Abstract
Background
Botulinum toxin (BT) is a nonsurgical alternative to lateral internal sphincterotomy (LIS). While there are promising results, there is still a gap in knowledge regarding long-term outcomes and the predictors for healing after BT.
Methods
Chronic anal fissure (CAF) patients treated with 100 IU BT with a minimum 5-year follow-up were analyzed retrospectively. Patients with persistent or recurrent fissures after their first BT injection were offered either a second BT injection or LIS. Healing was defined as complete symptom resolution with fissure epithelization. The primary outcome measure was recurrence-free healing rate with BT at 5 years. Predictors of healing were assessed by logistic regression analysis.
Results
The mean age was 33.8 ± 10 years, and 139 (69.5%) patients were female. The complete healing rate at 5 years was 73.8% and 26.2% for the patients that underwent LIS. Multivariate analysis for LIS likelihood revealed that female gender (odds ratio, OR: 0.48, 95% confidence intervals, CI 0.25–0.92, p = 0.028), absence of chronic constipation (OR: 0.09, 95% CI 0.03–0.25, p = < 0.0001), and shorter constipation duration (OR: 1.10, 95% CI 1.06–1.13, p = < 0.0001) were predictors for recurrent-free healing after BT at 5-years. A cutoff value of 10 months of constipation yielded an accuracy of 88% for predicting nonhealing with BT (AUC: 0.881). BT-related incontinence was mild and resolved within 2 months, while LIS resulted in 19.2% permanent incontinence at 5 years.
Conclusions
BT is an effective and safe treatment for CAF, with acceptable long-term outcomes and minimal incontinence risk. Constipation duration and gender are key predictors of healing, aiding patient selection. Male patients with prolonged constipation may benefit from earlier consideration of LIS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Anal fissure is a common and painful condition characterized by a tear in the distal anal canal, often accompanied by severe pain and bleeding. Chronic anal fissures (CAF), defined by symptoms persisting for more than 6 weeks, are frequently associated with elevated anal pressure [1]. Traditional treatment approaches include dietary modifications, topical agents, and surgical interventions, each carrying specific risks or not universally effective for all patients. In recent years, botulinum toxin (BT) injection has emerged as a nonsurgical alternative. The main reason for exploring nonsurgical treatments, despite having the gold standard of lateral internal sphincterotomy (LIS), is the associated risk of substantial permanent incontinence [2, 3].
Topical agents are the first-line treatment for CAF, achieving healing rates of 50–70% and late recurrence rates of 9–18% [2, 3]. Current guidelines recommend BT as the second-line treatment owing to its comparable efficacy to topical agents, with slightly better outcomes and fewer side effects as the second-line treatment [1, 4]. While the reported outcomes for healing and recurrence with BT are not as effective as those achieved with LIS, the incontinence rates are significantly lower (0–14%), and any impact on continence is transient [4].
If the long-term outcomes of BT are established and the predictive factors for healing are clarified, many complications and unnecessary costs could be avoided. Literature on BT is marked by significant heterogeneity in dosage, injection site, follow-up protocol, and duration, with varying efficacy and success rates. Additionally, there is a lack of data on factors that could predict healing with BT. This study aims to address these gaps by evaluating the 5-year outcomes of BT injection in patients with CAF and providing comprehensive insights into patient selection.
Methods
This research received approval from the institutional ethics committee (approval number: E-10840098-772.02-786). We retrospectively analyzed the prospectively collected the data of 1296 patients from three centers diagnosed with anal fissure between October 2017 and June 2024, starting with the initiation of anal BT treatment in our departments.
Our treatment protocol was structured as follows: patients presenting with acute anal fissure or patients presenting with CAF without prior treatment were initially advised to have a 6-week course of topical diltiazem, coupled with a high-fiber diet, laxatives, and warm sitz baths/showers. For those not responding to the initial regimen or experiencing recurrent fissures, the second-line intervention involved BT application. Patients with persistent or recurrent fissures post-first BT injection were offered either a second BT injection or LIS. Our LIS technique is open sphincterotomy up to the apex of the fissure [5]. For low-pressure fissures, flap surgeries were considered. Since 2019, a psyllium capsule supplement of 2× 500 mg/day was recommended to all patients evaluated for anal fissure, regardless of whether they experience constipation (Solgar Psyllium Husk Fibre, Solgar Inc., USA). This supplement was initiated as soon as the patient was evaluated at the first visit and continued for 2 months.
Diagnosis of CAF and healing was determined through patient history and rectal examination. Anal tone was evaluated by the surgeon and, when available, through anal manometry. Healing was defined as the complete resolution of symptoms and full epithelization or granulation of the fissure base, as assessed by the surgeon. “Nonhealing” referred to ongoing pain or bleeding after 2 months post-BT injection. “Recurrence” was defined as the return of symptoms after initial symptom resolution. Chronic constipation was assessed by the Rome IV criteria [6].
Inclusion criteria encompassed patients over 18 years with CAF who received 100 IU BT across four quadrants and completed a minimum follow-up of 5 years. Patients receiving combination therapies with BT, such as topical diltiazem, were excluded. Exclusion criteria included acute fissures, prior anal surgeries (e.g., LIS and hemorrhoidectomy), inflammatory bowel diseases, coexisting anorectal conditions (e.g., hemorrhoids and anal fistula), atypical fissures (e.g., lateral, multiple, or infected), low-pressure fissures (assessed by digital examination or anal manometry), comorbidities such as acquired immunodeficiency syndrome (AIDS), sexually transmitted diseases, tuberculosis, leukemia, and those who were breastfeeding or pregnant. In 2022, our BT protocol was modified to 50 IU administered through two quadrants, and these patients were excluded. Patients were thoroughly informed about the treatment outcomes and complications, and written consent was obtained for both the procedures and the use of their data for research purposes.
Botulinum toxin application
Procedures were conducted in the outpatient setting without anesthesia. A lyophilized 100 IU BT Type-A (BOTOX, Allergan, CA, USA) was reconstituted with 1 cc of saline. Using a 26-G needle, 25 units of the toxin were injected into each of the four quadrants at the 12, 3, 6, and 9 o’clock positions, targeting the internal anal sphincter.
Outcome measures
The primary outcome measure was the recurrence-free healing rate with BT at 5 years. The secondary outcome measure was the rate of incontinence at 5 years.
Follow-up
Patients were evaluated in the outpatient clinic on days 3 and 10 and at 1- and 2-months postprocedure. Assessments included symptoms, fissure epithelization (assessed by inspection), complications (hematoma/ecchymosis, abscess, and incontinence), and adherence to fiber supplementation.
After 2 months, patients with complete healing were advised to return if symptoms recurred. All patients were contacted by phone at 6, 12, 24, 48, and 60 months to check for incontinence and recurrence. Those reporting symptoms during phone follow-ups were invited for a clinical examination. The presence of incontinence was assessed at each visit and phone call by asking patients a simple yes-or-no question. Routine scoring was not performed. For patients who reported the presence of incontinence, the Cleveland Clinic Incontinence Score (CCIS) was conducted.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Categorical variables were presented as frequencies and percentages. The distribution of the continuous variables was evaluated using histograms. Variables that were normally distributed were reported as mean and standard deviation, and means were compared by the independent sample t-test. Skewed variables were reported as median, and range and means were compared by the Mann–Whitney U test. Predictors of BT outcome were investigated through the chi-squared test, Wilcoxon–Mann–Whitney test, and logistic regression models. Univariate logistic regression models were utilized to identify potential predictors of healing following BT treatment, as well as to assess the risk LIS. Variables with a p-value < 0.1 in univariate analysis were included in multivariable logistic regression models to determine independent predictors of healing at 5 years. Receiver operating characteristic (ROC) curves were generated to evaluate the predictive accuracy of significant factors, with the area under the curve (AUC) calculated for each model; p < 0.05 values were accepted as significant.
Results
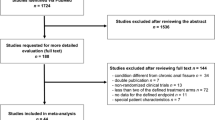
Among 1296 patients, 457 received BT treatment and 199 met the inclusion criteria (Fig. 1). The mean age was 33.8 ± 10 years, and 139 (69.8%) patients were female. The demographic and clinical characteristics are given in Table 1.
Complete healing with BT at 5 years
The complete healing rate at 2 months was 87.4% after the first BT injection. In total, 35 patients received a second injection for nonhealing or recurrent symptoms. Time-to-recurrence was 7.5 ± 3.6 months. After two BT injections, the overall complete healing rate at 5 years was 73.8% (n = 147). During the mean follow-up of 89 ± 9.3 months, 52 (26.2%) patients underwent LIS (Table 2).
Predictors of complete healing
Univariate analysis was conducted to identify predictors of healing with BT at 5 years, which can be also interpreted as the risk of LIS. Male gender (p = 0.026), longer symptom duration (p < 0.0001), chronic constipation (p < 0.0001), longer constipation duration (p < 0.0001), and absence of psyllium supplementation (p = 0.049) were associated with higher rates of LIS (Table 3).
The results of the multivariable model revealed that gender, chronic constipation, and constipation duration were independent predictors of BT outcome at 5 years (Table 4). Female patients (odds ratio, OR for LIS: 0.48 (95% confidence intervals, CI 0.25–0.92), p = 0.028) and patients without chronic constipation (OR for LIS: 0.09 (95% CI 0.03–0.25), p < 0.001)) were more likely to have complete healing with BT at 5 years. ROC analysis demonstrated that constipation duration (OR: 1.10 (95% CI 1.06–1.13), p < 0.001)) was the best predictor of BT outcome, with an area under curve (AUC) value of 0.881 (Table 4).
An adjusted model involving all significant factors was conducted (Table 5). Constipation duration alone was a superior predictor of LIS compared with the combined model. The AUC for the combined model was 0.869, which was very close to the AUC for constipation duration alone (AUC: 0.881) (Tables 4, 5). A cutoff value of 10 months of constipation yielded an accuracy of 88% for predicting unhealing with BT.
Incontinence at 5 years
Overall complication rate was 13%, including 1 (0.5%) abscess, 3 (1.5%) hematoma, and 22 (11%) incontinence, which were all managed conservatively. Among the patients who reported incontinence at 2 months, all of those in the BT group (n = 8) experienced temporary and mild incontinence (CCIS: 1–4), and none had symptoms at the end of the 5 years. In the LIS group, 14 (26.9%) patients reported incontinence at 2 months (CCIS: 1–13) and at 5 years, 10 (19.2%) patients had permanent incontinence (CCIS: 1–9), 2 (4%) had moderate incontinence, and 8 (15%) had mild degree of incontinence (Table 6).
Discussion
In this retrospective study, we assessed the efficacy and complications of BT treatment over a 5-year follow-up period. Our findings revealed a recurrence-free healing rate of 74%. The incontinence rate was 5.4% at 2 months posttreatment and decreased to 0% at 5 years. Despite these outcomes, 26% of patients eventually required LIS, which resulted in incontinence at varying degrees in 19% of cases. Beyond the primary and secondary endpoints, predictors of healing were also identified, with the strongest predictor of BT outcome being constipation duration. Risk factors for treatment failure were male gender, the presence of chronic constipation, and duration of constipation longer than 10 months.
Literature reports a highly heterogeneous healing rate for BT, ranging from 33% to 96% [7], as well as the follow-up durations, ranging from 2 weeks to 3 years in meta-analyses [4, 8, 9]. There are scarce data regarding long-term outcomes. A prospective study with 5-year follow-up by Ascanelli et al. [10] compared BT and LIS in 91 patients. Healing rates were 83.1% for BT and 96.8% for LIS at 6 months (p = 0.053). The authors reported that they observed all the recurrences within 6 months (16.9% for BT group) and performed a second BT injection in 8% and LIS in 8% [10]. By the end of the 5-year period, they found that 92% persisted in healing with either one or two BT injections. In our series, we observed 65.8% healing with the first BT injection, and this increased to 73.8% with the second injection. The mean time-to-recurrence after the first BT injection was 7.5 months and comparable with Ascanelli’s results. Other series giving 5-year results have reported healing rates between 64% and 85% [11, 12]. When compared with topical treatments, there is no evidence showing a superior efficacy of BT. In their comprehensive meta-analysis including 69 randomized trials, Jin et al. [9] concluded that LIS was the most effective treatment, while BT and medical treatments had equal outcomes regarding healing and recurrence at short and mid-term; however, LIS resulted in the highest rate of incontinence among all treatments. These results indicate that BT is a feasible second-line option with acceptable long-term outcomes, particularly in repeated applications.
Altered bowel movements, particularly constipation, is a well-known risk factor for chronic anal fissures (CAF). In a series of 1003 patients who underwent BT for CAF, half of them reported constipation [13]. Authors conducted a univariate analyses and found that pain after evacuation (p = 0.02), toxin dose (p < 0.0001), and injection site (p < 0.0001) were associated with healing, while dosage of toxin and injection site remained significant in multivariate analyses. High (50–100 IU) dosage of toxin resulted in higher rates of healing (83.1%) at 2 months when compared with medium (30 IU, 79.3%) and low dosages (≤ 25 IU, 64.8%) (p < 0.0001). Additionally, anterior injection site was correlated with higher healing than the posterior injection site (82 versus 59%, p < 0.0001). Interestingly, bowel movements and demographic variables showed no effect on healing [13]. In contrast to this study, gender, symptom duration, constipation, constipation duration, and fiber supplementation were significant factors in our univariate analysis. Other small series also reported that symptom duration was predictive for healing [14,15,16]. Given that Brisinda’s univariate and multivariate analyses focused on the 2-month outcomes, it makes sense that intervention-related factors were more prominent in their findings. In our series, patients were homogeneous regarding doses and injection sites. Regression models were conducted for 5-year outcomes. Our results highlight that patient-related factors may be more valuable predictive indicators and may serve as more valid criteria for selecting patients who will benefit from BT.
While analyzing our results, we aimed to test a model including age, gender, body mass index, sentinel pile, chronic constipation, constipation duration, and fiber supplementation to assist in patient selection for BT. This joint model did not truly improve the predictive value of constipation duration alone. Among 80 patients with constipation duration longer than 10 months, 40% underwent LIS, and most of them were male patients. Therefore, we believe that for male patients with prolonged constipation who do not respond to topical treatment, the option of LIS could be presented as a second-line treatment through decision-sharing. The latest UK guideline also recommend BT as a second-line treatment for both genders with a low grade of evidence, highlighting men who do not wish to undergo LIS [17].
The incidence of incontinence in our entire series was 11%. The median CCIS decreased over time and none of the patients had permanent complications in BT group. Compared with the 30–45% risk of minor and 11–22% risk of major incontinence reported in literature, we observed a relatively high rate of permanent incontinence after LIS (19.2%) [2, 3]. However, only two patients experienced moderate incontinence, while the others had mild incontinence. Incontinence observed after BT resolved spontaneously within 4 weeks in all patients.
The primary limitation of our study is its retrospective design, coupled with the absence of a control group. Assessing the healing of a fissure or incontinence by telephone is difficult, which constitutes a significant limitation of the study. Our incontinence rate after LIS is quite high, as all patients reporting any degree of incontinence were recorded as incontinent. Baseline CCIS and quality-of-life measures were not documented, and postprocedure assessments of pain, quality of life, and CCIS were not consistently recorded. Our results related to incontinence should be evaluated considering these factors. Additionally, we only had data of sentinel tag among descriptive elements of the fissure; fissure depth and papilla were missing, although they are likely to be predictive factors for treatment failure. Uncontrolled variables such as diet, lifestyle, and concurrent medications may have influenced the outcomes. The small number of patients receiving fiber supplements limits the interpretation of their effects. Furthermore, the 1 g/day psyllium capsule, the only available form in our country, lacks proven efficacy in improving bowel movements [18]. Finally, the administration of a second injection to some patients where BT was unsuccessful and the application of LIS to others, without being based on any criteria, may have introduced bias.
In conclusion, this study supports the use of BT as an effective and safe treatment for CAF, particularly for patients wishing to avoid surgery. The identification of key predictors of healing, including constipation duration and gender, may offer valuable guidance for clinicians in selecting patients. Further research should be performed to validate tailored treatment strategies.
Data availability
No datasets were generated or analyzed during the current study.
References
Davids JS, Hawkins AT, Bhama AR, Feinberg AE, Grieco MJ, Lightner AL et al (2023) The American Society of colon and rectal surgeons clinical practice guidelines for the management of anal fissures. Dis Colon Rectum 66:190–199. https://doi.org/10.1097/DCR.0000000000002664
Zbar AP, Pescatori M (2004) Functional outcome following lateral internal anal sphincterotomy for chronic anal fissure. Colorectal Dis 6:210–211. https://doi.org/10.1111/j.1463-1318.2004.00641.x
Al-thoubaity F (2020) Safety and efficacy of the treatment of chronic anal fissure by lateral internal sphincterotomy: a retrospective cohort study. Ann Med Surg 57:291–294. https://doi.org/10.1016/j.amsu.2020.08.010
Chen HL, Woo XB, Wang HS, Lin YJ, Luo HX, Chen YH et al (2014) Botulinum toxin injection versus lateral internal sphincterotomy for chronic anal fissure: a meta-analysis of randomized control trials. Tech Coloproctol 18:693–698. https://doi.org/10.1007/s10151-014-1121-4
Menteş BB, Ege B, Leventoglu S, Oguz M, Karadag A (2005) Extent of lateral internal sphincterotomy: up to the dentate line or up to the fissure apex? Dis Colon Rectum 48:365–370. https://doi.org/10.1007/s10350-004-0812-8
Drossman DA, Hasler WL (2016) Rome IV—Functional GI disorders: disorders of gut–brain interaction. Gastroenterology 150:1257–1261. https://doi.org/10.1053/j.gastro.2016.03.035
Bobkiewicz A, Francuzik W, Krokowicz L, Studniarek A, Ledwosiński W, Paszkowski J et al (2016) Botulinum toxin injection for treatment of chronic anal fissure: is there any dose-dependent efficiency? A meta-analysis. World J Surg 40:3064–3072. https://doi.org/10.1007/s00268-016-3693-9
Bonyad A, Zadeh RH, Asgari S, Eghbal F, Hajhosseini P, Ghadri H et al (2024) Botulinum toxin injection versus lateral internal sphincterotomy for chronic anal fissure: a meta-analysis of randomized control trials. Langenbecks Arch Surg 409:355. https://doi.org/10.1007/s00423-024-03484-9
Jin JZ, Bhat S, Park B, Hardy MO, Unasa H, Mauiliu-Wallis M et al (2022) A systematic review and network meta-analysis comparing treatments for anal fissure. Surgery (United States) 172:41–52. https://doi.org/10.1016/j.surg.2021.11.030
Ascanelli S, Rossin E, Aisoni F, Sette E, Chimisso L, Valpiani G et al (2024) Botulinum toxin injection for chronic anal fissure: a prospective controlled study with long follow-up. Minerva Surg 79:293–302. https://doi.org/10.23736/S2724-5691.24.10228-6
Barbeiro S, Atalaia-Martins C, Marcos P, Gonçalves C, Canhoto M, Arroja B et al (2017) Long-term outcomes of Botulinum toxin in the treatment of chronic anal fissure: 5 years of follow-up. United Eur Gastroenterol J 5:293–297. https://doi.org/10.1177/2050640616656708
De Robles MS, Young CJ (2022) Real world outcomes of lateral internal sphincterotomy vs botulinum toxin for the management of chronic anal fissures. Asian J Surg 45:184–188. https://doi.org/10.1016/j.asjsur.2021.04.027
Brisinda G, Fico V, Tropeano G, Altieri G, Chiarello MM (2024) Effectiveness and safety of botulinum toxin injection in the treatment of recurrent anal fissure following lateral internal sphincterotomy: cohort study. BJS Open. https://doi.org/10.1093/bjsopen/zrad156
Gandomkar H, Zeinoddini A, Heidari R, Amoli HA (2015) Partial lateral internal sphincterotomy versus combined botulinum toxin A injection and topical diltiazem in the treatment of chronic anal fissure: a randomized clinical trial. Dis Colon Rectum 58:228–234. https://doi.org/10.1097/DCR.0000000000000307
Minguez M, Herreros B, Espi A, GarciaGranero E, Sanchiz V, Mora F et al (2002) Long-term follow-up (42 months) of chronic anal fissure after healing with botulinum toxin. Gastroenterology 123:112–117. https://doi.org/10.1053/gast.2002.34219
Arslan NÇ, Özdenkaya Y (2019) Duration of the symptoms influence the outcome after botulinum toxin injection in anal fissure. Turk J Colorectal Dis 29:33–38. https://doi.org/10.4274/tjcd.galenos.2018.94547
Cross KLR, Brown SR, Kleijnen J, Bunce J, Paul M, Pilkington S et al (2023) The Association of Coloproctology of Great Britain and Ireland guideline on the management of anal fissure. Colorectal Dis 25:2423–2457. https://doi.org/10.1111/codi.16762
Van Der Schoot A, Drysdale C, Whelan K, Dimidi E (2022) The effect of fiber supplementation on chronic constipation in adults: an updated systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 116:953–969. https://doi.org/10.1093/ajcn/nqac184
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by C.A., T.B., Y.Y., O.B., and I.E.B. Statistical analysis was performed by M.K. The first draft of the manuscript was written by C.A., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Istanbul Medipol University ethics committee (approval number: E-10840098-772.02-786). Ethical approval was waived by the committee in view of the retrospective nature of the study, and all the procedures being performed were part of the routine care. They all gave written consent for prospective data collection and anonymous use in scientific research.
Informed consent
Written informed consent was obtained from all patients included in this study regarding the treatments they received. The consent form provides detailed information about the treatments administered and includes a statement of permission that declares the patients' data may be used in scientific purposes with their identities kept confidential.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arslan, C., Yildirim, Y., Kocak, M. et al. The 5-year outcomes and predictors of healing in chronic anal fissure treated with botulinum toxin: a retrospective analysis of 199 cases. Tech Coloproctol 29, 122 (2025). https://doi.org/10.1007/s10151-025-03162-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10151-025-03162-y