosis. Microbiol Spectr e0390523. https://doi.org/10.1128/spectrum.03905-23
Wang Y, Grunewald M, Perlman S (2020) Coronaviruses: an updated overview of their replication and Pathogenesis. Methods Mol Biol 2203:1–29. https://doi.org/10.1007/978-1-0716-0900-2_1
Wang Y, Moon A, Huang J, Sun Y, Qiu HJ (2022) Antiviral effects and underlying mechanisms of Probiotics as Promising antivirals. Front Cell Infect Microbiol 12:928050. https://doi.org/10.3389/fcimb.2022.928050
Wei X, Zhang L, Yang Y, Hou Y, Xu Y, Wang Z, Su H, Han F, Han J, Liu P et al (2022) LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep 39(9):110880. https://doi.org/10.1016/j.celrep.2022.110880
White KM, Rosales R, Yildiz S, Kehrer T, Miorin L, Moreno E, Jangra S, Uccellini MB, Rathnasinghe R, Coughlan L et al (2021) Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371(6532):926–931. https://doi.org/10.1126/science.abf4058
Wu Q, Patočka J, Kuča K (2018) Insect antimicrobial peptides, a Mini Review. Toxins (Basel) 10(11). https://doi.org/10.3390/toxins10110461
Wuerth KC, Falsafi R, Hancock REW (2017) Synthetic host defense peptide IDR-1002 reduces inflammation in Pseudomonas aeruginosa lung infection. PLoS ONE 12(11):e0187565. https://doi.org/10.1371/journal.pone.0187565
Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S et al (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30(4):343–355. https://doi.org/10.1038/s41422-020-0305-x
Xie F, Zan Y, Zhang X, Zhang H, Jin M, Zhang W, Zhang Y, Liu S (2020) Differential abilities of mammalian cathelicidins to inhibit bacterial biofilm formation and promote multifaceted Immune functions of neutrophils. Int J Mol Sci 21(5). https://doi.org/10.3390/ijms21051871
Xiong W, Li J, Feng Y, Chai J, Wu J, Hu Y, Tian M, Lu W, Xu X, Zou M (2021) Brevinin-2GHk, a peptide derived from the skin of Fejervarya limnocharis, inhibits Zika Virus infection by disrupting viral Integrity. Viruses 13(12). https://doi.org/10.3390/v13122382
Xu C, Wang A, Marin M, Honnen W, Ramasamy S, Porter E, Subbian S, Pinter A, Melikyan GB, Lu W et al (2021) Human defensins inhibit SARS-CoV-2 infection by blocking viral entry. Viruses 13(7). https://doi.org/10.3390/v13071246
Yang J, Zhang B, Huang Y, Liu T, Zeng B, Chai J, Wu J, Xu X (2021) Antiviral activity and mechanism of ESC-1GN from skin secretion of Hylarana guentheri against influenza a virus. J Biochem 169(6):757–765. https://doi.org/10.1093/jb/mvab019
Ye M, Liao Y, Wu L, Qi W, Choudhry N, Liu Y, Chen W, Song G, Chen J (2020) An oleanolic acid derivative inhibits hemagglutinin-mediated entry of Influenza A Virus. Viruses 12(2). https://doi.org/10.3390/v12020225
You Y, Liu H, Zhu Y, Zheng H (2023) Rational design of stapled antimicrobial peptides. Amino Acids 55(4):421–442. https://doi.org/10.1007/s00726-023-03245-w
Zannella C, Chianese A, Palomba L, Marcocci ME, Bellavita R, Merlino F, Grieco P, Folliero V, De Filippis A, Mangoni M et al (2022) Broad-spectrum antiviral activity of the Amphibian Antimicrobial peptide temporin L and its analogs. Int J Mol Sci 23(4). https://doi.org/10.3390/ijms23042060
Zeng B, Chai J, Deng Z, Ye T, Chen W, Li D, Chen X, Chen M, Xu X (2018) Functional characterization of a Novel lipopolysaccharide-binding antimicrobial and anti-inflammatory peptide in Vitro and in vivo. J Med Chem 61(23):10709–10723. https://doi.org/10.1021/acs.jmedchem.8b01358
Zhang M, Ouyang J, Fu L, Xu C, Ge Y, Sun S, Li X, Lai S, Ke H, Yuan B et al (2022) Hydrophobicity determines the bacterial killing rate of α-Helical antimicrobial peptides and influences the bacterial Resistance Development. J Med Chem 65(21):14701–14720. https://doi.org/10.1021/acs.jmedchem.2c01238
Zheng X, Yang N, Mao R, Hao Y, Teng D, Wang J (2024) Pharmacokinetics and pharmacodynamics of antibacterial peptide NZX in Staphylococcus aureus mastitis mouse model. Appl Microbiol Biotechnol 108(1):260. https://doi.org/10.1007/s00253-024-13101-w
Zou K, Zhang S, Yin K, Ren S, Zhang M, Li X, Fan L, Zhang R, Li R (2024) Studies on the in vitro mechanism and in vivo therapeutic effect of the antimicrobial peptide ACP5 against Trichophyton mentagrophytes. Peptides 175:171177. https://doi.org/10.1016/j.peptides.2024.171177
Acknowledgements
The study is funded by Binzhou Medical University Research Fund Project (Grant Number BY2021KYQD02).
Author information
Authors and Affiliations
Contributions
Ma YQ drafted the work and made substantial contributions to the conception or design of the workand Yang F wrote and revised articles and prepared graphs and charts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by J. Gonzalez-Lopez.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, F., Ma, Y. The application and prospects of antimicrobial peptides in antiviral therapy. Amino Acids 56, 68 (2024). https://doi.org/10.1007/s00726-024-03427-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00726-024-03427-0