Abstract
Background
Laparoscopic ventral mesh rectopexy (LVR) has gained increasing acceptance for the treatment of patients with a full-thickness rectal prolapse (RP), but literature on follow-up of at least 10 years is scarce. We studied recurrence rate, long-term functional results and quality of life in patients who had LVR for RP more than 12 years ago.
Method
The study population consisted of patients who could be contacted among the 175 who had undergone LVR for RP and whose short- and medium-term outcomes were published in 2012. We studied the long-term recurrence rate (Kaplan-Meier), functional outcome (Wexner and ODS scores), quality of life (EuroQol) and satisfaction of the patient through clinical examination(s), specific scores and questionnaires.
Results
Of the 175 patients, 14 patients had exclusion criteria, 57 had died, and 42 were lost to follow-up, leaving 62 patients for analysis. Seventeen patients presented with a recurrence (10.5%) at the 10-year follow-up. The only statistically significant risk factor for recurrence was recurrent RP (HR = 11.5 (2.54–52.2), P = 0.002). The median faecal incontinence score was 4 (0–10) and significantly worse in patients who had a recurrence [12 (7–13) vs 3 (0–9); P = 0.016]. The median obstructive defaecation score was 6 (3–12). The median quality of life score was 7 (6–8). Most patients who presented with a recurrence said they would undergo the operation again and recommended it, as would patients with no recurrence.
Conclusion
LVR for RP is a safe and efficient technique with sustainable long-term results that shows long-term efficacy at > 10 years after the operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic ventral mesh rectopexy (LVR), as described by D’Hoore et al., has gained increasing acceptance for the treatment of patients with a full-thickness rectal prolapse (RP) [1, 2]. The operation corrects the anatomical anomaly and strengthens the rectovaginal septum. Dissection is limited to the anterior aspect of the lower rectum followed by a mesh suspension to the sacral promontory. Avoidance of extensive rectal mobilization minimizes the risk of pelvic nerve damage and related constipation [1, 3]. We conducted a review of the literature on LVR for RP and concluded that, based on the published favourable outcome data in terms of a low complication rate, low de novo constipation rate, improvement of anal incontinence and low recurrence rate at mid and long term, LVR seems to emerge as an efficient procedure for the treatment of patients with RP [4].
Nevertheless, some recent questions about ventral rectopexy for RP are still debated, such as the interest in a robotic approach [5,6,7,8,9], using a biological mesh [10], the using glue [11] or staplers [12] for mesh fixation, the advantage of an associated sigmoid resection [13, 14], the alternative of suture rectopexy [15,16,17], or perineal stapled prolapse resection [18].
In this context and to serve as a reference against which future studies can be compared, we aimed to study the recurrence rate, long-term functional results, quality of life and satisfaction in patients who had undergone LVR for RP (but not for rectocele, enterocele or internal prolapse) > 10 years ago in a single large university hospital.
Methods
Patients and study design
Between May 1996 and June 2009, 206 patients were operated on for RP in our colorectal unit. Of these patients, 175 (17 male) of mean age 58 (range, 16–94) years had a LVR; 17 patients were operated on using the ViKY Robotic Scope Holder (Endocontrol Medical, Grenoble, France) as part of a prospective randomized study [5]; 3 patients were operated on with the Da Vinci robot (Intuitive Surgical, Inc., Sunnyvale, CA, USA) at the beginning of our experience in robotic surgery [6, 8].
For the 175 patients, RP had been present for on average 29 months (range, 2–744); one woman aged 62 had had RP since infancy. Fifteen patients were referred with a recurrence. RP was diagnosed in the standing, squatting and lateral decubitus position at rest and during straining. Dynamic video cysto-colpo-proctography in women or video proctography in men were performed in all patients before the operation to confirm exteriorized rectal prolapse and reveal any eventual associated pelvic floor disorder. The radiological technique has been described elsewhere [19]. During the same period, and essentially from 1996 to 2002, 14 other patients underwent ventral rectopexy by laparotomy and 17 underwent a perineal procedure under locoregional or local anaesthesia for RP. Reasons for an open or perineal approach are detailed in Supplementary material 1.
The short- and long-term technical results, with a mean follow-up of 74 (range, 24–181) months in the 175 patients with RP who underwent LVR, were published in 2012 [20]: there was no 30-day mortality and 5.1% severe morbidity grade IIIb in the Dindo-Clavien classification [21], and two patients presented with a recurrence of RP at month 6 and 24, giving a recurrence rate of 3% at 5 years [20].
This population served as a base for the present retrospective, single-centre study. The patients were contacted by phone from June 2020 onwards (i.e., > 10 years after the operation on the last patient). Those who responded were given verbal and written information about the study and if they agreed were asked to sign informed consent and complete a set of questionnaires. They were offered a consultation with a colorectal surgeon participating in the study. In the absence of a reply after many attempts, their family and general practitioner were contacted, before declaring death or loss to follow-up.
Exclusion criteria were patients who refused to participate, those deprived of liberty by judicial or administrative decision, under legal protection, or patients with missing data essential for the study.
Patients’ characteristics extracted from the prospectively maintained institutional database for the 2012 study included age, sex, body mass index (BMI), American Society of Anaesthesiologists (ASA) score, past history of hysterectomy, previous surgery for RP whatever the technique and approach, psychiatric disease including past anorexia nervosa, history of chronic constipation and/or obstructive defaecation syndrome (ODS) [22], faecal incontinence defined as a Jorge and Wexner score > 5 [23], onset of the prolapse defined as < 1 year, ≥ 1 but < 5 years and ≥ 5 years, conversion to laparotomy, and associated cystopexy and/or colpopexy and/or other procedure.
Surgical technique
The procedure was derived from the original open technique described by Loygue et al. in 1984 [24], modified by D’Hoore et al. [1] and extensively described as laparoscopic anterior rectopexy to the promontor [20]. Laparoscopy or a robotic approach was performed through four trocars, as shown on two recently published videos [25, 26] (Fig. S1). The procedure is extensively described in Supplementary material 2, and an operative view of the mesh fixation is shown in Fig. S2.
Endpoints and long-term assessments
The primary endpoint for this analysis was the recurrence rate calculated according to the Kaplan-Meier method. Recurrence was defined as the presence of RP on physical examination. Date of recurrence, date of surgical treatment if any, type of surgery and outcome were collected. Risk factors for recurrence were also studied.
Secondary endpoints were the long-term functional outcome, quality of life and satisfaction of the patient collected during the interview and clinical examination for those who had responded. Faecal incontinence was given by the Jorge and Wexner score. Transit constipation was studied through two questions: Are you constipated? What treatment do you use to treat constipation? ODS was studied through the validated ODS score [27]. Quality of life was studied using the validated EuroQoL scale EQ-5D-3L [28]. To be able to interpret this quality of life, we assessed the general health status of the patients with a visual analog scale (VAS) from 0 (the worst state of health imaginable) to 100 (the best). Patient satisfaction regarding the operation was collected using a VAS from − 5 (very disappointed) to + 5 (very satisfied) and through two questions: If you had to have the procedure again, would you do it (yes, perhaps, certainly not)? Would you recommend this rectopexy operation to family or friends?
Institutional review board (IRB) approval
The trial protocol was approved by the French Ethics Committee CPP Sud Méditerranée IV on October 2, 2020 (IRB no. 2020-A02293-36) and the French Health Authority (ANSM) was informed. The study was registered in the CHU Grenoble Alpes register of studies respecting the reference methodology MR003 of the National Commission for Informatics and Liberties (CNIL).
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the median and 25th–75th percentiles. A recurrence-free curve was generated using the Kaplan-Meier method. For patients who had died or were lost to follow-up, data were censored at the time of death or last documented follow-up.
Both univariate and multivariate analyses were conducted to identify factors associated with recurrence. A Cox model was employed to estimate hazard ratios and their confidence intervals. The multivariate analysis was adjusted for clinically significant variables, including age, sex, BMI, ASA score, history of hysterectomy, psychiatric disorders, chronic constipation and late onset of prolapse. Pvalue < 0.05 was considered statistically significant. Statistical analyses were performed using R version 4.3.3.
Results
Of the 175 patients who were treated by LVR for RP in our institution until June 2009 and selected, 14 patients had exclusion criteria. From the 161 eligible patients, 57 had died including 34 before 10 years of follow-up, and 42 were lost to follow-up, leaving 62 patients who signed the inform consent and responded to the questionnaires (Fig. 1). Characteristics of the 161 eligible patients are presented in Table 1.
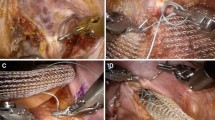
From the total cohort of 161 patients, 17 presented with a recurrent RP at 10-year follow-up, with a median (i.q.r.) interval time of 78 (10–120) months, giving a raw recurrence rate of 10.5%. Of these 17 patients, 8 had undergone redo ventral rectopexy (Fig. 2), 4 the Altemeier procedure and 3 the Delorme procedure, all with uneventful technical recovery except for one redo ventral rectopexy in a woman whose laparoscopy had been converted because of a presacral vein haemorrhage and reoperated at day 6 for adhesiolysis. The last two patients were not reoperated because of their short prolapse size with few symptoms and refusal of further surgery. The baseline characteristics of the patients with and without a recurrence are shown in Table 1.
Univariate analysis showed that recurrent RP [HR = 6.48 (2.37–17.7); P = 0.001] was a confounding factor for repeat recurrence. In multivariate analysis, the only statistically significant risk factor for recurrence was recurrent RP [HR = 11.5 (2.54–52.2); P = 0.002] (Table 2). In other words, redo rectopexy has higher risk for recurrence than primary operation. Age, sex, BMI, ASA score, history of hysterectomy, psychiatric disorders, chronic constipation and late onset of prolapse were not associated with risk of recurrence in this study. Excluding the 15 patients who were initially referred to us with recurrent RP gives a recurrence rate of 7.5%, which reflects the true recurrence rate for patients operated on for primary RP in our institution using the anterior rectopexy technique.
Functional results, quality of life and patient satisfaction for the 62 patients who responded to the questionnaires are shown in Table 3. The median (i.q.r.) Jorge and Wexner score was 4 (0–10) and significantly worse in patients who had a recurrence (P = 0.016). Median (i.q.r.) ODS score was 6 (3–12) with a tendency towards a worse score in the patients who had a recurrence. All patients without recurrence reported an improvement in their anorectal function. The median (i.q.r., min-max) quality of life score given by the EuroQol scale was 7 (6–8) and similar in both groups; general health status established at a median (i.q.r.) level of 70 (50–80) on the VAS was similar in both groups.
Despite more severe anal incontinence scores, a tendency towards difficulty in rectal evacuation and dissatisfaction, most patients who presented with a recurrence would undergo the operation again and recommend it to friends and family, as would patients with no recurrence (Table 3).
Discussion
The actuarial 10-year recurrence rate of 10.5% in this study, and 7.5% when patients referred for a recurrent RP were excluded from the analysis, is within the lower range of the published recurrence rates of the main series of abdominal rectopexy with follow-ups of at least 10 years [15, 29, 30]. In a multicentre pooled analysis of 643 individual patients including 320 patients with mesh rectopexy, published in 2005, data showed a 10-year recurrence rate of 28.9% [29]. In 2014, Foppa et al. reported a raw 10-year recurrence rate of 18% in 179 patients after laparoscopic suture rectopexy [15]. The 10-year RP recurrence rate following LVR in the study by Consten et al. was 8.2% (13 patients) [30], but eight patients developed symptomatic “internal rectal prolapse with or without enterocele” recurrence requiring surgical correction that we did not observe in our series, leaving a “true” recurrence rate for rectal prolapse requiring surgery of nearly 13% in this study.
The wide range of variation in recurrence rates after abdominal surgery for RP has several explanations that have been explored by DiGiuro et al. [31] and more recently discussed by van der Schans et al. [32]. The variation in recurrence rate first reflects differences in the length of follow-up between studies, with lower RP recurrence rates at shorter follow-up [4]. Articles reporting on LVR for RP describe time intervals to recurrence of between 6 and 90 months after surgery. The present study falls within this range with a median time to recurrence of 78 months. Recurrence developing within the first 36 months usually reflects technical failure, but not all studies report on this time interval [30]. Raftopoulos et al. showed that the 5-year recurrence rate after surgery can quadruple at 10 years [29]. In our series, the 5-year recurrence rate tripled beyond 10 years from 3 to 10.5%. Therefore, we agree with Consten et al. who insisted on the need to have long-term follow-up data to evaluate the ultimate efficacy of rectopexy [30]. A second important factor influencing the recurrence rate is the initial indication for surgery. Some cohorts included only external prolapse patients, others only internal rectal prolapse patients, or only enterocele/rectocele patients, or mixed series. While external prolapse is a clear diagnosis made on physical examination or defecography, internal rectal prolapse and colpocele diagnoses are less straightforward [31]. Radiological images of intussusception or rectocele at the end of rectal evacuation without complaint can even be considered normal [33]. The various definitions of recurrence could explain variations in recurrence rates among cohorts. The third cause suggested in the literature could be the nature of the mesh. Mackenzie et al. found that the only predictor of recurrence was the use of polyester mesh, which generated a significant twofold increase in recurrence rate, compared with the use of a polypropylene graft [34]. However, an analysis of 643 patients suggested that the surgical technique, such as the method of rectopexy and access, does not affect the recurrence rate [29]. It therefore seems appropriate to look further for other biases that could explain the variations in recurrence rate in the literature. An important factor is the heterogeneity amongst cohorts regarding the percentage of patients who had undergone previous surgery for RP (ranging from 0 to 41%) [32]. Perrenot et al. showed that patients with previous pelvic floor surgery or surgery for a recurrent RP had more recurrences of RP (21.0% versus 10.7%) [35]. We observed the same tendency, and for us this was the only risk factor for recurrence. Another factor relates to heterogeneity in the learning curve. Our retrospective series involved learning curves for LVR and for robotic surgery of RP; indeed, our current long-term recurrence rate is lower than that reported here. Some authors have argued the need for extensive rectal mobilization to minimize RP recurrence [36]. However, we observed recurrence rates even lower than for rectopexy techniques that require more extensive rectal mobilization [37,38,39]. Lastly, while some recurrences occur because of dehiscence of the mesh from the sacral promontory or the rectum, other factors can also contribute to failure of rectopexy, such as persistence of the cause responsible for or favouring the RP in the first place, for example, severe transit constipation, the practice of certain sports or professions, or specific conditions such as Ehlers Danlos disease, Marfan disease, or use of corticosteroids.
While the recurrence rate is the key measure of a successful outcome, patient reported endpoints are also important, including function and quality of life [36]. Surgical repair for RP also aims to improve anorectal function. We observed low median values for the Wexner and ODS scores. These findings correlate with the literature on LVR without posterior rectal mobilization, showing a mean decrease of 45% in faecal incontinence and a mean decrease of 40% in constipation [37]. We observed no new-onset constipation in our study. Consten et al. only noted new-onset constipation in 22 (2.4%) patients [30]. A systematic review on functional outcomes of different rectopexy techniques reported new-onset constipation in 5.5% to 10.5% cases for LVR without posterior rectal mobilization [37]. Mäkelä-Kaikkonen et al. studied the long-term functional results in a multicentre cohort of 330 patients operated on for external or internal rectal prolapse with a median follow-up of 44 months and demonstrated that subjective symptom relief was experienced by 86% of patients with external rectal prolapse [40]. In a recent systematic review and meta-analysis, Emile et al. found that the weighted mean rates of improvement in faecal incontinence and constipation after LVR were 79.3% and 71%, respectively [41]. We found in our study that patients with recurrent RP had a worse functional result than those without recurrence. Nevertheless, the patients with recurrence were overall satisfied with their care and presented similar quality of life scores. Singh et al. analysed the patients’ perception of their long-term outcome after LVR for various indications and reported that overall 63% of patients were satisfied with the outcome and 76% would recommend this procedure to others with similar symptoms [42]. For RP, we also found that the majority of patients having had a LVR in our institution would undergo the operation again and would recommend it to family and friends, even in the case of recurrence.
The principal limitation of this study is its retrospective character; nevertheless, very few events like complications and recurrence were missed by the analysis. Although the Kaplan-Meier method yields appropriate estimates for recurrence at various points in time, underestimation remains possible. A second limitation is the very long follow-up of the study, with the first patients having been operated on > 25 years ago. Many patients died or were lost to follow-up by the time of the analysis in 2020 and later, and this could have introduced a bias. The third major limitation is the absence of baseline functional evaluations of anal incontinence, constipation, obstructed defaecation and baseline quality of life. Some validated scores or questionnaires were not available at the beginning of the study period. These limitations should be considered in the interpretation of the long-term outcome and underline the necessity for the better functional and global evaluation of benign disorders such as RP.
Conclusion
LVR for RP is a safe and efficient technique with sustainable long-term results that shows long-term efficacy at > 10 years after the operation.
Data availability
The data collected and analysed in this study are available for academic use on reasonable request to the corresponding author and will be the object of a data release contract signed by the applicant and Grenoble Alpes University Hospital.
References
D’Hoore A, Cadoni R, Penninckx F (2004) Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 91:1500–1505
Reche F, Faucheron JL (2015) Laparoscopic ventral rectopexy is the gold standard treatment for rectal prolapse. Tech Coloproctol 19:565–566
Boons P, Collinson R, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Colorectal Dis 12:526–532
Faucheron JL, Trilling B, Girard E, Sage PY, Barbois S, Reche F (2015) Anterior rectopexy for full-thickness rectal prolapse: technical and functional results. World J Gastroenterol 21:5049–5055
Jarry J, Moreau Gaudry A, Long JA, Chipon E, Cinquin P, Faucheron JL (2013) Miniaturized robotic laparoscope-holder for rectopexy: first results of a prospective study. J Laparoendosc Adv Surg Tech A 23:351–355
Faucheron JL, Trilling B, Barbois S, Sage PY, Waroquet PA, Reche F (2016) Day case robotic ventral rectopexy compared with day case laparoscopic ventral rectopexy: a prospective study. Tech Coloproctol 20:695–700
Inaba CS, Sujatha-Bhaskar S, Koh CY, Jafari MD, Mills SD, Carmichael JC et al (2017) Robotic ventral mesh rectopexy for rectal prolapse: a single-institution experience. Tech Coloproctol 21:667–671
Trilling B, Sage PY, Reche F, Barbois S, Waroquet PA, Faucheron JL (2018) Early experience with ambulatory robotic ventral rectopexy. J Visc Surg 155:5–9
Postillon A, Perrenot C, Germain A, Scherrer M-L, Buisset C, Brunaud L et al (2020) Long-term outcomes of robotic ventral mesh rectopexy for external rectal prolapse. Surg Endosc 34:930–939
McLean R, Mercer-Jones M, Kipling M, Spoerer E (2018) Short and long-term clinical and patient-reported outcomes following laparoscopic ventral mesh rectopexy using biological mesh for pelvic organ prolapse: a prospective cohort study of 224 consecutive patients. Colorectal Dis 20:424–436
Silveira RK, Domingie S, Kirzin S, de Melo Filho DA, Portier G (2017) Comparative study of safety and efficacy of synthetic surgical glue for mesh fixation in ventral rectopexy. Surg Endosc 31:4016–4024
Huaulmé A, Jannin P, Reche F, Faucheron JL, Moreau-Gaudry A, Voros S (2020) Offline identification of surgical deviations in laparoscopic rectopexy. Artif Intell Med 104:101837
Carvalho E, Carvalho ME, Hull T, Zutshi M, Gurland BH (2018) Resection rectopexy is still an acceptable operation for rectal prolapse. Am Surg 84:1470–2147
Kalev G, Marquardt C, Schmerer M, Ulrich A, Heyl W, Schiedeck T (2023) Resection rectopexy as part of the multidisciplinary approach in the management of complex pelvic floor disorders. Innov Surg Sci 8:29–36
Foppa C, Martinek L, Arnaud JP, Bergamaschi R (2014) Ten-year follow up after laparoscopic suture rectopexy for full-thickness rectal prolapse. Colorectal Dis 16:809–814
Madbouly KM, Youssef M (2018) Laparoscopic ventral rectopexy versus laparoscopic Wells rectopexy for complete rectal prolapse: long-term results. J Laparoendosc Adv Surg Tech A 28:1–6
Thomas GP, Wong F, Vaizey CJ, Warusavitarne JH (2023) Laparoscopic modified mesh rectopexy: medium-term results of a novel approach for external rectal prolapse. Colorectal Dis 25:2378–2382
Mistrangelo M, Tonello P, Brachet Contul R, Arnone G, Passera R, Grasso L et al (2016) Perineal stapled prolapse resection for full-thickness external rectal prolapse: a multicentre prospective study. Colorectal Dis 18:1094–1100
Faucheron JL, Barot S, Collomb D, Hohn N, AngladeDubreuil D (2014) Dynamic cystocolpoproctography is superior to functional pelvic magnetic resonance imaging in the diagnosis of posterior pelvic floor disorders: results of a prospective study. Colorectal Dis 16:240–247
Faucheron JL, Voirin D, Riboud R, Waroquet PA, Noel J (2012) Laparoscopic anterior rectopexy to the promontory for full-thickness rectal prolapse in 175 consecutive patients: short- and long-term follow-up. Dis Colon Rectum 55:660–665
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC (2006) Functional bowel disorders. Gastroenterology 130:1480–1492
Jorge JMN, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36:77–97
Loygue J, Nordlinger B, Cunci O, Malafosse M, Huguet C, Parc R (1984) Rectopexy to the promontory for the treatment of rectal prolapse: report of 257 cases. Dis Colon Rectum 27:356–359
Faucheron JL, Mancini A, Reche F (2017) Hedrocele associated with full-thickness rectal prolapse: a very rare condition treated by ambulatory laparoscopic anterior rectopexy. Dis Colon Rectum 60:992–993
Kalifi M, Oyekashopefoluwa A, Faucheron JL (2024) Laparoscopic ventral mesh rectopexy for male patients - tips and tricks. Dis Colon Rectum 67(6):e362–e363. https://doi.org/10.1097/DCR.0000000000003104
Altomare DF, Spazzafumo L, Rinaldi M, Dodi G, Ghiselli R, Piloni V (2008) Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Colorectal Dis 10:84–88
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol group. Ann Med 33:337–343
Raftopoulos Y, Senagore AJ, Di Giuro G, Bergamaschi R, for the Rectal Prolapse Recurrence Study Group (2005) Recurrence rates after abdominal surgery for complete rectal prolapse: a multicenter pooled analysis of 643 individual patient data. Dis Colon Rectum 48:1200–1206
Consten ECJ, van Iersel JJ, Verheijen PM, Broeders IAMJ, Wolthuis AM, D’Hoore A (2015) Long-term outcome after laparoscopic ventral mesh rectopexy—an observational study of 919 consecutive patients. Ann Surg 262:742–748
DiGiuro G, Ignjatovic D, Brogger J, Bergamaschi R, on behalf of the Rectal Prolapse Recurrence Study Group (2006) How accurate are published recurrence rates after rectal prolapse surgery? A meta-analysis of individual patient data. Am J Surg 191:773–778
van der Schans EM, Boom MA, El Moumni M, Verheijen PM, Broeders IAMJ, Consten ECJ (2022) Mesh-related complications and recurrence after ventral mesh rectopexy with synthetic versus biologic mesh: a systematic review and meta-analysis. Tech Coloproctol 26:85–98
Trilling B, Faucheron JL (2016) New-onset rectoanal intussusceptions after laparoscopic ventral rectopexy: a normal image? Tech Coloproctol 20:885–886
Mackenzie H, Dixon AR (2014) Proficiency gain curve and predictors of outcome for laparoscopic ventral mesh rectopexy. Surgery 156:158–167
Perrenot C, Germain A, Scherrer ML, Ayav A, Brunaud L, Bresler L (2013) Long-term outcomes of robot-assisted laparoscopic rectopexy for rectal prolapse. Dis Colon Rectum 56:909–914
Bishawi M, Foppa C, Tou S, Bergamaschi R, for the Rectal Prolapse Recurrence Study Group (2016) Recurrence of rectal prolapse following rectopexy: a pooled analysis of 532 patients. Colorectal Dis 2016(18):779–784
Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP (2010) Systematic review on ventral rectopexy for rectal prolapse and intussusception. Colorectal Dis 12:504–512
Cadeddu F, Sileri P, Grande M, De Luca E, Franceschilli L, Milito G (2012) Focus on abdominal rectopexy for fullthickness rectal prolapse: meta-analysis of literature. Tech Coloproctol 16:37–53
Madiba TE, Baig MK, Wexner SD (2005) Surgical management of rectal prolapse. Arch Surg 140:63–73
Mäkelä-Kaikkonen J, Rautio T, Kairaluoma M, Carpelan-Holmström M, Kössi J, Rautio A et al (2018) Does ventral rectopexy improve pelvic floor function in the long term? Dis Colon Rectum 61:230–238
Emile SH, Elfeki H, Shalaby M, Sakr A, Sileri P, Wexner SD (2019) Outcome of laparoscopic ventral mesh rectopexy for full-thickness external rectal prolapse: a systematic review, meta-analysis, and meta-regression analysis of the predictors for recurrence. Surg Endosc 33:2444–2455
Singh S, Ratnatunga K, Bolckmans R, Iqbal N, Jones O, Lindsey I et al (2022) Patients’ perception of long-term outcome after laparoscopic ventral mesh rectopexy; single tertiary center experience. Ann Surg 276:e459–e465
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
J.L.F. participated in the study design. J.L.F., J.F., F.T., B.T. participated in the methodology. F.T., B.T., P.Y.S., D.D., J.L.F. participated in the data collection. M.B.,G.M.,A.R.,J.L.F.,P.Y.S.,B.T. participated in the data analysis/interpretation. M.B.,J.L.F.,A.F.,B.T. participated in the writing. M.B.,G.M.,A.R.,F.T.,P.Y.S.,B.T., J.L.F.participated in the critical revision/final approval.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a conflict of interest concerning this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous Communication: French Society of Digestive Surgery (SFCD) Meeting, Paris, 23–25 November 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Barra, M., Trilling, B., Mastronicola, G. et al. Long-term outcome of laparoscopic ventral rectopexy for full-thickness rectal prolapse: the PEXITY study. Tech Coloproctol 29, 68 (2025). https://doi.org/10.1007/s10151-024-03104-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10151-024-03104-0