Abstract
Purpose
This study aimed to evaluate the effectiveness of the laser-assisted new attachment procedure (LANAP) and low-level laser therapy (LLLT) on clinical, biochemical, and radiographic parameters when applied alongside scaling and root planing (SRP).
Methods
The study was designed as a randomized controlled, single-blind, parallel trial involving 68 patients diagnosed with periodontitis. The participants were divided into three groups: Group 1: SRP (control), Group 2: LANAP, and Group 3: LLLT. Clinical measurements, gingival crevicular fluid (GCF) samples, and standard periapical radiographs were obtained pre-treatment and at one- and three-month follow-ups. In GCF, interleukin-1beta (IL-1β), interleukin-10 (IL-10), and vascular endothelial growth factor (VEGF) were analyzed.
Results
In moderate (4–6 mm) and deep pockets (≥ 7 mm), laser-treated groups showed a significant reduction in pocket depth (PD) and clinical attachment level (CAL) compared to the control group. However, there were no statistically significant differences in biochemical markers between the groups. Group 2 demonstrated significant bone filling compared to the control group.
Conclusion
In deep pockets, laser-treated groups provide additional benefits to SRP. The application of LLLT positively affected recession (REC).
Trial registration number: NCT04694222
Date of registration: 01/01/2021
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Periodontitis is an infectious disease that begins as gingivitis caused by microbial dental plaque (MDP) and can lead to inflammation, attachment loss, and bone loss if left untreated [1]. Nonsurgical periodontal treatment (NSPT) procedures, primarily consisting of scaling and root planing (SRP), are critical for eliminating MDP, preventing disease progression, and promoting periodontal health [2]. The effectiveness of SRP may be influenced by factors such as initial pocket depth, root concavity, furcation involvement, tooth type, and the presence of improper restorations [3]. These factors can hinder the complete elimination of MDP, potentially leading to the recurrence of periodontal disease due to bacteria invading the gingival tissue and dentin tubules [2]. To address this issue, systemic and local antibiotics and antiseptics [4], photodynamic therapy [5], and ozone treatment [6] are often used along with SRP. However, due to the side effects and disadvantages of these treatments, alternative methods are being investigated.
Currently, there is significant interest in researching various types of lasers to enhance the effectiveness of SRP in treating periodontitis. The neodymium: yttrium–aluminum-garnet (Nd:YAG) laser, with a wavelength of 1,064 nm, is a distinguished type of laser due to its high hemoglobin and melanin absorption, excellent hemostasis, and deep penetration into soft tissues [7]. The Nd:YAG laser also exhibits strong bactericidal and detoxification effects, along with biomodulation and anti-inflammatory properties [8].
The laser-assisted new attachment procedure (LANAP) is a method recently used with the Nd:YAG laser that has achieved promising results in treating periodontitis [9]. LANAP is a step-by-step procedure conducted in three stages [10]. In the first stage, the laser is used to remove the diseased epithelium, specifically targeting bacteria associated with periodontal disease, existing dental calculus, and thermolabile toxins that can be destroyed by heat [11]. In the second stage, root planing is performed to remove any remaining calculus, infected cementum, and granulation tissues. The third stage involves reapplying the laser to promote stable blood clot formation, preventing bacterial infiltration into the gingival sulcus and avoiding the epithelium from developing down into the sulcus [11, 12]. This procedure is the only laser treatment shown to contribute to periodontal regeneration by promoting the formation of new bone, cementum, and periodontal ligament, as well as improving periodontal clinical parameters [13, 14]. However, there are only a few randomized controlled clinical trials (RCCT) in the literature investigating the effectiveness of LANAP in treating periodontitis [9, 15, 16]. Furthermore, there are no RCCTs evaluating the biochemical and radiographic effects of LANAP. Therefore, this study is the first RCCT evaluating the clinical data along with the biochemical and radiographic outcomes of LANAP.
Another laser application that can enhance the effectiveness of periodontal treatment is low-level laser therapy (LLLT) [17]. LLLT has several positive effects on tissues, including reducing inflammation, promoting wound healing, enhancing immune system, managing pain, and stimulating the growth of new cells and tissues [17,18,19]. Recent in vitro studies using Nd:YAG low-level laser have shown that laser application not only facilitates periodontal regeneration but also significantly enhances osteoblast migration and Adenosine triphosphate production [20,21,22]. However, few studies have investigated the use of the Nd:YAG laser for LLLT along with SRP [23,24,25,26]. Moreover, in these studies, laser application has been observed to generally contribute to periodontal healing using a fiber optic tip. Notably, no studies have evaluated the additional effects of LLLT on SRP using a larger diameter (950-µm) R24 biomodulation handpiece. This study thus aims to assess the pure biomodulation effect of LLLT using this larger diameter Nd:YAG laser handpiece.
The hypothesis of this study is that the two laser procedures (LANAP and LLLT), which have demonstrated periodontal regenerative efficacy, will enhance periodontal healing when applied adjunctively to SRP. The originality of this research lies in being the first study to simultaneously evaluate the effects of both laser therapies (LANAP and LLLT) on SRP, while also conducting a comprehensive assessment of the relative efficacy of these two procedures through clinical, biochemical, and radiographic outcomes.
Material and methods
Study design, patient populations, and sample size calculation
This prospective, parallel-design, single-blind, RCCT was conducted per the Helsinki Declaration of 1975, as revised in 2013. The study protocol was approved by the Research Ethics Committee of Izmir Katip Celebi University of Medical Sciences (Version No. 20.09.2017). It was also registered with the Turkish Medicines and Medical Devices Agency (No. 10643207–511.06-E.251329) and the ClinicalTrials.gov database (Reference No. NCT04694222).
A total of 68 patients (40 males and 28 females, aged 26–68 years; mean age: 49.0 years) with periodontitis were recruited between October 2017 and March 2018 from the Department of Periodontics, Faculty of Dentistry, Izmir Katip Celebi University, Turkey. Eligible participants were informed about the study’s purpose and procedures and asked to provide written consent by signing a Volunteer Consent Form.
Based on a power analysis conducted using G*Power 3.1, a sample size of 20 individuals was determined to yield 98% power, assuming a 0.53 mm change in clinical attachment level (CAL) at a significance level of α = 0.05, as per a previous study [16].
Determination of periodontal status and eligibility criteria
Patients were included based on the following criteria: diagnosis of periodontal stage II, III, or IV with grade B [27]; age between 25–70 years; systemically healthy; presence of at least 12 teeth; and having ≥ 4 teeth with pocket depth (PD) ≥ 5 mm, CAL ≥ 4 mm, and radiographic evidence of alveolar bone loss. Each quadrant had at least one tooth sampled. Exclusion criteria included smoking, pregnancy or lactation, recent antibiotic (last six months) or anti-inflammatory (last three months) use, systemic diseases, medication affecting periodontal condition, or restoration adjacent to sampled teeth.
Clinical parameters
Periodontal status was assessed using clinical measurements, including PD, CAL, and recession (REC), evaluated at six sites (mesiobuccal, midbuccal, distobuccal, mesiopalatinal, midpalatinal, and distopalatinal) of each tooth. Additionally, the plaque index (PI) [28], gingival index (GI) [29], and bleeding on probing (BOP) [30] were measured at four sites (mesiobuccal, midbuccal, distobuccal, and midpalatinal) of each tooth.
Standardization was ensured by using a calibrated manual periodontal probe (PCP15-Hu-friedy, USA) for all patients. Measurements were conducted by a masked, calibrated researcher to maintain blinding. Intra-examiner agreement was assessed for PD (k = 0.96), and calibration was repeated in 10 patients with measurements taken one hour apart. Baseline measurements were repeated at the first and third months by the same examiner.
Collection of samples
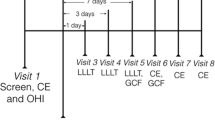
Following baseline clinical measurements, a second appointment was scheduled at least three days later for gingival crevicular fluid (GCF) collection. No periodontal procedures were performed before GCF collection to prevent influencing current periodontal status. The sampling was conducted during specific hours of the day, with saliva isolation achieved using sterile cotton rolls. Subsequently, supragingival plaque was removed, and tooth surfaces were lightly air-dried before paper strips (Periopaper®, OraFlow Inc., USA) were placed 1–2 mm into the gingival crevice for 30 s. Contaminated strips were excluded. The fluid volume was measured using a calibrated device (Periotron™ 8000 m; Oraflow Inc., USA), and strips from four quadrants were stored in coded polypropylene tubes (Eppendorf AG, Germany) at − 20 °C and subsequently at − 80 °C until analysis.
Radiographic examination
After GCF sampling, standard periapical radiographs were taken from the region believed to have the deepest bone defect among the samples before SRP and at first and third months post-treatment. The parallel technique described in Khojastehpour et al.’s [31] study was utilized for this. The periapical radiographs thus obtained were transferred to a computer, and measurements were conducted using a specialized software programFootnote 1 during evaluation. These measurements were performed following the methodology outlined by Eickholz et al. [32]. Changes in bone level were reported as a percentage.
Intervention, periodontal treatment, randomization, and allocation concealment
In the second appointment, after the GCF collection was completed, patients were grouped randomly using opaque sealed envelopes. A masked investigator opened these envelopes.
Out of the 350 individuals assessed, 68 were included in the study. These patients were divided into the following three groups according to the aforementioned randomization:
-
Group 1 (n = 24): receiving only scaling and root planing (control)
-
Group 2 (n = 22): receiving scaling and LANAP (LANAP)
-
Group 3 (n = 22): receiving scaling and root planing and LLLT (LLLT)
All treatments (SRP and laser procedures) were performed by the same investigator and completed within 24 h. After the initial samples were collected, patients in all groups received oral hygiene instructions, including the use of manual toothbrushes and dental floss or interproximal brushes. Before treatments, hand instruments were sharpened. Supragingival scaling was the first step for all groups.
For Group 1, root planing was conducted under local anesthesiaFootnote 2 using a combination of Gracey curettesFootnote 3 and ultrasonic instrumentsFootnote 4 until a smooth, clean surface was achieved for each tooth. Post-instrumentation, all supragingival surfaces were polished.
For Group 2, LANAP treatment followed scaling and polishing; it was performed in three stages:
-
(1)
In the first stage, the fiber-optic tip of the Nd:YAG laser (1,064 nm) Footnote 5 was placed parallel to the tooth’s long axis and inserted approximately 1 mm less than the PD. The fiber was slowly moved apically and laterally in a sweeping motion during the laser light emission. The laser was set at the following parameters: 3.0 W power, 180-µs (short pulse) pulse duration, and 20 Hz frequency, according to the preliminary study [14]. This ensure removal of the diseased epithelium toward the soft tissue wall of the periodontal pocket and significant hemostasis. Additionally, to prevent thermal damage and ensure standardization, the duration of the laser application was determined to be 10 s for each pocket.
-
(2)
In the second stage, full-mouth root planing was performed as in the control group.
-
(3)
In the third stage, an Nd:YAG laser fiber optic tip was applied at 4.0 W power, 320-µs (long pulse) pulse time, and 20 Hz frequency to achieve a stable fibrin clot. To avoid thermal damage to the gingiva during the laser application stages of LANAP treatment, physiological saline was applied with a blunt-tip 10 cc injector.
For Group 3, after SRP, LLLT was performed using the Nd:YAG laser (1,064 nm) with an R24 biomodulation handpiece tip (950-µm) at 1 W power, 10 Hz frequency, and 320-µs (long pulse) pulse duration. The spot size was 0,28 cm2 and energy density was calculated to be 106,15 J/cm2. The instrument was applied for 30 s per site (mesiobuccal, distobuccal, mesiolingual, and distolingual regions) at an average distance of 1 cm from the oral epithelial surface.
Patients in all groups received instructions on oral hygiene including the use of manual toothbrushes and dental floss or interproximal brushes. Mouth rinsing was discouraged post-treatment to avoid changes in results. After their periodontal treatments were completed, the patients were called for a follow-up two weeks later. Those with poor oral hygiene were motivated further; however, those who could not maintain optimal oral hygiene were dropped from the study. At the first and third months, patients were carefully re-evaluated to ensure they had performed effective plaque control.
Biochemical analysis
GCF levels of interleukin- 1beta (IL- 1β), interleukin- 10 (IL- 10), and vascular endothelial growth factor (VEGF) were analyzed using enzyme-linked immunosorbent assay (ELISA) kitsFootnote 6. The total amounts of biochemical markers in four samples collected over 120 s were calculated.
To analyze the GCF samples, polypropylene tubes containing frozen paper strips were kept at room temperature for at least 20 min before 400 μl of phosphate buffer was added to each tube. The samples were kept at 4 °C overnight before analysis to ensure the passage of GCF into the phosphate buffer in the paper strips. Homogenization was achieved by vortexing the tubes for one minute, two hours before analysis. Diluted GCF samples were pipetted with polypropylene tips and analyzed for each biochemical mediator per the manufacturer's instructions. After the reaction was stopped with a solution, the absorbance was measured spectrophotometricallyFootnote 7 at a wavelength of 450 nm. Cytokine concentrations were adjusted based on GCF volume and expressed in pg/ml.
Outcome variables
The primary outcome was the difference in the change of CAL between groups. Secondary outcome variables included differences among the groups in the following parameters: (i) PD, (ii) GI, (iii) PI, (iv) BOP, (v) mean levels of each biochemical parameter, and (vi) radiographic bone changes.
Statistical analysis
Descriptive statistics of clinical, laboratory, and radiographic data were presented as mean, standard deviation, median, minimum, maximum, frequency, and percentage values. The normality assumption of the quantitative data was checked using the Shapiro–Wilk test.
For normally distributed variables, the repeated-measures analysis of variance (ANOVA) method was employed for intra-group comparisons, with Bonferroni correction applied for pairwise comparisons. Inter-group comparisons were conducted using the one-way ANOVA method along with Tukey’s HSD (honestly significant difference) multiple comparison test. Statistical analyses for normally distributed data were performed using the SPSS 25.0 package program (IMB, Armonk, NY).
Since moderate and deep pocket variables (PD, CAL, and REC) did not follow a normal distribution, the non-parametric Brunner-Langer method (F1-LD-F1 model) was used to examine the time-dependent variation in these variables. Analyses were performed using R 3.5.2 software. If the time-dependent change was dissimilar across groups (interaction p < 0.1), time comparisons within each group were conducted using the Brunner-Langer method (LD-F1 model) with Bonferroni correction. Furthermore, inter-group comparisons were performed using the Kruskal–Wallis method, with pairwise group comparisons conducted via the Dunn test. The level of significance was set at p < 0.05 for all analyses.
Results
Clinical findings
Although the study initially enrolled 68 patients, it was completed with 60 participants, with each treatment group comprising 20 patients. Of the eight patients who discontinued participation, four failed to attend the first month’s appointment, and two missed the third month’s appointment. The remaining twos were excluded from the study for failing to comply with oral care recommendations (Fig. 1).
No adverse effects or complications related to SRP or laser treatments were reported throughout the study. The initial demographic characteristics of the patients are provided in Supplementary Table 1. At baseline, there were no statistically significant differences in the full-mouth periodontal pocket values of PI, GI, BOP, PD, and CAL across all groups (p > 0.05).
The PI, GI, and BOP values of all treated periodontal pockets (PD ≥ 4 mm) at each sampling time are presented in Supplementary Table 2. No statistically significant differences were observed between the groups at any time point (p > 0.05). Across all groups, significant reductions in PI, GI, and BOP values were noted at both the first and third months compared to baseline. However, in Group 3 (LLLT), a significant decrease in GI values was observed at the third month compared to the first month (p < 0.001).
Periodontal pockets were classified into two subgroups based on disease severity: moderate pockets (4–6 mm) and deep pockets (≥ 7 mm). A total of 2,378 moderate pockets were treated across all groups, with 759 in Group 1 (control), 859 in Group 2 (LANAP), and 723 in Group 3 (LLLT). Additionally, 569 deep pockets were identified, with 187 in Group 1, 219 in Group 2, and 163 in Group 3.
PD, CAL, and REC values for moderate pockets are shown in Table 1. In moderate pockets, a significant difference was observed in the inter-group comparisons of PD changes from baseline to the first month (p = 0.048). However, no significant differences were found in the pairwise comparisons (p > 0.05). Regarding CAL changes, a significant difference was observed only between Group 3 (LLLT) and Group 1 (control) (p = 0.007).
In the inter-group comparisons of PD changes from baseline to the third month, significant differences were found between the test groups (LANAP and LLLT) and Group 1 (control) (p = 0.002). Conversely, a significant difference in CAL was observed only between Group 3 (LLLT) and the other groups (p < 0.001). For REC changes, a significant difference was observed between Group 3 (LLLT) and the other groups (p < 0.001). As shown in Fig. 2a, REC was lower in Group 3 (LLLT), although the rate of change was minimal.
PD: Pocket Depth, CAL: Clinical Attachment Level, REC: Recession, 0: Baseline, Δ[0–3]: Changes in third month compared to baseline. The meanings of the symbols representing the statistically significant time-dependent changes between groups are as follows: * represents the difference of LLLT compared to the control. * represents the difference of LANAP compared to the control. † represents the difference of LLLT compared to LANAP. ‡ represents the difference of LANAP and LLLT compared to the control (Brunner-Langer method [F1-LD-F1 model]-R 3.5.2 software)
PD, CAL, and REC values of deep pockets are shown in Table 2. In deep pockets, intra-group evaluation revealed a significant reduction in PD in all groups at both the first and third months compared to baseline (p < 0.001). While the test groups showed a significant PD reduction at the third month compared to the first month, no significant reduction was observed in the control group. Statistically significant differences were found in the inter-group comparisons of PD changes (p < 0.001). Both from baseline to the first month and from the first to the third month, test groups exhibited a significantly greater PD reduction compared to the control group (p < 0.001). Consistent with this, test groups also demonstrated a statistically significant CAL gain compared to the control group (p < 0.001). A significant difference was observed in REC changes from baseline to the third month, particularly between Group 3 (LLLT) and the other groups. Group 3 (LLLT) exhibited a lesser degree of REC compared to the other groups (p < 0.001), consistent with the results observed in moderate pockets (Fig. 2b).
Biochemical findings
The GCF volume decreased over time in all groups; however, no statistically significant differences were observed between the groups. Intra-group and inter-group comparisons of the total amounts of GCF IL- 1β, GCF IL- 10, and VEGF at baseline, the first month, and the third month are summarized in Table 3.
GCF IL- 1β levels showed a statistically significant reduction across all groups throughout the study, but no significant differences were found between the groups (p > 0.05). Similarly, the total amounts of GCF VEGF did not exhibit statistically significant differences between groups (p > 0.05).
For IL- 10, a statistically significant difference was observed between the groups at baseline (p < 0.036); however, no significant differences were noted at the first and third months (p > 0.05).
Radiographic findings
Inter-group comparisons of the percentage changes in periodontal defects of all groups are presented in Table 4. At the first month, the highest bone filling was observed in Group 2 (LANAP), with a statistically significant difference compared to the control group (p = 0.021). By the third month, Group 2 (LANAP) continued to exhibit the highest bone filling; however, no statistically significant differences were found between the groups (p > 0.05).
Discussion
To the best of our knowledge, this is the first RCCT to assess the clinical, biochemical, and radiographic effects of LANAP and LLLT besides SRP. This study’s primary finding is that both LANAP and LLLT significantly reduced PD and improved CAL, particularly in deep pockets with LLLT also demonstrating a notable positive impact on REC.
In the inter-group comparisons of changes in moderate pockets, it was found that the test groups showed a statistically significant reduction in PD at the third month compared to baseline. However, only Group 3 (LLLT) achieved a significant CAL gain at the third month relative to the control group. Additionally, Group 3 consistently exhibited the lowest REC in both moderate and deep pockets, suggesting healing via clinical attachment gain rather than gingival recession. LLLT’s ability to stimulate keratinocyte activity, accelerate wound healing, and enhance immune responses through fibroblast proliferation, matrix synthesis, and neovascularization [33, 34] likely contributed to this outcome. Furthermore, LLLT has been shown to improve tensile strength and gingival margin stability, aiding in REC prevention [35].
Conversely, the results in deep pockets showed slight differences. In intra-group evaluations, all groups demonstrated statistically significant reductions in PD compared to baseline at both the first and third months. However, by the third month, the test groups exhibited a statistically significant decrease in PD, while the control group did not. This finding suggests that when only SRP is performed in deep pockets, periodontopathogens may not be completely eliminated, leading to recolonization over subsequent months.
In inter-group comparisons for deep pockets, LLLT (applied to the oral epithelial surface outside the gingival sulcus) resulted in statistically significant reductions in PD and gains in CAL compared to the control group, similar to LANAP (applied internally within the gingival sulcus). To date, no study has specifically evaluated the effect of LLLT applied to periodontal pockets externally. Since LLLT is administered to the oral epithelial surface outside the gingival sulcus, it is unlikely to exert the same antimicrobial effects or ability to remove diseased tissue as LANAP [36, 37]. The benefits of LLLT in deep pockets attributed to its positive effects on tissue repair. Several studies have reported that LLLT enhances blood circulation, raises interleukin- 8 (IL- 8) levels, and modulates immune system cells, thereby improvin the immune response [38, 39]. In this study, LLLT was employed for biomodulation, and its contribution to periodontal healing likely stems from its enhancement of the host immune response.
While Group 2 (LANAP) provided reductions in PD, it did not yield a significant difference in CAL gain in moderate pockets. However, it did result in statistically significant CAL gain and PD reduction in deep pockets, likely due to the additional contributions of LANAP. Katuri et al. [16] stated that in deep pockets, LANAP provides greater reductions in PD and gains in CAL compared to SRP alone. Cobb et al. [40] examined the effects of the Nd:YAG laser on the root surface and subgingival flora. Microbiological studies involving LANAP have demonstrated its efficacy in reducing periodontal pathogens and promoting healing in difficult-to-access periodontal areas [36, 37, 41]. Additionally, LANAP has been reported to improve the de-epithelialization of diseased sulcular epithelium, potentially eliminating invasive bacteria [16]. Since access is more challenging in deep pockets than in moderate ones, LANAP’s ability to provide effective PD reduction in deep pockets is particularly relevant for clinical practice.
Biochemically, this study revealed significant reductions in the levels of IL- 1β across all groups. IL- 1β, a key mediator in the inflammatory cascade associated with periodontal disease, showed a consistent decline throughout the study period, with all groups demonstrating reductions by the third month. IL- 1β levels have been shown to decrease even after SRP alone due to resolution of inflammation [42]. Laser therapies applied in conjunction with SRP have shown positive effects in reducing IL- 1β levels [1]. Although some studies found no significant effects [25], the current study observed a consistent decrease over time, aligning with previous reports that highlighted positive effects. The lack of inter-group differences could be attributed to that SRP alone is sufficient to lower IL- 1β levels, as suggested by the literature [43]. In addition, the relatively long time frame of sample collection, which may have masked potential differences in early inflammatory responses. Although IL- 10 is generally known as an anti-inflammatory cytokine, its relationship with periodontal disease remains unclear. Literature findings on the effects of SRP and laser therapies on IL- 10 levels have been inconsistent, reporting decreases [44], no changes [45], or increases [46]. In the present study, no statistical differences in IL- 10 levels were observed between groups at any time point, consistent with studies reporting no significant changes. The VEGF levels, which play a crucial role in angiogenesis and tissue healing, decreased over time across all groups, which likely reflects the overall resolution of inflammation and the subsequent healing process. No significant differences were observed between groups regarding VEGF levels at any time point. Previous studies have reported reductions in GCF VEGF levels after SRP [47]. While studies on laser applications noted statistically significant increases in VEGF levels with both LLLT [48] and high-dose laser applications such as LANAP [49], these evaluations were conducted in the early healing period (24–72 h). Differences might have diminished in this study, where samples were collected at later stages.
The biochemical markers measured in this study were selected to provide insights into the molecular mechanisms underlying clinical outcomes. Together, the clinical and biochemical findings suggest that both LANAP and LLLT provide significant clinical benefits, potentially through modulation of inflammatory cytokines and promotion of tissue regeneration. The reduction of IL- 1β is consistent with the clinical improvements in PD and CAL, highlighting the role of inflammation in periodontal disease progression and healing. The involvement of VEGF in angiogenesis and bone regeneration is supported by the clinical improvements observed in bone fill, particularly in the LANAP group, where early regenerative effects were most pronounced.. The more significant effects of LANAP on bone healing and tissue attachment in deeper pockets may be attributed to its ability to target both diseased tissue removal and tissue regeneration, while the beneficial effects of LLLT on reducing gingival recession and promoting healing in moderate pockets may be attributed to its anti-inflammatory and biomodulatory effects.
Radiographically, although bone filling was observed across all test groups, only Group 2 (LANAP) demonstrated a statistically significant difference compared to the control group. While no large-scale studies have specifically evaluated LANAP’s effects on bone filling, case reports suggest that it can promote bone regeneration [50, 51]. This regenerative capacity is attributed to LANAP’s ability to form a physical barrier skin to a membrane that inhibits epithelial growth, stimulates the release of precursor cells from periodontal ligament and alveolar bone, and promotes stable fibrin clot formation. This clot is thought to enhance regeneration by directing healing from apical to coronal regions [50]. Conversely, LLLT has been shown to increase the release of growth factors [48], stimulate precursor cell production [17], and contribute to periodontal regeneration by reducing osteoclastic activity in periodontal tissues [52]. Further, LLLT contributes to early-stage bone formation by promoting osteoblast and fibroblast proliferation [53]. These mechanisms likely explain the bone filling observed in laser-treated groups and highlight the positive effects of LLLT and LANAP on bone healing [54, 55]. No statistically significant differences were noted between groups regarding changes in bone levels at both the first and third months.
This study has several limitations. First, the timing of biochemical sample collection might have affected the findings. Future studies should investigate the early healing effects of laser therapies combined with SRP on biochemical markers in periodontal tissues. Additionally, while this study monitored bone filling in the deepest defects, it did not standardize defect types. Further research focusing on specific bone defect types is warranted. Finally, bone assessments were performed linearly in this study. Volumetric changes should be evaluated using advanced and precise methods in future investigations.
Conclusion
Both LANAP and LLLT have been shown to be effective adjunctive therapies to SRP in the management of periodontal disease. Additionally, both interventions contributed significantly to clinical outcomes, including the CAL gain and PD reduction, particularly in deep pockets. Notably, LLLT demonstrated a distinct advantage in minimizing gingival recession and promoting tissue healing, highlighting its potential as a valuable adjunctive treatment in periodontal therapy. Biochemically, a reduction in the inflammatory cytokine IL- 1β levels was observed in both treatment groups, indicating the ability of these therapies to control inflammation. However, no significant differences were found in VEGF and IL- 10 levels between the treatment groups, suggesting that these biomarkers did not vary significantly with treatment type. Additionally, both LANAP and LLLT treatments have demonstrated the potential for promoting bone gain. The significant advantages of these therapies suggest their potential to minimize the need for periodontal surgical interventions. However, further studies involving larger sample sizes and long-term outcome evaluations are necessary to confirm and expand upon these findings.
Data availability
No datasets were generated or analysed during the current study.
Notes
Adobe Systems Incorporated, San Jose, CA, USA.
Maxicaine forte, VEM Ilac, Turkey.
Hu-Friedy, USA.
EMS minipiezon, France.
Fotona Fidelis AT, USA.
E-Bioscience, USA.
Multiskan GO, Thermo Fisher Scientific, MA, USA.
References
Dommisch H, Kebschull M (2015) Chronic periodontitis. In: Carranza’s clinical periodontology, 12th edn. Elsevier, Missouri pp 309–319
Kinane DF, Lindhe J, Trombelli L (2008) Chronic periodontitis. In: Clinical periodontology and implant dentistry, 5th edn. Blackwell Munksgaard, Copenhagen pp 420–427
Dentino A, Lee S, Mailhot J, Hefti AF (2013) Principles of periodontology. Periodontol 61:16–53. https://doi.org/10.1111/j.1600-0757.2011.00397.x
Slots J (2002) Selection of antimicrobial agents in periodontal therapy. J Periodontal Res 37:389–398. https://doi.org/10.1034/j.1600-0765.2002.00004.x
Sgolastra F, Petrucci A, Severino M et al (2013) Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol 40:514–526. https://doi.org/10.1111/jcpe.12094
Jungbauer G, Moser D, Müller S et al (2021) The antimicrobial effect of cold atmospheric plasma against dental pathogens—a systematic review of in-vitro studies. Antibiotics 10:1–26. https://doi.org/10.3390/antibiotics10020211
Mizutani K, Aoki A, Coluzzi D et al (2016) Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000 71:185–212. https://doi.org/10.1111/PRD.12123
Grzech-Leśniak K, Sculean A, Gašpirc B (2018) Laser reduction of specific microorganisms in the periodontal pocket using Er:YAG and Nd:YAG lasers: a randomized controlled clinical study. Lasers Med Sci 33:1461–1470. https://doi.org/10.1007/s10103-018-2491-z
Dortaj D, Bassir SH, Hakimiha N et al (2021) Efficacy of Nd:YAG laser-assisted periodontal therapy for the management of periodontitis: a double-blind split-mouth randomized controlled clinical trial. J Periodontol 93(5):662–72. https://doi.org/10.1002/JPER.21-0242
Harris DM, Gregg RH, McCarthy DK et al (2004) Laser-assisted new attachment procedure in private practice. Gen Dent 52:396–403. https://doi.org/10.1016/j.marchem.2005.01.005
Sameer S, Mohan K, Pillai H et al (2015) Laser Assisted New Attachment Procedure in Periodontics. J Soc Periodontists Implantol Kerala 8:127–131
Atteya AF, Shafei RM, Jefri AAA (2017) Laser-assisted new attachment procedure – LANAP. Egypt J Hosp Med 69:1641–1645. https://doi.org/10.12816/0040113
Yukna R, Carr R, Evans G (2007) Histologic Evaluation of an Nd:YAG Laser-Assisted New Attachment Procedure in Human. Int J Periodontics Restorative Dent 27:577–587
Yukna RA (2022) Clinical evaluation of laser-assisted new attachment procedure® (LANAP®) surgical treatment of chronic periodontitis: a retrospective case series of 1-year results in 22 consecutive patients. J Periodontal Implant Sci 52:173–183. https://doi.org/10.5051/jpis.2202580129
Harris DM, Nicholson DM, McCarthy D et al (2014) Change in \clinical indices following laser or scalpel treatment for periodontitis. Proc SPIE 8929:1–9
Katuri KK, Bollepalli AC, Reddy Sunkireddy HK et al (2015) Clinical effectiveness of laser assisted new attachment procedure as an adjunct to non-surgical periodontal rreatment: a randomized study. J Int Oral Heal 7:57–62
Ishikawa I, Aoki A, Takasaki AA et al (2009) Application of lasers in periodontics: true innovation or myth? Periodontol 2000 50:90–126. https://doi.org/10.1111/j.1600-0757.2008.00283.x
Takemura S, Mizutani K, Mikami R et al (2023) Enhanced periodontal tissue healing via vascular endothelial growth factor expression following low-level erbium-doped: yttrium, aluminum, and garnet laser irradiation: in vitro and in vivo studies. J Periodontol 95:853–866. https://doi.org/10.1002/JPER.23-0458
Petrović MS, Kannosh IY, Milašin JM et al (2018) Clinical, microbiological and cytomorphometric evaluation of low-level laser therapy as an adjunct to periodontal therapy in patients with chronic periodontitis. Int J Dent Hyg 16:e120–e127. https://doi.org/10.1111/idh.12328
Wu N, Song J, Liu X et al (2023) Effect of an low-energy Nd: YAG laser on periodontal ligament stem cell homing through the SDF-1/CXCR4 signaling pathway. BMC Oral Health 23:1–11. https://doi.org/10.1186/s12903-023-03132-6
Wang L, Liu C, Wu F (2022) Low-level laser irradiation enhances the proliferation and osteogenic differentiation of PDLSCs via BMP signaling. Lasers Med Sci 37:941–948. https://doi.org/10.1007/s10103-021-03338-6
Tsuka Y, Kunimatsu R, Gunji H et al (2019) Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Lasers Med Sci 34:55–60. https://doi.org/10.1007/s10103-018-2586-6
Gómez C, Domínguez A, García-Kass AI, García-Nuñez JA (2011) Adjunctive Nd:YAG laser application in chronic periodontitis: clinical, immunological, and microbiological aspects. Lasers Med Sci 26:453–463. https://doi.org/10.1007/s10103-010-0795-8
Qadri T, Miranda L, Tuner J, Gustafsson A (2005) The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol 32:714–719. https://doi.org/10.1111/j.1600-051X.2005.00749.x
Eltas A, Orbak R (2012) Effect of 1,064-nm Nd:YAG laser therapy on GCF IL-1β and MMP-8 levels in patients with chronic periodontitis. Lasers Med Sci 27:543–550. https://doi.org/10.1007/s10103-011-0939-5
Ezber A, Taşdemir İ, Yılmaz HE et al (2023) Different application procedures of Nd:YAG laser as an adjunct to scaling and root planning in smokers with stage III grade C periodontitis: a single-blind, randomized controlled trial. Ir J Med Sci 192:457–466. https://doi.org/10.1007/s11845-022-02940-z
Tonetti MS, Greenwell H, Kornman KS, Tonetti M (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol 89:159–172. https://doi.org/10.1002/JPER.18-0006
Silness J, Löe H (1964) Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135. https://doi.org/10.3109/00016356408993968
Löe H, Silness J (1963) Periodontal disease in pregnancy I Prevalence and severity. Acta Odontol Scand 21:533–551. https://doi.org/10.3109/00016356309011240
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25:229–235
Khojastehpour L, Khosropanah H, Kharazifard MJ (2006) The effect of bite registration on the reproducibility of parallel periapical radiographs obtained with two-month intervals. J Dent 3:87–91
Eickholz P, Hörr T, Klein F et al (2004) Radiographic parameters for prognosis of periodontal healing of infrabony defects: two different definitions of defect depth. J Periodontol 75:399–407. https://doi.org/10.1902/jop.2004.75.3.399
Alves AC, Vieira R, Leal-Junior E et al (2013) Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther 15:R116. https://doi.org/10.1186/ar4296
Ren C, McGrath C, Jin L et al (2017) The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: a meta-analysis. J Periodontal Res 52:8–20. https://doi.org/10.1111/jre.12361
Akram Z, Vohra F, Javed F (2018) Low-level laser therapy as an adjunct to connective tissue graft procedure in the treatment of gingival recession defects: a systematic review and meta-analysis. J Esthet Restor Dent 30(4):299–306. https://doi.org/10.1111/jerd.12377
McCawley TK, McCawley MN, Rams TE (2021) Immediate effect of Nd:YAG laser monotherapy on subgingival periodontal pathogens: a pilot clinical study. J Periodontal Implant Sci 51:1–11. https://doi.org/10.5051/jpis.2100900045
Bechir ES (2023) The clinical and microbiological effects of LANAP compared to scaling and root planing alone in the management of periodontal conditions. Diagnostics 13:2450. https://doi.org/10.3390/diagnostics13142450
Ohta A, Abergel RP, Uitto J (1987) Laser modulation of human immune system: inhibition of lymphocyte proliferation by a gallium-arsenide laser at low energy. Lasers Surg Med 7:199–201. https://doi.org/10.1002/lsm.1900070211
Er H, Doganay S, Evereklioglu C et al (2002) Effects of L-NAME and Timolol on aqueous IL-1β, IL-6, IL-8, TNF-α and NO levels after Nd:YAG laser iridotomy in rabbits. Eur J Ophthalmol 12:281–286
Cobb CM, McCawley TK, Killoy WJ (1992) A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol 63:701–707. https://doi.org/10.1902/jop.1992.63.8.701
Chandra S, Shashikumar P (2019) Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci 10:56–63. https://doi.org/10.15171/jlms.2019.09
Hou L-T, Liu C-M, Rossomando EF (1995) Crevicular interleukin-1β in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J Clin Periodontol 22:162–167. https://doi.org/10.1111/j.1600-051X.1995.tb00128.x
Tsai C-C, Ho Y-P, Chen C-C (1995) Levels of interleukin-1β and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol 66:852–859. https://doi.org/10.1902/jop.1995.66.10.852
Gamonal J, Acevedo A, Bascones A et al (2000) Levels of interleukin-1β, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71:1535–1545
Goutoudi P, Diza E, Arvanitidou M (2004) Effect of periodontal therapy on crevicular fluid interleukin-1β and interleukin-10 levels in chronic periodontitis. J Dent 32:511–520. https://doi.org/10.1016/j.jdent.2004.04.003
da Cunha MG, Vitoretti LB, de Brito AA et al (2018) Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor. Oxid Med Cell Longev 2018:1–8. https://doi.org/10.1155/2018/6798238
Türer ÇC, Durmuş D, Balli U, Güven B (2017) Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum endocan, vascular endothelial growth factor-A, and tumor necrosis factor-alpha levels. J Periodontol 88:493–501. https://doi.org/10.1902/jop.2016.160279
Gkogkos AS, Karoussis IK, Prevezanos ID et al (2015) Effect of Nd:YAG low-level laser therapy on human gingival fibroblasts. Int J Dent 2015:1–7. https://doi.org/10.1155/2015/258941
Sağlam M, Köseoğlu S, Pekbağrıyanık T, Savran L (2017) Effects of high power-pulsed Nd : YAG laser irradiation on the release of transforming growth factor-beta (TGF- β) and vascular endothelial growth factor (VEGF) from human gingival fibroblasts. J Cosmet Laser Ther 19:469–474. https://doi.org/10.1080/14764172.2017.1342042
Brown IS (2013) Current advances in the use of lasers in periodontal therapy: a laser-assisted new attachment procedure case series. Clin Adv Periodontics 3:96–104. https://doi.org/10.1902/cap.2013.120087
Long B. (2016) Treating a diabetic patient with periodontal disease using the LANAP protocol: a case study. Dent İq
Zhang L, Chen W, Li Y et al (2020) Correction to: effect of 650-nm low-level laser irradiation on c-Jun, c-Fos, ICAM-1, and CCL2 expression in experimental periodontitis. Lasers Med Sci 35:41. https://doi.org/10.1007/s10103-019-02902-5
Kheiri A, Amid R, Kheiri L et al (2020) Effect of low-level laser therapy on bone regeneration of critical-size bone defects: a systematic review of in vivo studies and meta-analysis. Arch Oral Biol 117:104782. https://doi.org/10.1016/j.archoralbio.2020.104782
Aoki A, Mizutani K, Schwarz F et al (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 68:217–269. https://doi.org/10.1111/prd.12080
Stein A, Benayahu D, Maltz L, Uron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Lamster IB, Oshrain RL, Gordon JM (1986) Enzyme activity in human gingival crevicular fluid: considerations in data reporting based on analysis of individual crevicular sites. J Clin Periodontol 13:799–804. https://doi.org/10.1111/j.1600-051X.1986.tb00885.x
Acknowledgements
This study was supported by the Research Project Coordination of Izmir Katip Celebi University (project no. 2017-TDU-DİŞF- 0040). The authors thank Osman Sami Kırtıloğlu (Department of Cartography, Katip Celebi University, Izmir, Turkey) for his help in the radiographic analysis and Levent Savran (Department of Periodontology, Katip Celebi University, Izmir, Turkey) for his help in biochemical analysis.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the Research Project Coordination of Izmir Katip Celebi University (Project No. 2017-TDU-DİŞF- 0040). The authors have no financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Study conception: F.K.D., S.K., M.S. Study design: F.K.D., S.K. Data collection: F.K.D., S.G.K. Writing, original draft preparation: F.K.D. Supervision: F.K.D., S.K., M.S. Data analysis: O.F.D. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained from the ethics committee of Izmir Katip Celebi University of Medical Sciences (Version No. 20.09.2017). This study was registered with the Turkish Medicines and Medical Devices Agency (No. 10643207–511.06-E.251329). All procedures adhered to the tenets of the Declaration of Helsinki.
Consent to participate
All individuals eligible for the study were informed about the purpose of the study and all procedures to be carried out throughout the study, and then signed a Volunteer Consent Form.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaya Dadas, F., Genc Kocaayan, S., Saglam, M. et al. Evaluating efficacy of laser-assisted new attachment procedure and adjunctive low-level laser therapy in treating periodontitis: A single-blind randomized controlled clinical study. Lasers Med Sci 40, 208 (2025). https://doi.org/10.1007/s10103-025-04457-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-025-04457-0
Keywords
Profiles
- Omer Faruk Dadas View author profile