Abstract
Although the 5-year relative survival rates for resectable solid tumors have improved over the past few years, the risk of postoperative recurrence necessitates effective monitoring strategies. Recent advancements in molecular residual disease (MRD) testing based on circulating tumor DNA (ctDNA) analysis have shown considerable promise in the context of predicting recurrence; however, significant barriers to widespread clinical implementation remain—mainly, low awareness among healthcare professionals, high costs, and lack of standardized assays and comprehensive evidence. This position paper, led by the Japan Society of Clinical Oncology, aims to establish a common framework for the appropriate clinical use of MRD testing in a tumor type-agnostic manner. It synthesizes currently available evidence, reviews region-specific clinical trends, addresses critical clinical questions related to MRD testing, and offers recommendations to guide healthcare professionals, biotechnology and pharmaceutical companies, and regulatory authorities. These recommendations were developed based on a voting process involving 15 expert members, ensuring a consensus-driven approach. These findings underscore the importance of collaborative efforts among various stakeholders in enhancing the clinical utility of MRD testing. This project aimed to foster consensus and provide clear guidelines to support the advancement of precision medicine in oncology and improve patient outcomes in the context of perioperative care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
In Japan, approximately 380,000 people die annually from malignant cancer, making it the leading cause of death. The 5-year relative survival rates for patients with solid tumors confined to primary sites and regions were 92.4% and 58.1%, respectively, whereas that for patients with distant metastases was 15.7% [1]. As resectable solid tumors confined to primary sites and regions are expected to be curable, operative treatment, in combination with chemotherapy and radiotherapy, is the recommended standard of care in these cases.
Recently, it has been reported that the detection of molecular residual disease (MRD) using circulating tumor DNA (ctDNA) analysis may be useful in assessing the postoperative recurrence risk of solid tumors. Some MRD tests are already in clinical use in Europe and the U.S., and the granting of regulatory approval and inclusion in insurance coverage is expected in Japan in the future. Nonetheless, although MRD testing is expected to improve the clinical management of resectable solid tumors, the following issues regarding its clinical implementation need to be addressed:
-
1.
Awareness of MRD testing is low among both medical professionals and patients.
-
2.
A variety of MRD testing assays and methods are not standardized.
-
3.
The high cost of MRD testing has a significant impact on medical economics.
-
4.
There is a lack of high-level evidence from large-scale studies, including clinical studies and randomized trials.
-
5.
Consistent interpretation of the data is difficult because the standard of care can vary considerably depending on tumor type.
-
6.
There are currently no guidelines for the appropriate clinical use of MRD testing.
Therefore, it is necessary to formulate a common view of MRD testing in a tumor type-agnostic manner and prepare detailed guidelines for decision-making regarding its appropriate clinical use by medical professionals, biotechnology and pharmaceutical companies, regulatory authorities, and patients.
The objective of this document is to discuss and interpret the current evidence on MRD testing comprehensively and to reach a common view of its appropriate clinical use in a tumor type-agnostic manner.
Overview
Chapter 2 provides an overview of ctDNA analysis and MRD testing.
Chapter 3, particularly region-specific clinical trends, provides a comprehensive review of the current evidence for each tumor type.
Chapter 4 presents Clinical Questions (CQs) related to the appropriate clinical use of MRD testing, as well as the corresponding current tumor type-agnostic recommendations. With regard to these recommendations, we prioritized consensus building to obtain common views while also considering that the evidence for standards of care and MRD testing vary depending on the tumor type. Therefore, it should be noted that the details of the recommendations must be interpreted with reference to the relevant guidelines and other requirements pertinent to the respective tumor types.
Finally, in Chapter 5, the reference data and information on the latest MRD assays are provided.
When this position paper was planned, MRD testing had not obtained regulatory approval. The Japanese insurance system did not cover it, and certain recommendations for CQs are based on insufficient evidence at this time. Therefore, the descriptions and recommendations provided here could change significantly with the accumulation of future evidence. Considering that regulatory approval for MRD testing was not taken into account in preparing these recommendations and that they are based on existing evidence and consensus among the Working Group members, this document is characterized as a "position paper on appropriate clinical use."
Overview
ctDNA
Liquid biopsy refers to the use of body fluid samples for analysis of tumor-derived biomolecules. Various body fluids, such as blood, saliva, urine, cerebrospinal fluid, bile, and pancreatic juice, are used as specimens for analysis. Of these, blood can be collected repeatedly in a minimally invasive manner. Moreover, blood sample collection methods are well standardized, and the blood contains biomolecules derived from many organs. Therefore, blood sample analysis has been clinically used for many tumor types [2, 3].
The presence of extracellular DNA, also called cell-free DNA (cfDNA), in blood was first reported in 1948 [4]. Although it is present only in trace amounts (1–10 ng in 1 mL of plasma) in healthy individuals, its amount is reported to increase under certain conditions, such as cancer and infection, and after cerebral infarction, traumatic injury, and excessive exercise. ctDNA is a form of cfDNA released into the blood as a result of the apoptosis or necrosis of cancer cells. The reported variant allele frequency (VAF) of ctDNA in cfDNA is approximately 0.1–10% [2]. Since it was first reported in 1994 that KRAS mutations identical to those in tumor tissues were detectable in the plasma cfDNA of a patient with pancreatic cancer, research on ctDNA has advanced rapidly [5].
As ctDNA is present in plasma only in trace amounts, standardization of preanalytical conditions is essential for appropriate analysis [6]. An important consideration in this regard is that if leukocytes are lysed due to sample blood coagulation, the resulting increase in normal cfDNA levels makes it difficult to detect ctDNA; therefore, blood collection tubes containing anticoagulants need to be used for such analyses. Ethylenediaminetetraacetic acid (EDTA) is the preferred anticoagulant, whereas heparin is not appropriate. Furthermore, to remove blood cells that may dilute cfDNA, it is important to ensure that the initial centrifugation of the blood collected in EDTA-containing collection tubes is performed within a few hours of sample collection. Specially designed collection tubes that minimize the incorporation of leukocyte-derived cfDNA are often used, and blood samples can be stored at room temperature for up to 14 days. For other precautions regarding ctDNA analysis according to the specific methodology used, please refer to the position paper of the Japanese Promotion Council for Laboratory Testing [6].
As ctDNA has a half-life of less than an hour, it essentially reflects the real-time whole-body tumor status in clinical practice [2, 3]. Therefore, whereas biopsies are more invasive and genomic analysis of tissue biopsies is associated with the problem of spatial and temporal genomic heterogeneity in tumor tissues, ctDNA has the advantage of allowing real-time, minimally invasive, and simpler genomic analysis of the entire tumor. In one study, a comparison of tumor tissue- and ctDNA-based genomic analyses of unresectable gastrointestinal cancer showed that ctDNA analysis had a significantly higher success rate (99.9% vs 89.4%, P < 0.001), required fewer days for analysis (7 days vs 19 days, P < 0.001), and had a higher enrollment rate in the genomic analysis clinical study (9.5% vs 4.1%, P < 0.001). Moreover, the response rates for both analyses were comparable (20.0% vs 16.7%, P = 0.69), further highlighting the utility of ctDNA for genomic analysis [7].
MRD
MRD can represent “molecular residual disease,” “minimal residual disease,” or “measurable residual disease,” and a literature search revealed that MRD was used for “minimal residual disease” in 74%, “molecular residual disease” in 21%, and “measurable residual disease” in 5% of reports, highlighting the discrepancies in the definition of this term [8]. “Minimal residual disease” or “measurable residual disease” can be defined as a form of hematologic malignancy that is below the limit of detection of optical microscopy but is detectable using techniques such as multi-parameter flow cytometry and next-generation sequencers (NGS). Accordingly, it is considered an important hematologic prognostic factor for malignancy and is recommended to be evaluated over time during treatment [9]. In 2018, the US Food and Drug Administration (FDA) approved blinatumomab therapy for patients with adult acute lymphocytic leukemia and minimal residual disease after chemotherapy. Therefore, minimal residual disease has been established as a new condition requiring therapeutic intervention.
Conversely, “molecular residual disease” can be defined as evidence of recurrence at the molecular level that is detectable prior to the clinical, biological, or radiological evidence of recurrence. It is evaluated by detecting ctDNA in the postoperative recurrence-free state of solid tumors. The term “molecular residual disease” was first reported in 2008 in the context of detection after resection for colorectal cancer and its correlation with recurrence [10]. Since then, research on tumor type-agnostic MRD in solid tumors has advanced rapidly. This document provides expert opinions on the evaluation and appropriate clinical use of MRD—defined as “molecular residual disease”—by ctDNA analysis in solid tumors as a whole. MRD is the presence of detectable ctDNA in the absence of findings suggesting postoperative recurrence on imaging, and cases of ctDNA detected before and after surgery (referred to as preoperative ctDNA-positive and postoperative MRD-positive, respectively) are distinguished.
ctDNA analysis is used for both comprehensive genomic profiling (CGP) and MRD testing. CGP refers to the comprehensive analysis of a large number of genomic alterations using NGS or other methods (reported limit of detection [LOD] for VAF, 0.1–1%). On the other hand, MRD testing uses techniques such as molecular barcoding, BEAMing (beads, emulsions, amplification, and magnetics), and droplet digital polymerase chain reaction (ddPCR) to analyze a smaller number of specific genes or molecular markers (LOD, about 0.01–0.1%). It is characterized mainly by its precision [3]. Therefore, CGP is mainly used for decision-making regarding drug therapies for genomic alterations detected in patients with advanced, recurrent cancer, whereas MRD testing is mainly used to evaluate the risk of recurrence and diagnose recurrence at the molecular level in an apparently recurrence-free state after curative-intent treatment. Both CGP and MRD testing utilize ctDNA analysis; however, it is necessary to understand the aforementioned differences when considering their clinical applications.
MRD testing can be classified into two types: tumor-informed, which requires tumor tissue, and tumor-naïve, which does not require tumor tissue [3]. In tumor-naive assays, prespecified gene panels are used to detect MRD by analyzing epigenomic alterations in ctDNA without using any tumor tissue. For tumor-informed assays, two modes of testing are available: detection of MRD using a patient-specific panel designed based on the genomic analysis of tumor samples obtained by biopsy or surgery and detection of variants using tumor tissue and a standard gene panel. Tumor-informed assays that use a patient-specific panel are highly sensitive but require more time and depend on the quality and quantity of the tumor specimen. However, tumor-naive assays do not require tumor tissue, and the results can, therefore, be obtained faster.
As of August 2024, no clinical MRD assay has been approved in Japan. In the US, Signatera (Natera Inc., Austin, TX, USA) and RaDaR (NeoGenomics Inc., Fort Myers, FL, USA) tumor-informed assays using patient-specific panels and Guardant Reveal (Guardant Health Inc., Redwood City, CA, USA) tumor-naive assays are covered by Medicare and Medicaid reimbursement under the approval of the Centers for Medicare and Medicaid Services (CMS). Signatera and RaDaR are also certified with the Conformité Européenne (CE) marking in Europe and are available for clinical use there. For more information on the individual assays, please refer to "Chapter 5. Reference data."
MRD testing: Japanese and international guidelines
The Precision Medicine Working Group of the European Society of Medical Oncology (ESMO) discussed the clinical use of MRD testing in its 2022 report on the use of ctDNA assays. It states that the specificity of MRD-positive recurrence is 90% and that the lead time from MRD detection to clinical recurrence may be as high as 6 months. Although the report acknowledged the clinical validity of MRD testing in terms of recurrence prediction, it also pointed out the importance of developing ultrasensitive MRD assays with LOD lower than 0.01% because the sensitivity is currently inadequate (at less than 50%) [11]. Furthermore, regarding the clinical utility of MRD testing, the report highlighted the importance of demonstrating prognostic improvement and safe treatment simplification by therapeutic interventions based on MRD testing results through randomized clinical trials. The report does not mention concrete CQs or recommendation levels regarding MRD testing because the available evidence was considered insufficient. Similarly, the 2024 US NCCN Guidelines Colorectal Cancer Panel did not recommend the routine use of MRD testing because the data on curative therapeutic interventions for MRD-positive patients were considered insufficient. Accordingly, enrollment in clinical studies on MRD testing is increasing [12]. ctDNA analysis was also covered by panels for other tumor types; however, there was no mention of MRD testing in August 2024.
On the other hand, the 2023 "Molecular Testing for Colorectal Cancer Treatment (5th Edition)" in Japan specified the following basic requirements: "Panel tests for the detection of the minimal residual tumor should be performed in colorectal cancer patients who have undergone curative-intent resection, for treatment selection according to the risk of recurrence" [13]. Notably, MRD testing is "strongly recommended" as a technique that can be performed repeatedly to identify high-risk groups for recurrence because its clinical utility has been demonstrated in prospective phase II clinical studies.
In summary, although there were differences in the recommendation levels for MRD testing among the Japanese, American, and European guidelines, there was agreement to a certain degree that while MRD testing has some clinical validity, further evidence is required to establish its clinical utility, such as in terms of improving prognosis. Currently, several randomized clinical studies are ongoing worldwide to evaluate the utility of therapeutic interventions based on MRD testing results, warranting the need for a position paper that reviews the updated results in a timely manner.
Particulars: regions-specific clinical trends
Objectives
This position paper serves as a guideline for the appropriate clinical use of tumor-type agnostic MRD testing. MRD testing was first reported for colorectal cancer in 2008 [10]. Since then, considerable evidence has accumulated on this topic, and it is expected to be more widely used clinically in the future. However, some tumor types lack adequate evidence, and more comprehensive clinical studies are required.
To reach a consensus regarding tumor type-agnostic MRD testing, it is necessary to fully understand and discuss the clinical trends corresponding to each region. In this chapter, experts in each field comprehensively reviewed the currently available evidence and ongoing clinical studies to promote the appropriate clinical development and use of MRD testing in each region.
Gastrointestinal cancer
Status of evidence accumulation
A PubMed literature search using the keywords “colorectal cancer,” “gastric cancer,” “esophageal cancer,” and “circulating tumor DNA" OR “surgery” for each of them yielded 513, 131, and 86 reports published till June 2024, respectively. Although there were some limitations in terms of the search terms, most reports were related to colorectal cancer. Furthermore, 157, 54, and 18 reports were published before 2019; 224, 49, and 41 were published between 2020 and 2022; and 132, 28, and 27 were published in or after 2023, respectively, showing an increasing trend in the number of reports for these cancers year by year. This indicates that there is growing concern about and interest in MRD testing based on ctDNA analysis for gastrointestinal cancers.
Major literature reports (Table 1)
Colorectal cancer
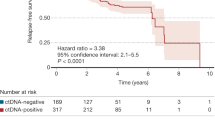
Colorectal cancer is the most studied gastrointestinal cancer in terms of MRD evaluation using ctDNA. One of the reasons for this is that colorectal cancer is an easily detectable cancer with high levels of ctDNA shedding into the blood. As described in the commentary on CQ1, the clinical validity of MRD testing is evaluated based on parameters such as sensitivity and specificity. Many studies have indicated that MRD positivity after curative-intent treatment predicts future clinical recurrence with high sensitivity and specificity, thereby demonstrating the clinical validity of MRD testing for this cancer type. Tie et al. evaluated MRD testing using the tumor-informed Safe-SeqS method in a prospective observational study that included 230 stage II patients and 96 stage III patients. They reported that postoperative and post-adjuvant treatment MRD positivity was a significant risk factor for recurrence [14, 15]. Parikh et al. used the Guardant Reveal assay in a prospective observational study and reported that the sensitivity and specificity for recurrence in postoperative stage I–IV patients were 55.6% and 100%, respectively (N = 103) [16]. Mo et al. reported a high risk of recurrence among postoperative MRD-positive patients using the ColonAiQ assay to detect cfDNA methylation in a prospective observational study (N = 299 stage I–III patients) [17]. Faulkner et al. conducted a meta-analysis of 37 studies (N = 3002 patients). They reported that postoperative MRD positivity was associated with an increased risk of recurrence regardless of cancer stage, including the presence or absence of adjuvant chemotherapy and assay type, and that it could serve as an independent indicator of poor prognosis (hazard ratio [HR]: 6.92, 95% confidence interval [CI] 4.49–10.64]) [18]. Studies in other countries have shown that MRD testing using ctDNA analysis may be useful. In the randomized phase II DYNAMIC study, 455 patients with pathological stage II were randomized into two groups: the standard group, wherein conventional clinicopathological factors were used as the eligibility criteria for adjuvant chemotherapy, and the MRD-guided group (based on MRD test results at 4 and 7 weeks after surgery). The results showed that the MRD-guided group was non-inferior to the standard group in terms of recurrence-free survival and had significantly reduced adjuvant chemotherapy use [19]. In the GALAXY study, a prospective observational study that included clinical stage II–IV patients, postoperative MRD positivity was significantly associated with an increased risk of recurrence in 2240 patients included in the interim analysis (HR: 11.99 [95% CI 10.02–14.35]). It was also suggested that adjuvant chemotherapy for postoperative MRD-positive patients may reduce the risk of recurrence (adjusted HR: 0.23 [95% CI 0.15–0.35]) [20]. Therefore, the utility of MRD for predicting recurrence has been reported in many studies, and several large-scale prospective studies on MRD are ongoing.
However, not all studies have indicated the utility of MRD. In the randomized phase II/III NRG-GI005 (COBRA) study, which used the Guardant Reveal for MRD testing, patients with stage II colon cancer and a low risk of recurrence were assigned to two groups: the MRD-guided group, in which adjuvant chemotherapy was administered to postoperative MRD-positive patients, and the standard group, in which follow-up was conducted based on clinicopathological factors. Of the 635 enrolled patients, 16 tested positive for MRD postoperatively, and resolution of MRD at 6 months was observed in three of seven patients (43%) in the untreated follow-up group. In contrast, in the group receiving adjuvant chemotherapy, resolution of MRD at 6 months was observed in 1 of 9 patients (11%). Therefore, owing to the failure to achieve the primary objective for phase II, the study was discontinued [21]. The results of this study indicate that MRD testing may not be demonstrably useful, depending on the accuracy of the assay and the specificity of the patient population. Therefore, all study results should be interpreted with caution.
Gastric cancer
Yang et al. reported that MRD positivity after curative-intent surgery, as assessed using a tumor-informed assay, was associated with recurrence and shortened recurrence-free survival (HR: 14.78 [95% CI 7.991–61.29]) in stage I–III patients (N = 46 patients) [22]. In the CRITICS study, Leal et al. analyzed ctDNA using a tumor-naive assay (VariantDx). They reported that preoperative ctDNA negativity was significantly more frequent among responders (P = 0.03). Recurrence-free survival was significantly shortened in postoperative MRD-positive patients (HR: 21.8 [95% CI 3.9–123.1]) [23]. Huffman et al. analyzed postoperative MRD positivity using the Signatera test. They reported that it was associated with a risk of recurrence and significantly shortened recurrence-free survival (HR: 23.6 [95% CI 10.2–66.0]) (N = 125 patients) [24]. Mi et al. conducted a meta-analysis of eight studies on gastric cancer (N = 423 patients). They reported that patients who were positive for ctDNA/MRD perioperatively were at a higher risk of recurrence. Those who were positive for preoperative ctDNA and postoperative MRD had significantly worse recurrence-free survival (HR: 6.37 [95% CI 2.70–15.01]) and overall survival (HR: 4.58 [95% CI 1.68–12.49]) [23]. Therefore, MRD has been reported to be useful for predicting the recurrence of gastric cancer. Prospective clinical studies, including MRD testing, have been conducted, and the accumulation of evidence and advancements in precision medicine applications are anticipated in the future.
Esophageal cancer
Owing to the nature of esophageal cancer treatment, there are very few reports on simple perioperative ctDNA/MRD evaluation, and most reports involve evaluations under neoadjuvant chemotherapy/chemoradiation therapy (CRT), adjuvant therapy, and curative-intent CRT. Azad et al. reported that ctDNA positivity after CRT significantly increased the risk of progressive disease and death (HR: 18.7 [95% CI 1.1–316.5]) and predicted recurrence earlier with higher sensitivity (71.4%) and specificity (100%) than clinical recurrence on imaging in squamous cell esophageal cancer (N = 45 patients) [25]. Ng et al. reported that MRD positivity at 6 months postoperatively was associated with overall survival (HR: 7.84 [95% CI 1.87–32.97]) in patients who underwent curative-intent resection (N = 74) [26]. Zhang et al. conducted a meta-analysis of 22 studies (N = 1144 patients) and reported that postoperative MRD positivity was significantly associated with overall survival (HR: 3.87 [95% CI 2.86–5.23]) and progression-free survival (HR: 4.28 [95% CI 3.34–5.48]) [27]. The utility of preoperative ctDNA and postoperative MRD testing has also been sporadically reported for esophageal cancer. Due to the epidemiological landscape of incidence, clinical studies are ongoing, mainly in Asian countries such as Japan and China. In Japan, neoadjuvant chemotherapy has been adopted as the standard of care. However, the impact of neoadjuvant chemotherapy on the interpretation of postoperative MRD results has not been clarified, and further studies are required.
Expected future clinical development (Table 2)
Colorectal cancer
Several randomized clinical studies based on MRD testing are currently ongoing in Japan. The VEGA study is a randomized phase III study that aims to verify the non-inferiority of follow-up observation compared with CAPOX adjuvant therapy in high-risk stage II/low-risk stage III postoperative MRD-negative patients. The ALTAIR study is a randomized phase III study verifying the superiority of FTD/TPI over placebo in MRD-positive patients under recurrence surveillance. The AURORA study is a randomized phase II study on verifying the superiority of mFOLFOXIRI plus bevacizumab therapy to mFOLFOX6 therapy in patients who were MRD-positive after surgery for oligometastases (screened in the COSMOS-CRC-03 study) [28, 29]. These studies are expected to have a significant impact on the direction of future clinical development of MRD testing. Nonetheless, MRD test results still require careful interpretation, as several important issues remain to be resolved, such as with regard to the accuracy of MRD assays and the appropriate selection of target patient populations. Attention should also be paid to how MRD testing using ctDNA analysis can be incorporated into colorectal cancer treatment in the future. To fully realize the benefits of precision medicine based on MRD testing, obtaining high-level evidence, therapeutic development strategies, and efforts are required, with a view to its use in clinical practice.
Gastric cancer
Several randomized studies are being conducted on postoperative MRD testing in gastric cancer, although all of them are small-scale studies (N = 46–80 patients each), and no large-scale studies have been initiated to date. Nevertheless, several prospective observational studies have been conducted and have demonstrated the utility of MRD testing and the predictive ability of ctDNA analysis in response to neoadjuvant chemotherapy.
It has been noted that for recurrence in the form of peritoneal dissemination, which is characteristic of gastric cancer, the detection sensitivity may be reduced due to low ctDNA levels [30]. To resolve this issue, it is desirable to develop more sensitive assays and establish an MRD testing method that can complement sensitivity. In the future, large-scale prospective studies are expected to help verify the clinical validity of MRD testing and to promote the development of precision medicine approaches.
Esophageal cancer
In addition to neoadjuvant CRT, curative-intent treatment with CRT is the standard of care for esophageal cancer. Research focusing on the utility of MRD testing during and after curative intent treatment is also underway, mainly in Asian countries. In Japan, the DISCOVER study (jRCT1071240001) is being conducted to evaluate ctDNA-positive status before, during, and after surgery using Guardant Reveal. There are three groups (N = 200 patients) in this study: preoperative untreated, neoadjuvant chemotherapy, and neoadjuvant CRT groups. The results of the study need to be interpreted with caution because of the complexity of the treatment strategy; however, it could demonstrate the clinical validity of MRD testing in esophageal cancer, further promoting the development of relevant precision medicine strategies.
Lung cancer
Status of evidence accumulation
A PubMed search of the literature, excluding case reports, using the keywords circulating tumor DNA (or ctDNA, minimally residual disease, molecular residual disease), surgery (or neoadjuvant, chemoradiation), and lung cancer yielded 685 reports published as of May 2024. After narrowing them down to those relevant to MRD testing after curative-intent treatment in patients with lung cancer, 41 reports remained; specifically, six retrospective studies, 30 prospective observational studies, and five meta-analyses, with no randomized studies reported to date.
Major literature reports (Table 1)
The prospective observational TRACERx study by Abbosh et al. found that MRD positivity was significantly associated with poor prognosis in patients with stage I–III non-small cell lung cancer in the UK. [31]. In this study, tumor-informed ctDNA analysis was performed on 197 patients who underwent surgery using probes targeting a median of 200 (range 72–201) tumor-specific genomic alterations per patient, which were divided into MRD-positive and MRD-negative groups based on their status within 120 days of surgery and before the start of adjuvant therapy, to compare their prognosis. The MRD positivity rate within 120 days of surgery was 25%, indicating a significantly higher risk of recurrence in the MRD-positive group than in the MRD-negative group (HR: 6.8 [95% CI 3.7–12.3]). In addition, the median time from MRD detection to clinical recurrence during recurrence surveillance (lead time) was 119 days (range 0–1137 days). It should be noted that the preoperative ctDNA positivity rate was reported to be higher for histological cancer types other than adenocarcinoma than for adenocarcinoma.
In 2023, Chen et al. reported similar results in their prospective observational study of 181 patients with stage I–III non-small cell lung cancer who underwent surgery in China. In this study, tumor-informed ctDNA analysis targeted up to 50 tumor-specific genomic alterations per patient. The HR for MRD-positive recurrence-free survival at 30 days after surgery was 8.86 (95% CI 3.7–21.1), and the median lead time from MRD detection to diagnosis of clinical recurrence was 299 days [32].
In their meta-analysis of literature published till April 2022 (14 articles, N = 1051 patients), Chen et al. found that the HR for the risk of recurrence in postoperative MRD-positive patients was 6.52 (95% CI 5.08–8.36) [33].
In contrast, in the randomized IMpower 010 study comparing atezolizumab and best supportive care in patients who underwent cisplatin-based adjuvant chemotherapy after curative-intent resection for stage IB–IIIA non-small cell lung cancer, atezolizumab treatment prolonged disease-free survival (HR: 0.66 [95% CI 0.50–0.88]) [34] in the primary analysis population (stage II–IIIA patients with PD-L1 expression 1% or higher). MRD testing using Signatera was performed as an exploratory analysis of the IMpower 010 study data and revealed that the postoperative MRD positivity rate increased with the advancement of the disease stage (stage IB: 9%; stage II: 14%; and stage IIIA: 29%) and that the HR for MRD-negative stage II–IIIA patients relative to that for MRD-positive patients was 0.72 (95% CI 0.52–1.00) in the atezolizumab group and 0.61 (95% CI 0.39–0.94) in the best supportive care group. Therefore, MRD positivity is consistently a poor prognostic factor [35]. In the overall primary analysis population, disease-free survival was prolonged regardless of postoperative MRD status (HR for MRD-positive patients: 0.54 [95% CI 0.31–0.93]; HR for MRD-negative patients: 0.57 [95% CI 0.36–0.90]). Disease-free survival was prolonged in MRD-negative patients with PD-L1 expression ≥ 50% (HR: 0.35 [95% CI 0.16–0.75]) but not in those with PD-L1 expression 1–49% (HR: 0.78 [95% CI 0.43–1.42]). This suggests that treatment de-escalation may be used in some patients, depending on the MRD status [36].
In addition, the utility of MRD testing has been reported for recurrence surveillance after curative-intent CRT. Pan et al. performed MRD testing after curative-intent CRT on 139 patients, mainly with unresectable locally advanced clinical stage III non-small cell lung cancer. Regarding progression-free survival, the HR for the risk during progression surveillance in MRD-negative patients was 0.18 (95% CI 0.12–0.28) [37]. Similarly, many other studies have reported the utility of MRD testing for recurrence surveillance after curative-intent treatment.
Several observational studies have analyzed the efficacy of adjuvant therapy in association with postoperative MRD positivity. Shen et al. conducted a meta-analysis covering reports published up to May 2022 and showed that the HR for the efficacy of adjuvant therapy in MRD-positive patients was 0.27 (95% CI 0.17–0.44). In contrast, in MRD-negative patients, the HR was 1.51 (95% CI: 0.81–2.79), which did not confirm the efficacy of adjuvant therapy [38].
Expected clinical development in the future (Table 2)
Several randomized clinical studies based on postoperative MRD testing are currently underway. Although no data from randomized studies are currently available for lung cancer, published observational and retrospective studies have consistently reported the utility of MRD testing for recurrence prediction. While awaiting the results of randomized studies, the utility of MRD testing is expected for future clinical development of precision onco-surgery.
Breast cancer
Status of evidence accumulation
A PubMed search using the keywords for breast cancer, ctDNA, and operable (or early) yielded 651 reports published as of May 2024. Reviews and meta-analyses were excluded, after which the search was further narrowed to studies conducted within the past 10 years using the keywords retrospective, observational, and clinical trials, which yielded 18, 10, and 42 reports, respectively. Thereafter, combined with hand-searched literature, reports considered essential were reviewed.
Major literature reports (Table 1)
Nader-Marta et al. conducted a meta-analysis to evaluate the associations between preoperative ctDNA positivity/postoperative MRD positivity and disease-free and overall survival in patients with stage I–III breast cancer (3174 reports, 57 studies; N = 5779 patients) [39]. MRD-positive patients under recurrence surveillance had a poor prognosis for disease-free survival (HR, 14.04; 95% CI 7.55–26.11); patients who tested positive for ctDNA after neoadjuvant chemotherapy also had a poor prognosis (HR, 7.69; 95% CI 4.83–12.24). For overall survival, recurrence surveillance had an HR of 9.19 (95% CI 3.26–25.90), and after neoadjuvant chemotherapy, the HR was 2.72 (95% CI 1.44–5.14); similar results were obtained using multivariate analysis. The reported lead time was 10.81 months (range 0–58.9 months). Furthermore, the association between ctDNA positivity, disease-free survival, and overall survival was particularly higher in the tumor-informed assay than in the tumor-naive assay.
In a multicenter, randomized phase II c-TRAK TN interventional study, patients who underwent triple-negative breast cancer surgery followed by MRD testing using digital PCR every 3 months for 12 months were compared to those in the intervention group, in which patients with no clinical recurrence at the time of MRD-positive conversion were administered pembrolizumab and to the follow-up observation group. The primary endpoints were MRD detection and MRD-negative conversion rates during pembrolizumab treatment. The MRD positivity rate was 27.3% (44 of 161 patients, 95% CI 20.6–34.9) at 12 months. Seven patients experienced recurrence while remaining MRD-negative, and in 72% of patients in the intervention group, clinical recurrence was observed when MRD-positive conversion was identified. Five patients in the intervention group received pembrolizumab, but none of them continuously maintained MRD-negative status. Recurrence had already been confirmed by imaging in many cases by the time of MRD-positive conversion. Therefore, the results highlight several issues that need to be addressed and suggest the importance of performing MRD testing frequently from the early postoperative period onwards, using an assay with higher sensitivity [40].
Several similar reports have been published on the risk of recurrence in MRD-positive patients involving either ctDNA analysis after neoadjuvant chemotherapy or MRD testing after surgery and during recurrence surveillance. Data for all studies were reported according to subtype, and the risk of recurrence was consistently high in patients with preoperative ctDNA positivity or postoperative MRD positivity, irrespective of the setting and subgroup.
Expected future clinical development (Table 2)
Several clinical trials are being conducted to evaluate MRD over time during recurrence surveillance and whether new therapeutic interventions performed at the time of MRD-positive conversion can improve prognosis. Based on the results of these studies, the development of more personalized precision medicine strategies that focus on patients with poor prognoses is expected in the future. One representative clinical study is the LEADER study, and some of its results were reported at the San Antonio Breast Cancer Symposium in 2023. This study enrolled 191 patients with hormone receptor-positive, HER2-negative breast cancer. At the median follow-up time point of 12 months, 10.1% (17 patients) of 168 patients who were eligible for ctDNA analysis using Signatera were MRD-positive. Among these 17 MRD-positive patients, the percentage of patients with no recurrence on imaging or clinically at the time of MRD-positive conversion (true molecular relapse) was 70.6% (12 of 17 patients) [41]. Future results are expected to determine whether the addition of CDK4/6 inhibitors improves the prognosis of these patients. Issues to be addressed when conducting such clinical trials include the low MRD positivity rate of 10%, which makes the number of patients insufficient to enable randomization. Approximately 30% of the patients already had clinical relapse by the time of MRD-positive conversion, lack of information regarding the appropriate MRD testing interval and the appropriate drugs to be used in the intervention group.
Urologic cancer
Status of evidence accumulation
A PubMed search using the keywords prostate cancer, bladder cancer, and renal cell carcinoma, in combination with circulating tumor DNA and surgery for each of them, yielded eight, 20, and 11 reports, respectively, as of June 2024.
Major literature reports (Table 1)
Prostate cancer
Recurrence surveillance for prostate cancer is being conducted using the tumor-specific marker prostate-specific antigen; therefore, there have been a relatively limited number of reports on MRD testing in prostate cancer. However, some studies have reported that the detection of ctDNA before treatment affects the recurrence-free survival of patients undergoing total prostatectomy for localized prostate cancer. Pope et al. performed tumor-informed preoperative ctDNA analysis using whole genome sequencing (INVAR) to increase sequencing depth further. They showed that both biochemical recurrence-free survival (HR: 3.3 [95% CI 1.4–8.1]) and recurrence-free survival (HR: 2.8 [95% CI 1.1–7.1]) were significantly shorter in preoperative ctDNA-positive patients than in ctDNA-negative patients [42]. There are also other reports on preoperative ctDNA analysis with targeted sequencing focusing on some genes.
Urothelial cancer
It has been reported that more ctDNA is released in urothelial cancer than in other cancer types [43]. Therefore, evidence of MRD is most abundant in the context of the urinary system. Bladder cancer accounts for 90% of all urothelial cancers and has been studied using targeted sequencing with ddPCR. In recent years, multiple clinical studies using Signatera, a tumor-informed assay, have been conducted. Christensen et al. performed MRD testing using Signatera before and after curative-intent cystectomy. They reported that the postoperative MRD positivity rate was 26.6%, the recurrence rate in postoperative MRD-positive patients was 76%, the sensitivity and specificity for recurrence were extremely high (100% and 98%, respectively), and the median lead time to clinical recurrence in MRD-positive patients under recurrence surveillance was 96 days [44]. In the IMvigor010 study, Powles et al. performed MRD testing using Signatera. They reported improvements in recurrence-free survival (HR: 0.58 [95% CI 0.43–0.79]) and overall survival (HR: 0.59 [95% CI 0.41–0.86]) in the atezolizumab group as compared with those in the follow-up group of postoperative MRD-positive patients (37%) [45]. However, there are only two reports on MRD for upper urinary tract urothelial cancer originating from the renal pelvis and ureter. Furthermore, Christensen et al. used ctDNA analysis of urine and plasma to consider the characteristics of bladder cancer [46]. Specifically, they performed ddPCR of driver genes (FGFR3 and PIK3CA) and reported significantly lower recurrence-free survival duration in urinary ctDNA-positive patients. A small-scale study on upper urinary tract urothelial cancer, which occurs at a low incidence, also used postoperative ddPCR and target-sequencing-based MRD analysis.
Renal cancer
Renal cancer is known to be a low ctDNA-shedding type, along with head and neck cancer and melanoma. The number of reports related to MRD is limited, and there are some reports on perioperative methylation analysis of specific genes, considering the low levels of ctDNA. Buttner et al. reported significantly shortened recurrence-free survival (HR: 5.89 [95% CI 1.46–23.8]) in the group with high SHOX2 promoter methylation frequency, which is reported to be associated with prognosis in other cancer types [47].
Expected future clinical development (Table 2)
Prostate cancer
As of June 2024, no studies have aimed at detecting postoperative MRD. However, if the MRD detection rate improves with the advancement of sequencing technology, it is expected to promote further investigations, such as whether perioperative ctDNA/MRD status is associated with recurrence-free survival rate and other clinical prognosis indicators independent of PSA.
Urothelial cancer
Based on the results of the abovementioned IMvigor010 study, the IMvigor011 study is being conducted to evaluate the efficacy of atezolizumab as an adjuvant therapy for MRD-positive patients with muscle layer-invasive bladder cancer. This study is expected to determine the utility of adjuvant therapy depending on the presence or absence of postoperative MRD.
Renal cancer
In August 2022, pembrolizumab was included in the national health insurance reimbursement coverage as the first adjuvant therapy for renal cell carcinoma. Therefore, MRD testing may be required to identify the groups that truly benefit from adjuvant therapy, as in the IMvigor011 study on urothelial cancer. Newer technologies, such as whole-genome sequencing and cfDNA methylation analysis, are expected to improve the MRD detection rate in the future [48].
Hepatobiliary and pancreatic cancer
Status of evidence accumulation
A PubMed literature search using the keywords molecular residual disease, circulating tumor DNA, and surgery yielded 369 reports published until February 2024. After combining the results of a subsequent manual search, 412 reports were reviewed. Hepatocellular carcinoma, biliary tract cancer, and pancreatic cancer were reported in the hepatobiliary and pancreatic regions (11, 5, and 21 cases, respectively).
Major literature reports (Table 1)
Pancreatic cancer
In the field of pancreatic cancer, which has the largest number of reports, most studies have used MRD testing based on ctDNA analysis of KRAS mutations. Lee et al. performed ctDNA analysis of KRAS mutations before and after resectable pancreatic cancer surgery. They reported that the preoperative ctDNA positivity rate was 62.2%, and the postoperative MRD positivity rate was 37.1% [49]. Preoperative ctDNA-positive patients had worse recurrence-free survival (HR: 4.1 [95% CI 1.8–9.0]) and overall survival (HR: 4.1 [95% CI 1.6–10.5]) than ctDNA-negative patients. The overall survival of the positive patients was comparable to that of the unresected patients. In addition, postoperative ctDNA-positive patients had poor recurrence-free survival (HR: 5.4 [95% CI 1.9–15.2]) and overall survival (postoperative, HR: 4.0 [95% CI 1.2–13.6]). According to a meta-analysis of KRAS-mutant MRD-positive patients who underwent pancreatectomy, the prognosis was poor. However, the HR tended to be lower than that for other cancer types (HR: 3.32 [95% CI 2.19–5.03]) [50]. Lee et al. conducted an interventional study (the DYNAMIC-Pancreas study, which used tumor-informed ctDNA analysis for MRD testing) in postoperative pancreatic cancer patients, where adjuvant chemotherapy was administered for 6 months in the MRD-positive group and for 3–4 months in the MRD-negative group. They reported that the prognosis was significantly better in the latter (recurrence-free survival: 13 vs 22 months, HR: 0.28). However, as the median recurrence-free survival of 22 months was still poor, they concluded that reduction of the adjuvant therapy period for MRD-negative patients should not be recommended [51]. In Japan, neoadjuvant chemotherapy is adopted as the standard of care; however, its impact on the interpretation of postoperative MRD results has not been clarified and will require further investigation.
Hepatocellular carcinoma
Patients with postoperative MRD-positive hepatocellular carcinoma patients have also been reported to have a poor prognosis. Ye et al. used ctDNA for MRD testing within 7 days of surgery. They reported that MRD-positive patients (N = 23 of 96, 24.0%) who underwent resection for hepatocellular carcinoma had a significantly poor prognosis in terms of recurrence-free survival (HR: 6.074 [95% CI 2.648–13.929]) and overall survival (HR: 4.829 [95% CI 1.508–15.466]) [52]. Furthermore, MRD positivity was revealed to be an independent prognostic factor for previously identified risk factors such as high alpha-fetoprotein (AFP) levels, microscopic vascular invasion, and Barcelona Clinic Liver Cancer (BCLC) stage.
Biliary tract cancer
Patients with postoperative MRD-positive biliary tract cancer have also been reported to have a poor prognosis, although the number of reports is limited. King et al. reported a significantly poor recurrence-free survival (HR: 7.4 [95% CI 2.6–4758]) in a study on ctDNA analysis of 12 perioperative MRD-positive biliary tract cancers (N = 3/9, 33%) [53].
Expected future clinical development (Table 2)
Pancreatic cancer
The prognosis of pancreatic cancer is extremely poor, and effective treatment options are limited. Therefore, MRD testing is expected to be an effective technique for facilitating multidisciplinary treatment strategies for this cancer [54]. However, at present, most reports on MRD testing for pancreatic cancer use ctDNA analysis for KRAS mutations, raising the issue of low sensitivity. The development of highly sensitive assay methods, such as a tumor-naive methylation-based ctDNA detection assay and a tumor-informed assay based on whole-exome and whole-genome analyses, is in progress [55,56,57]. Regarding the development of perioperative treatments based on ctDNA testing, many studies have reported that the postoperative overall survival in preoperative ctDNA-positive patients is very poor, comparable to that in patients with distant metastases. Therefore, the use of ctDNA testing to determine eligibility for surgery or neoadjuvant therapy and for efficacy assessment has been considered [1, 5, 58, 59]. Regarding the postoperative period, as the DYNAMIC-Pancreas study concluded that reduction in the duration of adjuvant therapy is not recommended for MRD-negative patients, treatment intensification for MRD-positive patients should be discussed rather than treatment simplification for MRD-negative patients [3]. A randomized clinical study (NCT05802407) is ongoing in China to verify the efficacy of modified adjuvant chemotherapy in MRD-positive patients under recurrence surveillance [60].
Hepatocellular carcinoma
Several large-scale prospective studies are ongoing to verify the clinical validity of MRD testing in hepatocellular carcinoma [57] [61,62,63,64,65]. Current clinical studies on adjuvant therapy, in comparison with follow-up observational groups, include the IMbrave050 study on atezolizumab plus bevacizumab, the CheckMate9DX study on nivolumab, the KEYNOTE-937 study on pembrolizumab, and the EMERALD-2 study on durvalumab plus bevacizumab. The IMbrave050 study on patients at high risk of recurrence showed no significant difference in recurrence-free survival in the subgroup with a tumor diameter ≤ 5 cm (HR: 1.06 [95% CI 0.65–1.74]) [66]. For patients who undergo surgery for hepatocellular carcinoma, identifying those at high risk of recurrence is considered to be an issue, and MRD testing may be useful in this regard. In addition, as radiofrequency ablation is the standard of care for patients with hepatocellular carcinoma, treatment development based on MRD testing in patients who have undergone curative-intent non-operative treatment is also expected.
Biliary tract cancer
Similar to pancreatic cancer, the prognosis of biliary tract cancer is poor, and effective treatment options are limited. Therefore, MRD testing is expected to be an effective technique to establish multidisciplinary treatment strategies. Furthermore, prospective studies assessing the clinical validity of MRD testing are also in progress. However, the number of these studies is very limited [57] [67, 68], and there have been no interventional studies on perioperative treatments based on MRD testing. Therefore, future prospective studies are expected to help evaluate the clinical validity of MRD testing, potentially leading to advancements in treatment development.
Gynecological cancer
Status of evidence accumulation
A PubMed literature search using the keywords ovarian cancer, cervical cancer, and uterine cancer in combination with circulating tumor DNA for each of them yielded 143, 92, and 50 articles, respectively, published till May 2024. However, reports on MRD testing are limited, and there are even fewer prospective studies (three studies on ovarian cancer, one on endometrial cancer, and three on cervical cancer). Notably, there has been only one confirmatory study on cervical cancer.
Major literature reports (Table 1)
Ovarian cancer
The reported preoperative ctDNA positivity rate in patients with ovarian cancer ranges from 58.6% to 93%. Heo et al. performed ctDNA analysis using a tumor-naive assay for nine genes in patients who underwent debulking surgery for ovarian cancer (N = 22) and reported a preoperative ctDNA positivity rate of 69.2% [69]. Patients who were ctDNA-positive before treatment initiation and 6 months later had worse progression-free survival (HR: 10.7 [95% CI 4.4–25.9]) than those who were ctDNA-negative before treatment initiation and those with negative ctDNA conversion 6 months later. In contrast, Hou et al. performed perioperative ctDNA analysis in stage I–IV patients using Signatera. They reported a preoperative ctDNA positivity rate of 73% and a postoperative MRD positivity rate of 33% [70]. In patients eligible for recurrence surveillance based on MRD testing, both the sensitivity and specificity for recurrence in MRD-positive patients were 100%, with a mean lead time from MRD positivity to clinical recurrence of 10 months. At the end of curative treatment, MRD-positive patients had significantly worse recurrence-free survival than MRD-negative patients (HR: 17.6 [95% CI 3.2–97.4]). Kallio et al. performed perioperative ctDNA analysis in stage I–IV patients using a tumor-informed assay. The ctDNA positivity rate before treatment initiation was 93% [71]; furthermore, at the time of the final testing during the treatment period, the MRD-positive patients had significantly worse progression-free survival (HR: 5.63) and overall survival (HR: 8.22) than the MRD-negative patients.
Endometrial cancer
Ashley et al. performed ctDNA analysis in stage I–IV patients using a tumor-naive assay of 129 genes and reported that the preoperative ctDNA positivity rate was 22%, and the postoperative MRD positivity rate was only 6.7% [72]. Both preoperative ctDNA-positive patients (HR: 11.14 [95% CI 2.72–45.59]) and postoperative MRD-positive patients (HR: 15.56 [95% CI 2.16–112.16]) had significantly worse progression-free survival.
Cervical cancer
Both surgery and radiation therapy are used as curative treatments for cervical cancer. Jeannot et al. used ddPCR of the E7 gene of human papillomavirus (HPV) for ctDNA analysis. The positivity rate was 63% before treatment [73]; at the end of treatment, MRD-positive patients had significantly shorter progression-free survival than MRD-negative patients (HR: 10.95 [95% CI 2.94–40.7]). The mean lead time to clinical recurrence in the MRD-positive group under recurrence surveillance was 10 months. Han et al. conducted a prospective study on CRT in patients with stage IB–IVA cervical cancer [74] and reported HPV ctDNA positivity before treatment in 70 (93.3%) of 75 patients. Three of the five patients who tested negative underwent cervical HPV screening and were HPV-negative. The progression-free survival of MRD-positive patients after treatment was significantly shorter than that of MRD-negative patients (HR, 8.58 [95% CI 3.56–20.71]). In addition, analysis using next-generation HPV sequencing instead of ddPCR showed significantly shorter progression-free survival in MRD-positive patients after treatment than in MRD-negative patients (HR: 4.19 [95% CI 1.76–9.98]).
Expected clinical development in the future (Table 2)
Ovarian cancer
The GALAXY-OV study (UMIN000050754), a prospective, observational study evaluating the efficacy of MRD testing using the tumor-informed Signatera assay in advanced ovarian cancer, is currently ongoing. In addition, the Nir-Bev study (jRCT2031220732), a randomized phase II study comparing niraparib maintenance therapy and niraparib plus bevacizumab combination maintenance therapy in MRD-positive patients enrolled in the GALAXY-OV study after neoadjuvant chemotherapy plus interval debulking surgery (IDS), is currently ongoing. These interventional studies are expected to clarify the utility of MRD status as a prognostic factor in advanced ovarian cancer, the natural history of MRD-positive patients during adjuvant chemotherapy and maintenance therapy, and the utility of bevacizumab added to niraparib maintenance therapy in post-IDS-MRD-positive-patients who-are-considered to be at high risk of recurrence. Other ongoing studies include observational MRD monitoring studies and a study in which a neoantigen polypeptide vaccine was administered to patients who underwent ovarian cancer surgery and whose MRD status is being monitored (NCT06341907).
Endometrial cancer
A study (NCT06341855) comparing follow-up and additional treatment in postoperative MRD-positive patients was initiated in China.
Cervical cancer
Ongoing studies include an observational MRD monitoring study and a study in which adjuvant treatment consisting of CRT plus anti-PD-1 antibody therapy with or without chemotherapy was administered after surgery (NCT05872724).
Head and neck cancer
Status of evidence accumulation
A PubMed search for literature published till June 2024 using the keywords circulating tumor DNA and head and neck cancer yielded 72 reports, whereas a search with the keywords head and neck cancer, HPV, and plasma yielded 53 reports, and a search with the keywords nasopharyngeal carcinoma, EBV (Epstein–Barr virus), and plasma yielded 399 reports.
Major literature reports (Table 1)
Nasopharyngeal cancer
Numerous studies have been conducted on MRD testing through the detection of EBV-DNA in the blood using quantitative PCR and other methods. Lin et al. assessed the significance of EBV-DNA in the blood as a prognostic factor in patients with locally advanced nasopharyngeal cancer who underwent curative-intent radiation therapy and reported that the prognosis of MRD-positive patients was significantly worse (2-year recurrence-free survival: 28.6% vs 84.2%) than that of MRD-negative patients [75]. Chen et al. conducted a study on recurrence surveillance using blood EBV–DNA in patients who had completed radiation therapy. They reported that the sensitivity and specificity for recurrence were 82.3% and 80.0%, respectively, with a median lead time of 2.3 months [76]. Peng et al. conducted a meta-analysis and reported that the sensitivity and specificity of blood EBV DNA detection for recurrence were 85.8% and 89.0%, respectively [77].
The significance of personalization of nasopharyngeal cancer treatment based on MRD testing has not yet been established. The NPC 0502 study, a randomized phase III study including 104 patients who were MRD-positive after curative CRT, compared the MRD-guided group (six cycles of adjuvant chemotherapy with gemcitabine plus cisplatin) and the non-adjuvant chemotherapy and follow-up groups and found no difference between the groups in the primary endpoint of the 5-year recurrence-free survival rate (49.3% vs 54.7%, HR: 1.98 [95% CI 0.63–1.89]) [78]. We speculate that the reason for the absence of a significant difference was the adjuvant chemotherapy regimen used in the MRD-guided group.
HPV-related oropharyngeal cancer
There are reports from Japan and other countries on MRD testing using qPCR for detecting high-risk HPV DNA in the blood. Chera et al. used blood HPV DNA detection for recurrence surveillance after CRT and reported a median lead time of 6.6 months. The sensitivity and specificity for recurrence, by defining two consecutive MRD-positive results as recurrence, were 100% for both, and MRD-positive patients had a significantly worse prognosis than MRD-negative patients (2-year recurrence-free survival: 50% vs 100%) [79]. Hanna et al. used NavDx®, which detects tumor-derived HPV-DNA, and reported that the sensitivity and specificity for recurrence in MRD-positive patients were 87.3% and 99.4%, respectively [80]. Jensen et al. conducted a meta-analysis and reported that the sensitivity and specificity for recurrence determined based on blood HBV-DNA in MRD-positive patients were 54% and 98%, respectively [81]. Regarding the reasons for the low sensitivity in this meta-analysis, the authors considered that there were issues regarding tumor volume, blood collection, and storage methods.
The significance of MRD testing for personalized treatment of HPV-associated oropharyngeal cancer has not yet been established. A phase II study (NCT05307939) on the omission, delay, or reduction of adjuvant radiation therapy in postoperative MRD-negative patients is currently in progress. However, the cohort in which the initiation of adjuvant radiation therapy was delayed or omitted until MRD-positive conversion in the population with pathologically intermediate risk factors was terminated prematurely due to a failure to demonstrate the utility of MRD testing [82].
HPV-unrelated head and neck squamous cell carcinoma
Several attempts have been made to use ctDNA analysis to identify somatic genomic alterations in patients with HPV-unrelated head and neck squamous cell carcinoma. Honore et al. evaluated the utility of an NGS-based tumor-naive assay (with 26 genes, including two HPV genes) in locally advanced cancer (N = 53; 17 HPV-related and 36 HPV-unrelated) and reported that the prognosis of MRD-positive patients was significantly worse than that of MRD-negative patients (2-year progression-free survival, 23.53% vs 86.6%) [83]. Flach et al. evaluated the utility of RaDaR in cases of resection for HPV-unrelated head and neck squamous cell carcinoma (N = 17). They reported that clinical recurrence occurred in all six MRD-positive patients, with a lead time of 108 to 253 days [84].
Expected future clinical development (Table 2)
Nasopharyngeal cancer
The personalization of adjuvant chemotherapy based on MRD testing after curative-intent radiation therapy has been considered, and several clinical studies are ongoing. Of these, a particularly important one is the NRG-HN001 study, in which adjuvant chemotherapy with cisplatin plus 5-FU and adjuvant chemotherapy with gemcitabine plus paclitaxel were compared in the MRD-positive patients who underwent curative-intent radiation therapy and also adjuvant chemotherapy with cisplatin plus 5-FU and follow-up observation were compared in MRD-negative patients.
HPV-related oropharyngeal cancer
Several prospective interventional studies on the personalization of adjuvant chemotherapy based on MRD testing and recurrence surveillance using MRD testing after postoperative radiation therapy are ongoing. The NCT05307939 study enrolled patients who were pathologically high-risk and underwent radiation therapy after debulking surgery. The phase II SURVEILLE-HPV study is being conducted to compare two groups—one in which patients undergo follow-up observation at a conventional visiting frequency and the other in which MRD testing is introduced while visiting frequency is reduced and in the case of positive conversion MRD testing is performed every two months and magnetic resonance imaging (MRI) with positron emission tomography/computed tomography (PET/CT) is performed every four months.
HPV-unrelated head and neck squamous cell carcinoma
For locally advanced head and neck squamous cell carcinoma, both HPV-related and HPV-unrelated, the ongoing NeckTAR study aims to compare MRD testing using a tumor-informed assay in the presence or absence of residual lesions based on PET/CT. Further evidence regarding the significance of MRD testing in HPV-unrelated head and neck squamous cell carcinoma and the development of novel MRD-based treatments are expected in the future.
Skin cancer
Status of evidence accumulation
A PubMed search for literature published until September 2024 using the keywords circulating tumor DNA, MRD, melanoma, non-melanoma skin cancer, squamous cell carcinoma, basal cell carcinoma, extramammary Paget disease, cutaneous adnexal carcinoma, and Merkel cell carcinoma in addition to manual literature search for references in review articles on the same theme yielded 10 reports for melanoma and one report for non-melanoma skin cancer. Literature focusing on circulating tumor DNA in uveal melanoma was excluded because its primary treatment differs from other melanoma subtypes.
Major literature reports (Table 1)
Melanoma
All available reports were observational studies. Most studies first confirmed the presence of driver mutations, such as BRAF and NRAS, in tumor tissues and then tested for these mutations in blood samples using ddPCR [85,86,87,88,89,90]. Other methods used in these studies included BEAMing in one study [91], real-time PCR in one study [92], and the tumor-informed Signatera assay in two studies [93, 94]. Tan et al. used ddPCR for MRD testing of postoperative patients with stage III melanoma with a BRAF, NRAS, TERT, TP53, or KIT mutation (N = 133 patients). The MRD positivity rates before and after surgery were 35% and 24%, respectively; with regard to the risk assessment of recurrence-free survival, the HR was 2.9 (95% CI 1.5–5.6) for preoperative ctDNA-positive patients and 10 (95% CI 4.3–24) for postoperative MRD-positive patients [87]. Eroglu et al. used Signatera for MRD testing of 69 patients with melanoma (30 postoperative patients at stage III and 39 patients with unresectable disease or distant metastasis). The postoperative MRD positivity rate in the stage III group was 17%, and the HR for distant metastasis-free survival in MRD-positive patients relative to MRD-negative patients was 10.77 (95% CI 1.77–65.57) [94].
In observational studies that evaluated MRD (N = 10), the postoperative MRD positivity rate was generally around 20% to 30%, although there were considerable variations between studies, possibly due to differences in sample sizes, stages, assessment time points, and assays used. Although the HR for recurrence-free survival in MRD-positive patients ranged from 2 to 10, it was generally correlated with poor prognosis. The sensitivity for recurrence also ranged from approximately 10% to 80% but tended to be low in general. In contrast, the specificity was approximately 80% to 100%, tending to be high. Therefore, the development of assays with superior detection sensitivity is required.
Non-melanoma skin cancer
Non-melanoma skin cancers include cutaneous squamous cell carcinoma, basal cell carcinoma, extramammary Paget disease, cutaneous adnexal carcinoma, and Merkel cell carcinoma. In an observational study of Merkel cell carcinoma, Akaike et al. used the Signatera assay for MRD testing (N = 319 patients with stage I–III Merkel cell carcinoma). Among the 84 patients who became clinically negative for the disease after curative-intent surgery or radiation therapy, the 1-year recurrence-free survival rate was 26% for MRD-positive patients and 79% for MRD-negative patients (HR, 7.4; 95% CI 2.7–20.3) [95].
Expected future clinical development (Table 2)
Melanoma
Most of the ongoing prospective studies are observational studies using the MRD positivity rate as an endpoint. Clinical studies verifying treatment strategies to intensify or simplify perioperative treatment based on the MRD status of patients with curatively resectable melanoma are expected. The DETECTION study (NCT04901988) was a randomized phase II/III study to compare recurrence surveillance in stage IIB/C melanoma with BRAF, NRAS, or TERT promoter mutations between the standard group (in which follow-up observation was performed until recurrence without regard for MRD test results) and the MRD-guided group (in which MRD-positive patients received nivolumab) [96]. However, the DETECTION study was prematurely terminated because surgery alone was no longer a standard of care since adjuvant anti-PD-1 became available for stage IIB/C melanoma in 2021. The ClearMe study (NCT06319196) is a phase II study of postoperative stage III/IV melanoma with MRD clearance as the endpoint. In this study, MRD testing was performed using the tumor-informed RaDaR assay, and MRD-positive patients were randomized to either adjuvant nivolumab plus relatlimab combination therapy or adjuvant nivolumab therapy [97]. It is anticipated that the establishment of a high-sensitivity assay will lead to an increase in clinical studies verifying MRD-based treatment strategies in the future.
Non-melanoma skin cancer
Perioperative systemic therapy has not been approved for non-melanoma skin cancers. Therefore, no ongoing interventional studies have verified MRD-guided perioperative treatment strategies. If observational studies demonstrate the utility of MRD testing, as in the case of Merkel cell carcinoma, it is expected that clinical studies will be conducted in the future to verify MRD-guided treatment strategies.
Clinical questions (CQs)
The Position Paper on Appropriate Clinical Use of MRD Testing comprehensively covers resectable solid tumors and is an aggregation of expert opinions on the respective tumor types. Although there may be conflicting or weak evidence for some tumor types, we prioritized consensus building in developing the recommendations to share the utility of MRD testing with patients. Therefore, although there may be cases where the details of the recommendation may require different interpretations depending on the tumor type, we hope that these recommendations will be interpreted appropriately with reference to the guidelines for the respective tumor types before being used clinically.
Determination of the recommendation level
In the development of this guideline, CQs were established to address clinically relevant questions, and evidence that provides the rationale for answers to these CQs was collected by manual search. Based on these results, 15 Working Group members voted to determine the recommendation level for each CQ (Table 3). Voting was conducted based on the strength of the evidence for each CQ, including the expected benefits and losses for the patients. Regulatory approval and insurance reimbursement status in Japan regarding medical treatments (including indications for examinations and treatment) were not considered in the voting process and are noted as remarks in the commentaries, as needed.
The results of the recommendation level for each CQ were determined by voting as follows:
-
(1)
SR (Strongly recommended) if SR votes were 70% or more,
-
(2)
R (Recommended) if (1) was not met and SR + R votes were 70% or more,
-
(3)
ECO (Expert consensus opinion) if (1) and (2) were not met and SR + R + ECO votes were 70% or more,
-
(4)
NR (Not recommended) if NR votes were 50% or more, regardless of (1) to (3), and.
-
(5)
N/A (Recommendation level not applicable) if none of (1) to (4) were met.
Reference data
Available MRD assays for solid tumors
In this position paper, we discussed the appropriate clinical use of MRD testing for solid tumors in a tumor type-agnostic manner. Various companies and academic institutions are working on the development of techniques for detecting MRD, some of which are commercially available in other countries as laboratory-developed tests (LDTs). The details of representative assays and their current availability (as of July 2024) are summarized in Tables 7 and 8, based on publicly available information from PubMed and the respective companies. However, assay names, names of the companies possessing the rights, and actual approval statuses may change with time. Furthermore, because information regarding the characteristics, performance, and indications of each assay is constantly being updated, it is important to confirm the latest information before actual clinical application. It should also be noted that the utility of MRD testing can differ depending on tumor type and stage. Therefore, each assay must be used after appropriate deliberation, depending on the target tumor type and clinical situation. Further technical innovation and accumulation of clinical evidence are expected to lead to the determination of the optimal assay for each case, thereby contributing to decision-making regarding treatment strategies.
References
Cancer Information Service, National Cancer Center Japan (2024) Cancer Statistics (National Cancer Registry) [Available from: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html]
Wan JCM, Massie C, Garcia-Corbacho J et al (2017) Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17(4):223–238
Corcoran RB, Chabner BA (2018) Application of cell-free DNA analysis to cancer treatment. N Engl J Med 379(18):1754–1765
Mandel P, Metais P (1948) Nuclear acids in human blood plasma. C R Seances Soc Biol Fil 142(3–4):241–243
Sorenson GD, Pribish DM, Valone FH et al (1994) Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol Biomark Prev 3(1):67–71
Subcommittee for Gene-related Testing, Japanese Promotion Council for Laboratory Testing (2022) Opinion on quality assurance of circulating tumor DNA (ctDNA) testing by liquid biopsy 2022
Nakamura Y, Taniguchi H, Ikeda M et al (2020) Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 26(12):1859–1864
Aziz K (2024) Understanding the Most Common Definition of MRD in Medical Literature - CRC Minimal Residual Disease 2024 [updated 2024–05–29. Available from: https://crcmrd.com/?p=198588
Japanese Society of Hematology (2023) Practical Guidelines for Hematological Malignancies, 2023
Diehl F, Schmidt K, Choti MA et al (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 14(9):985–990
Pascual J, Attard G, Bidard FC et al (2022) ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol 33(8):750–768
NCCN Clinical Practice Guidelines in Oncology, Version 4 (2024) pMMR/MSS Colon Cancer 2024 [Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf]
Japanese Society of Medical Oncology (2023) Molecular testing for colorectal cancer treatment. Cancer Sci 115:1014–1021
Tie J, Wang Y, Tomasetti C et al (2016) Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 8:346ra92
Tie J, Cohen JD, Wang Y et al (2019) Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol 5:1710–1717
Parikh AR, Van Seventer EE, Siravegna G et al (2021) Minimal residual disease detection using a plasma-only circulating tumor DNA assay in patients with colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res 27:5586–5594
Mo S, Ye L, Wang D et al (2023) Early detection of molecular residual disease and risk stratification for stage I to III colorectal cancer via circulating tumor DNA methylation. JAMA Oncol 9:770–778
Faulkner LG, Howells LM, Pepper C et al (2023) The utility of ctDNA in detecting minimal residual disease following curative surgery in colorectal cancer: a systematic review and meta-analysis. Br J Cancer 128:297–309
Tie J, Cohen JD, Lahouel K et al (2022) Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 386:2261–2272
Nakamura Y, Watanabe J, Akazawa N et al (2024) ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med. https://doi.org/10.1038/s41591-024-03254-6
Morris VK, Yothers G, Kopetz S et al (2024) Phase II results of circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy in patients with stage II colon cancer: NRG-GI005 (COBRA) phase II/III study. J Clin Oncol 42:5–5
Yang J, Gong Y, Lam VK et al (2020) Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis 11:346
Mi J, Wang R, Han X et al (2023) Circulating tumor DNA predicts recurrence and assesses prognosis in operable gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 102:e36228
Huffman BM, Aushev VN, Budde GL et al (2022) Analysis of circulating tumor DNA to predict risk of recurrence in patients with esophageal and gastric cancers. JCO Precis Oncol 6:e2200420
Azad TD, Chaudhuri AA, Fang P et al (2020) Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology 158:494-505.e6
Ng HY, Ko JMY, Lam KO et al (2023) Circulating tumor DNA dynamics as prognostic markers in locally advanced and metastatic esophageal squamous cell carcinoma. JAMA Surg 158:1141–1150
Zhang Y, Du H, Wang N et al (2024) An update of clinical value of circulating tumor DNA in esophageal cancer: a systematic review and meta-analysis. BMC Cancer 24:129
Taniguchi H, Nakamura Y, Kotani D et al (2021) CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci 112:2915–2920
Oki E, Nakanishi R, Ando K et al (2024) Recurrence monitoring using ctDNA in patients with metastatic colorectal cancer: COSMOS-CRC-03 and AURORA studies. ESMO Gastrointest Oncol 3:100034
Sullivan BG, Lo A, Yu J et al (2023) Circulating tumor DNA is unreliable to detect somatic gene alterations in gastrointestinal peritoneal carcinomatosis. Ann Surg Oncol 30:278–284
Abbosh C, Frankell AM, Harrison T et al (2023) Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616(7957):553–562
Chen K, Yang F, Shen H et al (2023) Individualized tumor-informed circulating tumor DNA analysis for postoperative monitoring of non-small cell lung cancer. Cancer Cell 41(10):1749-1762.e6
Chen D, Guo J, Huang H et al (2023) Prognostic value of circulating tumor DNA in operable non-small cell lung cancer: a systematic review and reconstructed individual patient-data based meta-analysis. BMC Med 21(1):467
Felip E, Altorki N, Zhou C et al (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-smallcell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398:1344–1357
Zhou C, Das Thakur M, Srivastava MK et al (2021) (2021) IMpower010: biomarkers of disease-free survival (DFS) in a phase 3 study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann Oncol 32(suppl_7):S1373–S1391
Felip E et al (2022) IMpower010: ctDNA status in patients (pts) with resected NSCLC who received adjuvant chemotherapy (chemo) followed by atezolizumab (atezo) or best supportive care (BSC). ESMO Immuno-Oncology Congress 2022, Abstract 10
Pan Y, Zhang JT, Gao X et al (2023) Dynamic circulating tumor DNA during chemoradiation therapy predicts clinical outcomes for locally advanced non-small cell lung cancer patients. Cancer Cell 41(10):1763-1773.e4
Shen H, Jin Y, Zhao H et al (2022) Potential clinical utility of liquid biopsy in early-stage non-small cell lung cancer. BMC Med 20(1):480
Nader-Marta G, Monteforte M, Agostinetto E et al (2024) Circulating tumor DNA for predicting recurrence in patients with operable breast cancer: a systematic review and meta-analysis. ESMO Open 9(3):102390
Turner NC, Swift C, Jenkins B et al (2023) Results of the c-TRAK TN trial: a clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate- and high-risk early-stage triple-negative breast cancer. Ann Oncol 34(2):200–211
Medford AJ, Scarpetti L, Niemierko A, Isakoff SJ, Moy B, Wander SA et al (2022) Cell-free DNA monitoring in a phase II study of adjuvant endocrine therapy with CDK 4/6 inhibitor ribociclib for localized HR+/HER2− breast cancer (LEADER). 2022 SAN ANTONIO BREAST CANCER SYMPOSIUM, PD17–03
Pope B, Park G, Lau E et al (2024) Ultrasensitive detection of circulating tumour DNA enriches for patients with a greater risk of recurrence of clinically localised prostate cancer. Eur Urol 85(4):407–410
Zill OA, Banks KC, Fairclough SR et al (2018) The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res 24(15):3528–3538
Christensen E, Birkenkamp-Demtroder K, Sethi H et al (2019) Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 37(18):1547–1557
Powles T, Assaf ZJ, Davarpanah N et al (2021) ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 595(7867):432–437
Christensen E, Birkenkamp-Demtroder K, Nordentoft I et al (2017) Liquid biopsy analysis of FGFR3 and PIK3CA hotspot mutations for disease surveillance in bladder cancer. Eur Urol 71(6):961–969
Buttner T, Zarbl R, Krausewitz P et al (2024) Hypermethylated SHOX2 in circulating cell-free DNA post renal cell carcinoma surgery as TNM-independent biomarker for recurrence risk. Am J Transl Res 16(1):304–313
Nuzzo PV, Berchuck JE, Korthauer K et al (2020) Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med 26(7):1041–1043
Lee B, Lipton L, Cohen J et al (2019) Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol 30(9):1472–1478
Alqahtani A, Alloghbi A, Coffin P et al (2023) Prognostic utility of preoperative and postoperative KRAS-mutated circulating tumor DNA (ctDNA) in resected pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Surg Oncol 51:102007
Lee BJT, Wang Y, Cohen JD et al (2024) The potential role of serial circulating tumor DNA (ctDNA) testing after upfront surgery to guide adjuvant chemotherapy for early stage pancreatic cancer: the AGITG DYNAMIC-pancreas trial. J Clin Oncol 42(16_suppl):107
Ye K, Fan Q, Yuan M et al (2022) Prognostic value of postoperative circulating tumor DNA in patients with early- and intermediate-stage hepatocellular carcinoma. Front Oncol. https://doi.org/10.3389/fonc.2022.834992
King G, Cohen SA, Chiorean EG et al (2023) Prospective longitudinal tumor-informed ctDNA in resectable biliary tract cancers. Ann Oncol 34(suppl_2):S215–S232
Pietrasz D, Sereni E, Lancelotti F et al (2022) Circulating tumour DNA: a challenging innovation to develop “precision onco-surgery” in pancreatic adenocarcinoma. Br J Cancer 126(12):1676–1683
Integrated genomic and epigenomic analysis of circulating tumor DNA for pancreatic cancer 2024 [Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000046195]
Multicenter study of circulating tumor DNA in patients with pancreatic cancer using a personalized panel 2024 [Available from: https://center6.umin.ac.jp/cgi-open-bin/icdr/ctr_view.cgi?recptno=R000049738]
A multi-institutional collaborative study aiming to elucidate the spatiotemporal molecular profiles for patients with malignant tumor 2024. [Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000061608]
Sausen M, Phallen J, Adleff V et al (2015) Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun 6(1):7686
Hadano N, Murakami Y, Uemura K et al (2016) Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br J Cancer 115(1):59–65
The Value of Molecular Residual Disease Monitoring Based on ctDNA in Resected Pancreatic Cancer (MAP-02) 2024 [Available from: https://clinicaltrials.gov/study/NCT05802407]
Integrated genomic and epigenomic analysis of circulating tumor DNA from hepatocellular carcinoma patients 2024 [Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000047611&type=summary&language=J]
Prediction of Hepatocellular Carcinoma Recurrence After Curative Treatment by Monitoring Circulating Tumor DNA (REMNANT) 2024 [Available from: https://clinicaltrials.gov/study/NCT05375370?term=NCT05375370&rank=1]
Prediction of Hepatocellular Carcinoma Recurrence After Curative Treatment by Longitudinal Monitoring MRD Based on ctDNA 2024 [Available from: https://clinicaltrials.gov/study/NCT06157060?term=NCT06157060&rank=1]
Cell-free DNA From Junction of Hepatitis B Virus Integration in HCC Patients for Monitoring Post-resection Recurrence 2024 [Available from: https://clinicaltrials.gov/study/NCT05823584?term=NCT05823584&rank=1]
Biomarkers and Molecular Mechanism Study of Hepatocellular Carcinoma After Radical Resection and Conversion Therapy 2024 [Available from: https://clinicaltrials.gov/study/NCT05823584?term=NCT05823584&rank=1 ]
Qin S, Chen M, Cheng AL et al (2023) Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet 402(10415):1835–1847
Integrated analysis of genomic and epigenomic alterations in circulating tumor DNA in patients with biliary tract cancer 2024 [Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000060172]
Feasibility Study of Multi-Platform Profiling of Resected Biliary Tract Cancer 2024 [Available from: https://clinicaltrials.gov/study/NCT04561453]
Heo J, Kim Y-N, Shin S et al (2024) Serial circulating tumor DNA analysis with a tumor-naive next-generation sequencing panel detects minimal residual disease and predicts outcome in ovarian cancer. Cancer Res 84(3):468–478
Hou JY, Chapman JS, Kalashnikova E et al (2022) Circulating tumor DNA monitoring for early recurrence detection in epithelial ovarian cancer. Gynecol Oncol 167(2):334–341
Kallio HM, Savolainen K, Virtanen T et al (2024) Sensitive circulating tumor DNA-based residual disease detection in epithelial ovarian cancer. Life Sci Alliance 7(6):e202402658
Ashley CW, Selenica P, Patel J et al (2023) High-sensitivity mutation analysis of cell-free DNA for disease monitoring in endometrial cancer. Clin Cancer Res 29(2):410–421
Jeannot E, Latouche A, Bonneau C et al (2021) Circulating HPV DNA as a marker for early detection of relapse in patients with cervical cancer. Clin Cancer Res 27(21):5869–5877
Han K, Zou J, Zhao Z et al (2024) Clinical validation of human papilloma virus circulating tumor DNA for early detection of residual disease after chemoradiation in cervical cancer. J Clin Oncol 42(4):431–440
Lin JC et al (2004) Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. New Engl J Med 350(24):2461–2470. https://doi.org/10.1056/NEJMoa032260
Chen FP et al (2020) Prognostic potential of liquid biopsy tracking in the posttreatment surveillance of patients with nonmetastatic nasopharyngeal carcinoma. Cancer 126(10):2163–2173. https://doi.org/10.1002/cncr.32770
Peng H et al (2019) Clinical value of a plasma Epstein–Barr virus DNA assay in the diagnosis of recurrent or metastatic nasopharyngeal carcinoma: a meta-analysis. Biosci Rep 39(9):BSR20190691. https://doi.org/10.1042/BSR20190691
Chan ATC et al (2018) Analysis of plasma Epstein–Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol 36(31):3091–3100. https://doi.org/10.1200/JCO.2018.77.7847
Chera BS, Kumar S, Shen C et al (2020) Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol 38(10):1050–1058. https://doi.org/10.1200/JCO.19.02444
Hanna GJ et al (2023) Negative predictive value of circulating tumor tissue modified viral (TTMV)-HPV DNA for HPV-driven oropharyngeal cancer surveillance. Clin Cancer Res 29(20):4306–4313. https://doi.org/10.1158/1078-0432.CCR-23-1478
Jensen KK et al (2018) Circulating human papillomavirus DNA as a surveillance tool in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Clin Otolaryngol 43(5):1242–1249. https://doi.org/10.1111/coa.13136
Chen L et al (2024) Early disease recurrence following post-operative HPV ctDNA directed active surveillance in oropharyngeal carcinoma: outcomes of a prospective pilot study. Int J Radiat Oncol Biol Phys 118(5):e91. https://doi.org/10.1016/j.ijrobp.2024.02.003
Honoré N, van Marcke C, Galot R et al (2023) Tumor-agnostic plasma assay for circulating tumor DNA detects minimal residual disease and predicts outcome in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 34(12):1175–1186. https://doi.org/10.1016/j.annonc.2023.09.3102
Flach S et al (2022) Liquid bIOpsy for miNimal rESidual diSease detection in head and neck squamous cell carcinoma (LIONESS): a personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br J Cancer 126(8):1186–1195. https://doi.org/10.1038/s41416-022-01716-7
Lee RJ, Gremel G, Marshall A et al (2018) Circulating tumor DNA predicts survival in patients with resected high-risk stage II/III melanoma. Ann Oncol 29(2):490–496
Lee JH, Saw RP, Thompson JF et al (2019) Pre-operative ctDNA predicts survival in high-risk stage III cutaneous melanoma patients. Ann Oncol 30(5):815–822
Tan L, Sandhu S, Lee RJ et al (2019) Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Ann Oncol 30(5):804–814
Braune J, Keller L, Schiller F et al (2020) Circulating tumor DNA allows early treatment monitoring in BRAF- and NRAS-mutant malignant melanoma. JCO Precis Oncol 4:20–31
Forschner A, Niessner H, Sinnberg T et al (2022) Circulating tumor DNA (ctDNA) in the detection of relapse in melanoma patients with adjuvant anti-PD-1 therapy. J Dtsch Dermatol Ges 20(6):867–871
Gouda MA, Polivka J, Huang HJ et al (2022) Ultrasensitive detection of BRAF mutations in circulating tumor DNA of non-metastatic melanoma. ESMO Open 7(1):100357
Rowe SP, Luber B, Makell M et al (2018) From validity to clinical utility: the influence of circulating tumor DNA on melanoma patient management in a real-world setting. Mol Oncol 12(10):1661–1672
Giunta EF, De Falco V, Vitiello PP et al (2022) Clinical utility of liquid biopsy to detect BRAF and NRAS mutations in stage III/IV melanoma patients by using real-time PCR. Cancers (Basel) 14(13):3053
Brunsgaard EK, Bowles TL, Asare EA et al (2023) Feasibility of personalized circulating tumor DNA detection in stage II and III melanoma. Melanoma Res 33(3):184–191
Eroglu Z, Krinshpun S, Kalashnikova E et al (2023) Circulating tumor DNA-based molecular residual disease detection for treatment monitoring in advanced melanoma patients. Cancer 129(11):1723–1734
Akaike T, Thakuria M, Silk AW et al (2024) Circulating tumor DNA assay detects Merkel cell carcinoma recurrence, disease progression, and minimal residual disease: surveillance and prognostic implications. J Clin Oncol 42(26):3151–3161
Lee R, Rothwell DG, Jackson R et al (2022) DETECTION phase II/III trial: Circulating tumor DNA-guided therapy for stage IIB/C melanoma after surgical resection. J Clin Oncol 40(16):TPS9603
Saldanha EF, Spiliopoulou P, Huenike K et al (2024) 226TiP CLEAR-Me: interception trial to detect and clear molecular residual disease in patients with high-risk melanoma. Ann Oncol 35:S306
Japanese Association of Medical Sciences Guidelines for Genetic Testing and Diagnosis in Medical Care 2011 [Available from: https://jams.med.or.jp/guideline/genetics-diagnosis.pdf]
Services CfMM. MolDX: Minimal Residual Disease Testing for Cancer 2021 [Available from: https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdId=38779&ver=4]
Dawood ZS, Alaimo L, Lima HA et al (2023) Circulating Tumor DNA Imaging and Carcinoembryonic Antigen: Comparison of Surveillance Strategies Among Patients Who Underwent Resection of Colorectal Cancer. Syst Rev Meta-analysis Ann Surg Oncol 30(1):259–274. https://doi.org/10.1245/s10434-022-12641-7
Emiloju OE, Storandt M, Zemla T et al (2024) Tumor-informed circulating tumor DNA for minimal residual disease detection in the management of colorectal cancer. JCO Precis Oncol 8:e2300127
Li CL, Ho MC, Lin YY et al (2020) Cell-free virus-host chimera DNA from hepatitis B virus integration sites as a circulating biomarker of hepatocellular cancer. Hepatology 72(6):2063–2076
Zhao L, Jiang L, Liu Y et al (2022) Integrated analysis of circulating tumour cells and circulating tumour DNA to detect minimal residual disease in hepatocellular carcinoma. Clin Transl Med 12:e793
Jiang J, Ye S, Xu Y et al (2020) Circulating tumor DNA as a potential marker to detect minimal residual disease and predict recurrence in pancreatic cancer. Front Oncol 10:1220
Kitahata Y, Kawai M, Hirono S et al (2022) Circulating tumor DNA as a potential prognostic marker in patients with borderline-resectable pancreatic cancer undergoing neoadjuvant chemotherapy followed by pancreatectomy. Ann Surg Oncol 29:1596–1605
Hata T, Mizuma M, Motoi F et al (2023) Prognostic impact of postoperative circulating tumor DNA as a molecular minimal residual disease marker in patients with pancreatic cancer undergoing surgical resection. J Hepatobiliary Pancreat Sci 30:815–824
Chao A, Chen SJ, Chen HC et al (2023) Mutations in circulating tumor DNA detected in the postoperative period predict poor survival in patients with ovarian cancer. Biomed J 46:100563
Fakih M, Sandhu J, Wang C et al (2022) Evaluation of comparative surveillance strategies of circulating tumor DNA, imaging, and carcinoembryonic antigen levels in patients with resected colorectal cancer. JAMA Netw Open 5(3):e221093
Hussung S, Akhoundova D, Hipp J et al (2021) Longitudinal analysis of cell-free mutated KRAS and CA 19–9 predicts survival following curative resection of pancreatic cancer. BMC Cancer 21(1):49
Watanabe F, Suzuki K, Tamaki S et al (2019) Longitudinal monitoring of KRAS-mutated circulating tumor DNA enables the prediction of prognosis and therapeutic responses in patients with pancreatic cancer. PLoS ONE 14:e022736
Minato T, Ito S, Li B et al (2021) Liquid biopsy with droplet digital PCR targeted to specific mutations in plasma cell-free tumor DNA can detect ovarian cancer recurrence earlier than CA125. Gynecol Oncol Rep 38:100847
Japanese Society for Cancer of the Colon and Rectum (ed) (2024) The JSCCR Guidelines 2024 for the Treatment of Colorectal Cancer
The Japan Lung Cancer Society (ed) (2022) Guidelines for Diagnosis and Treatment of Lung Cancer/Malignant Pleural Mesothelioma/Thymic Tumor 2022, version 7
Japanese Breast Cancer Society (ed) (2022) Clinical Practice Guidelines for Breast Cancer, 2022 edition (version 5), vol. 2, Epidemiology and Diagnosis"
Signatera FAQ (Frequently Asked Questions): @nateragenetics; 2024 [Available from: https://www.natera.com/oncology/signatera-advanced-cancer-detection/faq/]
Guardant Reveal™ - Guardant Health AMEA 2024 [Available from: https://www.guardanthealthamea.com/guardant-reveal/]
Henriksen TV, Reinert T, Christensen E et al (2020) The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol Oncol 14(8):1670–1679
Cohen SA (2023) Kinetics of postoperative circulating cell-free DNA and impact on minimal residual disease detection rates in patients with resected stage I–III colorectal cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2023.41.4_suppl.5
Jackson-Spence F, Toms C, O’Mahony LF et al (2023) IMvigor011: a study of adjuvant atezolizumab in patients with high-risk MIBC who are ctDNA+ post-surgery. Future Oncol 19(7):509–515
Chen G, Peng J, Xiao Q et al (2021) Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J Hematol Oncol. https://doi.org/10.1200/JCO.2023.41.4_suppl.5
Kotani D, Oki E, Nakamura Y et al (2023) Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med 29:127–134
Leal A, van Grieken NCT, Palsgrove DN et al (2020) White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun 11:525
Nakano K, Koh Y, Yamamichi G et al (2022) Perioperative circulating tumor DNA enables the identification of patients with poor prognosis in upper tract urothelial carcinoma. Cancer Sci 113(5):1830–1842
Reinert T, Henriksen TV, Christensen E et al (2019) Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol 5(8):1124–1131
Scholer LV, Reinert T, Orntoft MW et al (2017) Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res 23(18):5437–5445
Henriksen TV, Demuth C, Frydendahl A et al (2024) Unraveling the potential clinical utility of circulating tumor DNA detection in colorectal cancer-evaluation in a nationwide Danish cohort. Ann Oncol 35(2):229–239
Bellmunt J, Hussain M, Gschwend JE et al (2021) Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22(4):525–537
Chidharla A, Rapoport E, Agarwal K et al (2023) Circulating tumor DNA as a minimal residual disease assessment and recurrence risk in patients undergoing curative-intent resection with or without adjuvant chemotherapy in colorectal cancer: a systematic review and meta-analysis. Int J Mol Sci 24(12):10230
Baxter NN, Kennedy EB, Bergsland E et al (2022) Adjuvant therapy for stage II colon cancer: ASCO guideline update. J Clin Oncol 40(8):892–910
(2024) The potential role of serial circulating tumor DNA (ctDNA) testing after upfront surgery to guide adjuvant chemotherapy for early stage pancreatic cancer: The AGITG DYNAMIC-Pancreas trial. J Clin Oncol 42(16_suppl):107–107. https://doi.org/10.1200/JCO.2024.42.16_suppl.107
Xia L, Mei J, Kang R et al (2022) Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res 28(15):3308–3317
Moding EJ, Liu Y, Nabet BY et al (2020) Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer 1(2):176–183
Wang X, Yu N, Cheng G et al (2022) Prognostic value of circulating tumour DNA during post-radiotherapy surveillance in locally advanced esophageal squamous cell carcinoma. Clin Transl Med. https://doi.org/10.1002/ctm2.1116
Acknowledgements
This position paper was led by the Japan Society of Clinical Oncology (JSCO) in coordination with the Japanese Society of Medical Oncology (JSMO) and the Japan Surgical Society (JSS). The authors thank Natsuki Fukuda for her dedicated work at the JSCO administrative office.
Funding
This work was funded by the JSCO and the National Cancer Center Research and Development Fund (Project No. 2024-A-05, to Shin Kobayashi).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yoshiaki Nakamura reports honoraria from Chugai Pharmaceutical and research funding from Genomedia, Guardant Health Japan, Tempus Labs, Roche Diagnostics, Daiichi Sankyo, PRA Health Sciences, and Chugai Pharmaceutical. Hideaki Bando reports honoraria from Taiho Pharmaceutical, Ono Pharmaceutical, and Eli Lilly Japan and research funding from Ono Pharmaceutical. Eiji Oki reports honoraria from Bristol-Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, Takeda Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo, and Bayer Yakuhin and research funding from Guardant Health Japan. Hidehito Horinouchi reports honoraria from AstraZeneca, Chugai Pharmaceutical, and AbbVie and research funding from AbbVie, AstraZeneca, Chugai Pharmaceutical, and Bristol Myers Squibb. Yukinori Ozaki reports honoraria from Pfizer, Kyowa Kirin, Daiichi Sankyo, Eli Lilly Japan, and Chugai Pharmaceutical. Hiroji Iwata reports honoraria from AstraZeneca and Daiichi Sankyo and research funding from AstraZeneca, Daiichi Sankyo, Pfizer, and Chugai Pharmaceutical. Akihiro Ohba reports honoraria from Ono Pharmaceutical and research funding from Ono Pharmaceutical, Novartis Pharma, and Chugai Pharmaceutical. Taigo Kato reports honoraria from MSD. Hideaki Miyake reports honoraria from Janssen Pharmaceutical, Takeda Pharmaceutical, MSD, Eisai, Astellas, Sanofi, Pfizer, Sysmex, Ono Pharmaceutical, Astellas, and AstraZeneca; research funding from Ono Pharmaceutical, MSD, and Pfizer; and scholarship donations from PDRadiopharma, Takeda Pharmaceutical, and FUJIFILM. Masafumi Ikeda reports honoraria from Taiho Pharmaceutical, Novartis Pharma, Chugai Pharmaceutical, Nihon Servier, MSD, AstraZeneca, Teijin Pharma, Incyte Biosciences Japan, Eisai, and Takeda Pharmaceutical and research funding from MSD, Eisai, Chiome Bioscience, Syneos Health Clinical, Novartis Pharma, Eli Lilly Japan, Chugai Pharmaceutical, Bristol-Myers Squibb, Nihon Servier, Merus N.V., INVITAE, Delta-Fly Pharma, AstraZeneca, J-Pharma, Merck Biopharma, and Chiome Bioscience. Tatsuyuki Chiyoda reports research funding from Takeda Pharmaceutical and JSR. Kosei Hasegawa reports honoraria from Sanofi, MSD, Takeda Pharmaceutical, Chugai Pharmaceutical, and AstraZeneca and research funding from MSD and Takeda Pharmaceutical. Kenjiro Namikawa reports honoraria from Novartis Pharma. Takayuki Yoshino reports honoraria from Chugai Pharmaceutical, Takeda Pharmaceutical, Merck Biopharma, Bayer Yakuhin, Ono Pharmaceutical, MSD K.K, and Sumitomo Corp.; research funding from Amgen, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, FALCO biosystems, Genomedia, Medical & Biological Laboratories, Merus N.V., Molecular Health GmbH, MSD, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer Japan, Roche Diagnostics, Sanofi, Sysmex, Taiho Pharmaceutical, and Takeda Pharmaceutical. Kiyoshi Hasegawa reports honoraria from Eisai and Chugai Pharmaceutical, research funding from Mochida Pharmaceutical and FUJIFILM Healthcare Laboratory, and scholarship donations from Taiho Pharmaceutical. The other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kobayashi, S., Nakamura, Y., Hashimoto, T. et al. Japan society of clinical oncology position paper on appropriate clinical use of molecular residual disease (MRD) testing. Int J Clin Oncol 30, 605–654 (2025). https://doi.org/10.1007/s10147-024-02683-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02683-0