Abstract
Background
Sentinel lymph node biopsy (SLNB), a standard surgical procedure for clinically axillary-negative breast cancer patients, significantly reduces complications compared with axillary lymph node dissection, but it is still a relatively invasive procedure with some complications, affecting patient’s quality of life. To identify patients who might benefit from avoiding SLNB, this study aimed to develop a nomogram for predicting sentinel lymph node metastasis (SLNM) in breast cancer patients using the SEER database. Methods: We identified breast cancer patients whose 1–5 lymph nodes were examined in the SEER database as those who underwent SLNB. Patients were randomly assigned to the training and validation cohorts at a 3:1 ratio. Univariate and multivariate logistic regression were used to evaluate the relationships between SLNM and patients' clinicopathological characteristics. A nomogram was constructed, and its performance was validated via ROC curves, calibration curves, and decision curve analysis. Results: Age, race, primary site, T stage, M stage, histological grade, pathological type, estrogen receptor status, and progesterone receptor status are independent predictive factors for SLNM in patients with breast cancer. We successfully developed a predictive nomogram for sentinel lymph node status, with AUC values of 0.711 and 0.700 for the training and validation cohorts, respectively. Conclusion: Our study successfully established an SLNM nomogram that provides richer predictive information. The model exhibits good clinical efficacy and serves as a reference value for populations potentially exempt from SLNB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignant tumor currently affecting women's health worldwide [1]. Establishing a predictive model for patients with breast cancer can provide a crucial reference for individualized clinical diagnosis and treatment decisions, such as the determination of surgical approaches and the development of adjuvant treatment plans. A nomogram is a visual predictive tool. It quantifies the risk of clinical events on the basis of various risk factors and generates numerical probabilities of clinical events [2]. Nomograms are among the most common forms used in studies of breast cancer LNM/prognosis prediction models.
Sentinel lymph node biopsy (SLNB), a landmark advance in breast surgery in the 1990s, has become the most common and important method for assessing axillary lymph node (ALN) status in patients with cN0 breast cancer, following refinements from trials such as NSABP-32, MILAN, and ALMANAC [3,4,5,6]. The results of SLNB often guide the decision to perform axillary lymph node dissection (ALND) [7,8,9,10]. Compared with ALND, SLNB has avoided many unnecessary axillary injuries in patients and has made significant contributions to reducing postoperative pain, arm swelling, activity impairment, muscle weakness, and other complications. However, studies have shown that SLNB, which is still a relatively invasive clinical procedure, has few but persistent complications, affecting the quality of life of some postoperative patients [11, 12]. Therefore, other potentially wider-reaching lymph node biopsy methods, such as targeted axillary dissection (TAD) [13, 14] and marking the axillary node with a radioactive iodine seed (MARI) [15], are being increasingly considered. Research suggests that these more targeted axillary lymph node assessment methods may further reduce surgical complications without compromising accurate ALN evaluation under specific conditions [16].
In fact, the evolving landscape of systemic treatment for breast cancer has also significantly impacted the surgical approaches to ALN assessment. In the pre-targeted therapy era, ALND was deemed essential not only for staging but also for local control, as adjuvant chemotherapy and endocrine therapy provided limited systemic protection [17]. The advent of HER2-directed therapies (e.g., trastuzumab) in the early 2000s marked a paradigm shift, enabling systemic eradication of micrometastases and reducing reliance on extensive nodal surgery [18]. More recently, immunotherapy (e.g., pembrolizumab in triple-negative breast cancer) and antibody–drug conjugates (e.g., trastuzumab deruxtecan) have further diminished the prognostic weight of nodal status by achieving unprecedented pathological complete response rates and metastatic burden reduction [19,20,21]. These advancements underscore a critical trend: As systemic therapies improve, the necessity of invasive nodal staging diminishes for select patients. For instance, some prospective randomized trials are exploring the possibility of omitting SLNB in patients with early breast cancer [22, 23]. The SOUND trial [22] is one of the important studies that has received widespread attention. The results revealed that there were no statistically significant differences in distant disease-free survival, DFS, OS, the axillary metastasis rate, or the distant metastasis rate between SLNB group and no axillary surgery group. Therefore, the conclusion was reached that "omitting axillary surgery is not inferior to SLNB for specific conditions of breast cancer patients." In the future, the publication of more related research results, including the BOOG 2013–08 trial [24], will provide more medical evidence for omitting SLNB.
Materials and methods
Database
We conducted research on the SEER database. The SEER database deleted all the breast cancer lymph node surgery information in 2011, lacked readily available SLNB/ALND information. To address this issue in the SEER database, we utilized the research findings of Bilimoria et al. [25]. This study extracted patient information from the National Cancer Database (NCDB) for 97,314 patients in the SLNB-alone group and the SLNB-with-completion ALND group. The median number of lymph nodes examined was 3 (interquartile range, 25) for the former group and 13 (918) for the latter. On the basis of these data, if the number of examined lymph nodes was ≤ 5, the patient was considered to have undergone SLNB alone. Many other studies have also used this conclusion as a reference [26,27,28].
Patients
Our retrospective analysis was conducted on 117,895 breast cancer patients from the SEER (Stat 8.4.2) database between January 2010 and December 2015. The inclusion criteria were as follows: (1) 1–5 lymph node examinations; (2) primary breast cancer; and (3) American Joint Committee on Cancer (AJCC) 7th edition T stage T1-T3 breast cancer. The exclusion criteria were as follows: (1) bilateral breast cancer; (2) neoadjuvant therapy; (3) histological grade IV; (4) AJCC 7th M stage cM0(i +); and (5) incomplete clinicopathological data. The lymph node status was determined on the basis of the "Regional nodes positive 1988" field in the SEER database.
We extracted the following clinicopathological information from the SEER database: age, race, sex, primary site, laterality, T stage, M stage, histological grade, pathological type, breast cancer subtype, ER status, PR status, human epidermal growth factor receptor 2 (HER2) status, and lymph node metastasis status.
Construction and validation of the nomogram
Using R Foundation, 4.2.2, patients were randomly assigned to the training and validation cohorts at a 3:1 ratio for the development and validation of a nomogram. We ultimately identified 9 significant predictive factors suitable for inclusion in the nomogram: age, race, primary site, T stage, M stage, histological grade, pathological type, ER status, and PR status. We assessed the sensitivity and specificity of the predictive model via the area under the receiver operating characteristic (ROC) curve (AUC), visualized the model calibration through 1000 bootstrap repetitions of the calibration curve, and evaluated the clinical applicability of the nomogram via decision curve analysis (DCA).
Statistical analyses
We used chi-square tests to organize detailed patient baseline information, summarizing the relationships between various influencing factors and sentinel lymph node status. We used univariate logistic regression analysis to verify the correlation, preliminarily excluding factors with no statistically significant impact on SLNM. Then, via multivariate logistic regression, we calculated odds ratios (ORs) to identify significant predictive factors independently associated with SLNM in our study population. ORs are presented with 95% CIs. We also performed Omnibus tests and Hosmer–Lemeshow tests to assess preliminary model goodness-of-fit.
Statistical analyses were performed via IBM SPSS (version 26.0) statistical software. All tests were two sided, and P < 0.05 was deemed significant. Random population grouping and the creation of a nomogram were performed via the R Foundation, 4.2.2 and other packages (car, rms, pROC, Hmisc, and rmda).
Results
Exploratory results of scientific grouping of pathology type
We first extracted the pathological type classification field "Site record–rare tumors" from the SEER database, which was not mentioned in previous studies. This field, which is based on the Surveillance of Rare Cancer in Europe (RARECARE), categorizes breast cancer into 7 main types (Table 1). We performed univariate analysis of each pathological classification. The results revealed that the SLNM rate for Paget's disease was the highest, at 19.4%, which was higher than those of invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC). We decided to combine the "Site record-rare tumors" field and the commonly used "ICD-O-3 Hist/behav" field into a new classification method. While retaining the meaningful classification method of the former, we established additional screening criteria: 1) screening out clinically known special types of cancers that are extremely easy or difficult to metastasize through the lymphatic system and 2) screening out special pathological types with large-sample sizes and significantly different SLNM rates than established subgroups. Finally, the predictive factor "pathological type" in this study was divided into 7 subgroups: IDC, ILC, IDC mixed with ILC, mucinous carcinoma, Paget's disease, IMPC, and other types.
Exploratory results of the scientific grouping of age
We grouped age by 5-year intervals. On the basis of the definition of the International Young Breast Cancer Expert Group (BCY) [29, 30], we divided the population under 40 years of age into one group. Owing to the small-sample size (469) for individuals ≥ 90 years old, we did not further subdivide this age group. On this basis, we created a trend graph (Fig. 1). The age of 80 years is a turning point where the curve trend changes significantly. A greater age but still < 80 years is correlated with a lower SLNM rate, which aligns with our clinical experience. However, in those over 80 years of age, the trend reversed, contradicting our clinical experience. Moreover, within the 40–79 age range, the maximum slope of the curve occurs at 60 years, indicating a significant difference between the 40–59 and 60–79 age groups. Therefore, this study divided patients into four groups according to age: < 40, 40–59, 60–79, and ≥ 80 years.
Patient characteristics
From 20,102,015, this study ultimately included 117,895 breast cancer patients, who were randomly divided into a training cohort (88,422 patients) and a validation cohort (29,473 patients). The detailed clinicopathological characteristics are presented in Table 2.
14,645 breast cancer patients (12.4%) had positive sentinel lymph nodes. The SLNM rate in patients ≥ 80 years old was 14.9%, which was higher than that in the 40–59 and 60–79 age groups and lower than the < 40-year-old group. Japanese patients had the lowest SLNM rate, followed by Chinese patients. "Other" races were similar to "White," while Black patients had the highest rate. Only 584 were male, and their SLNM rate was higher than that of females. The inner quadrant of the breast had a lower overall SLNM rate. The outer quadrants had higher rates, and the axillary tail showing even higher rates. The central part, including the nipple, had the highest percentage. The larger the tumor diameter is, the higher the SLNM rate. In addition, M1 patients had a significantly higher SLNM rate than did M0 patients. A lower tumor histological grade was associated with lower SLNM rates. Among pathology types, mucinous carcinoma had the lowest SLNM rate, IMPC had the highest rate. Among subtypes, triple-negative breast cancer (TNBC) had the lowest SLNM rate, followed by luminal A, HER2-enriched, and luminal B subtypes. ER-positive, PR-positive, and HER2-positive patients had a higher SLNM rate than did negative patients. Laterality was the only factor without a statistically significant difference, with similar rates for the left and right sides.
Univariate logistic regression analysis
The ORs of each subgroup obtained from the univariate logistic regression analysis (Table 3) were consistent with the ratio of SLNM rates between subgroups from the chi-square test, successfully validating the correlation between each influencing factor and SLNM. Laterality, which does not affect SLNM (P = 0.489), was first excluded. Age, race, sex, primary site, T stage, M stage, histological grade, pathological type, subtype, ER status, PR status, and HER2 status were statistically significantly predictive of positive lymph nodes (P < 0.001).
Multivariate logistic regression analysis
The factors screened by univariate analysis were included in multivariate logistic regression analysis to further screen independent predictive factors associated with SLNM. The training cohort results (Table 4) revealed that sex and HER2 status had p values of 0.479 and 0.227, indicating that these factors are not independent predictors of SLNM and were excluded from the final predictive model. Moreover, we excluded breast cancer subtypes as confounding factors for ER status, PR status, and HER2 status. The remaining factors had p < 0.001 in the training cohort. Therefore, this study ultimately identified 9 independent predictive factors influencing SLNM: age, race, primary site, T stage, M stage, histological grade, pathological type, ER status, and PR status. Importantly, T2, T3, M1, and IMPC had significantly higher OR values than did the other factors, suggesting that T stage, M stage, and pathological type play more prominent roles in influencing SLNM rates. In addition, Omnibus tests and Hosmer–Lemeshow tests were performed to evaluate the model. The Hosmer–Lemeshow test performed well on both the training cohort (p = 0.172) and the total population (p = 0.192), indicating good model fit. The results of the Omnibus test (P < 0.001) showed that the entire model is effective and accurately predicts SLNM.
Construction and validation of the nomogram
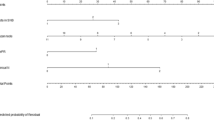
On the basis of the results of multivariate analysis, we developed a nomogram (Fig. 2) to predict SLNM in patients with breast cancer. By summing the scores of each factor, we can predict the probability of SLNM in every specific patient. Overall, Japanese and Chinese patients with no distant metastasis and T1mic, grade 1, and mucinous tumors in the central position of the breast had a lower risk of SLNM, whereas Black patients with distant metastasis and T3, grade 2&3, and IMPC tumors were more likely to have SLNM.
The AUCs for the training and validation cohorts (Fig. 3) were 0.711 (95% CI: 0.706–0.716) and 0.700 (95% CI: 0.691–0.708), respectively, indicating good discriminatory ability of the nomogram in differentiating sentinel lymph node status. Moreover, we performed 1000 bootstrap repetitions on the training and validation cohorts to obtain two calibration curves (Fig. 4). The calibration curves showed good agreement between the actual SLNM rate and the predicted rate, suggesting good calibration of the nomogram. The DCA results (Fig. 5) also showed that this nomogram is a reliable clinical tool for predicting SLNM in breast cancer patients.
Discussion
In this study, we developed a predictive nomogram to assess the probability of SLNM in breast cancer patients. Nine significant predictive factors with independent effects were ultimately identified. ROC curves, calibration curves, and clinical decision analysis of both the training and validation cohorts demonstrated that this nomogram has high sensitivity and specificity in predicting SLNM in patients with breast cancer and is a reliable clinical tool.
Age is one of the key objects explored in this study. On the basis of practical clinical experience, older patients tend to have a lower SLNM rate. However, our study data revealed that this pattern only exists in patients under 80 years of age. In patients over 80 years old, a greater age was associated with a higher SLNM rate, which is a novel finding. Although we cannot enumerate all previous prediction model studies on the relationship between age and LNM, the results of all single-center studies reviewed thus far support this clinical experience [31,32,33,34,35,36,37]. We believe that this is related mainly to the absolute shortage of patients aged ≥ 80 years; for this reason, few studies have categorized the population aged ≥ 80 years as a separate group. In fact, according to the findings of our study, previous studies' age-grouping methods essentially mixed the lowest SLNM rate group (60–79 years old) with the ≥ 80-year-old group. This naturally diluted the unique characteristics of the ≥ 80 age group, indicating that previous research underestimates the importance of the ≥ 80 age group. Therefore, it is necessary to conduct more systematic research on this population to fully explore the authenticity of the newly discovered pattern and its underlying reasons. This will become one of our key future research directions. On the basis of the patient baseline information statistically derived from this study, we can preliminarily analyze the possible reasons for this new finding: 1. Patients ≥ 80 years old have characteristics such as relatively larger tumors, a greater proportion of distant metastasis, and lower tumor differentiation. 2. This may also be related to the fact that elderly patients are less likely to detect the disease promptly, and by the time they seek medical attention, breast cancer may have significantly progressed. We similarly explored the scientific grouping of pathological types and ultimately categorized them into 7 types. The results revealed that mucinous adenocarcinoma was the least likely to metastasize via sentinel lymph nodes. The characteristics of mucinous adenocarcinoma, including lower histological grade, better differentiation, and lower LNM rates, have also been confirmed in multiple studies [36, 38, 39]. Compared with the most common pathological type “IDC,” Paget's disease [40] and IMPC [31, 41] are considered to have a high tendency for LNM, requiring greater clinical vigilance to avoid overlooking such lymphatic progression. Moreover, we found that the pathological type of IDC mixed with ILC had a significantly higher SLNM rate than did pure IDC and pure ILC. No previous research has focused on a detailed study of this type, which will also be a key focus of our future research.
Regarding race, as most previous studies categorized it into White, Black, and others, we referenced Iqbal J et al.'s earlier study [42] on the basis of the SEER database and additionally screened Chinese and Japanese populations, whose sample sizes were large enough and exhibited significantly different LNM rates than did other races. The results revealed that the SLNM rates for Chinese and Japanese patients were significantly lower than those for Black and White patients. SLNM risk positively correlates with tumor size, as reported in nearly all relevant studies by researchers such as Rivadeneira [43]. This conclusion, along with the conclusion that "M1 patients have a much higher LNM rate than M0 patients," applies in almost all scenarios. Our study also revealed that patients with primary tumors located in the axillary tail and nipple/central regions of the breast were more likely to experience SLNM, requiring attention to these relatively unique primary tumor locations. Research by Gou et al. [44] indicated that the axillary tail is an independent factor contributing to LNM. Another survey revealed that tumors in the central and nipple regions were associated with LNM [45]. With respect to breast cancer subtypes, Reyal et al. [46] reported that the TNBC had the lowest SLNM rate, and the HER2-enriched subtype had the highest SLNM rate. A study based on the SEER database [47] suggested that while the risk of LNM in the TNBC is significantly lower than that in the luminal A subtype, there is no significant difference between the other subtypes. In fact, our univariate logistic regression analysis revealed statistically significant differences in SLNM between subtypes. However, in our multivariate logistic regression analysis, subtype was not considered a significant predictive factor. The above results indicate that there may indeed be differences in SLNM between different breast cancer subtypes, while TNBC is generally considered the least likely to metastasize to lymph nodes. However, when factors with greater influence on SLNM are included in the predictive model, the overall differences between subtypes are further reduced, which is the primary reason for the discrepancy between the univariate and multivariate analysis results for breast cancer subtypes.
Compared with previous studies, our nomogram has several advantages. First, almost all previous prediction model studies for SLNM were small-sample, single-center studies. Owing to limitations in the geographic area and absolute number of people included in these studies, their predictive value is questionable. Although two articles [48, 49] used the National Cancer Database (NCDB) for large-sample prediction model studies, they both limited the enrolled population to ductal carcinoma in situ (DCIS) and did not establish an SLNM model for a broader population. Therefore, on the basis of the conclusions of Bilimoria et al. [25]., we screened the population considered to have undergone SLNB in the SEER database, leveraging the advantages of this database's large, multicenter sample size. This allowed us to build a prediction model for SLNM in a wider population of patients with breast cancer. Compared with previous similar studies, our prediction information is richer, the prediction model is larger, and it can be better applied in clinical practice. Moreover, before conducting clinicopathological feature analysis, we first explored the scientific grouping of each predictive factor. This is a novel approach compared with previous SEER database-based predictive model studies. Taking "pathological type" as an example, most existing SEER database-based studies simply divide it into three categories: IDC, ILC, and others. Reducing the subgroups of a prediction factor often leads to higher AUC values and better model discrimination, which is why few researchers choose grouping methods beyond the "three-category" approach. However, the "site record–rare tumors" field appears to have not been studied previously. On the basis of the clinical value of pathological type, sample size, and significant differences in SLNM rates, we successfully created a new classification method. Similarly, we conducted a scientific exploration of the "age" factor on the basis of the inherent objective relationship between age and SLNM, resulting in an age grouping that was more suitable for this study. We believe that evaluating a predictive model solely on the basis of AUC values is insufficient. Many studies prioritize increasing AUC values, often altering factor groupings to achieve this goal, potentially misclassifying groups with high discrimination into one category, and leading to erroneous conclusions.
This nomogram holds significant potential for clinical translation and optimizing axillary management in breast cancer. By enabling personalized risk stratification (e.g., identifying low-risk patients who may safely avoid SLNB), it aligns with global trends toward surgical de-escalation, potentially reducing complications like lymphedema by 15–20% [19, 22]. Its integration with multimodal data—such as imaging (MRI/PET-CT) and targeted therapies (e.g., HER2 blockade)—supports dynamic treatment planning [18], while cost-effectiveness analyses suggest substantial savings in resource-limited settings [21]. Moreover, the nomogram’s framework can be augmented with deep learning algorithms trained on radiopathomic data, potentially elevating AUC to > 0.85. [20]
Although the nomogram showed several advantages and significant potential, this study had some limitations. Our study utilized the conclusions of Bilimoria KY et al. to define breast cancer patients with 1–5 lymph nodes examined in the SEER database as patients who underwent SLNB. This approach is similar to a double-edged sword. While this allowed us to successfully establish a predictive model for SLNM in breast cancer patients on the basis of the SEER database, this definition inevitably contains bias. We could not perfectly match the actual SLNB population, which is a major limitation of our study. Moreover, several knowledge gaps remains. First, we could not obtain other important information from the SEER database, such as vascular invasion, despite its proven importance in axillary LNM in some single-center studies [50, 51]. Second, external validation in non-Western populations (e.g., Chinese cohorts) is pending, and the model’s generalizability requires further confirmation. Since our nomogram is based on a population of White and Black individuals, efforts are needed to minimize selection bias. Finally, the nomogram’s AUC (0.700–0.711) suggests moderate discriminative power. Incorporating predictive factors such as vascular invasion and molecular biomarkers (e.g., circulating tumor DNA or immune characteristics) may improve the accuracy of the model's prediction.
Overall, although SLNB has significantly fewer surgical complications than ALND, trials such as INSEMA (NCT02466737) [52] still demonstrate persistent complications, including pain, lymphedema, and functional impairment, evident within the first month after surgery. Therefore, in clinical practice, we should strive to improve axillary lymph node assessment while ensuring patient quality of life. We developed this nomogram to more accurately predict the individual probability of SLNM, with the goal of tentatively screening patients who were predicted to be SLN (-). This may provide valuable evidence for potential SLNB exemptions, achieving broader benefits. Over the next five years, we anticipate that predictive models will increasingly integrate multiomics data (genomic, radiomic, and clinicopathological) to achieve higher precision. Moreover, artificial intelligence-driven tools could dynamically update risk predictions based on real-world data, enabling adaptive clinical decision-making. Our study serves as a foundational step toward these goals, emphasizing the need for collaborative efforts to validate and refine such models in prospective trials.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63.
Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70.
Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the Almanac trial. J Natl Cancer Inst. 2006;98:599–609.
Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8.
Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–9.
Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(5):561–4.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: breast, version 2. (2008).
Natarajan S, Taneja C, Cady B. Evolution of lymphadenectomy in surgical oncology. Surg Oncol Clin N Am. 2005;14:447–59.
Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American college of surgeons oncology group trial Z0011. J Clin Oncol. 2007;25:3657–63.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53.
Sener SF, Winchester DJ, Martz CH, et al. Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer. 2001;92(4):748–52.
Rietman JS, Dijkstra PU, Geertzen JH, et al. Short-term morbidity of the upper limb after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast carcinoma. Cancer. 2003;98(4):690–6.
Simons JM, van Nijnatten TJA, van der Pol CC, et al. Diagnostic accuracy of radioactive iodine seed Placement in the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer. JAMA Surg. 2022;157(11):991–9.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–82.
van Hemert AKE, van Duijnhoven FH, Vrancken Peeters MTFD. This house believes that: MARI/TAD is better than sentinel node biopsy after PST for cN+ patients. Breast. 2023;71:89–95.
Rizzo A, Cusmai A, Acquafredda S, et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 2022;18(18):2301–9.
Guven DC, Erul E, Kaygusuz Y, et al. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer. 2023;31(12):624.
Caputo R, Buono G, Piezzo M, et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front Oncol. 2024;26(14):1362641.
Rizzo A, Schipilliti FM, Di Costanzo F, et al. Discontinuation rate and serious adverse events of chemoimmunotherapy as neoadjuvant treatment for triple-negative breast cancer: a systematic review and meta-analysis. ESMO Open. 2023;8(6):102198.
Vitale E, Rizzo A, Santa K, et al. Associations between “cancer risk”, “inflammation” and “metabolic syndrome”: a scoping review. Biology (Basel). 2024;13(5):352.
Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European institute of oncology of Milan (SOUND: sentinel node vs observation after axillary UltraSouND). Breast. 2012;21(5):678–81.
Hersh EH, King TA. De-escalating axillary surgery in early-stage breast cancer. Breast. 2022;62(Suppl 1):S43–9.
Van Roozendaal LM, Vane MLG, van Dalen T, et al. Clinically node negative breast cancer patients undergoing breast conserving therapy, sentinel lymph node procedure versus follow-up: a Dutch randomized controlled multicentre trial (BOOG 2013–08). BMC Cancer. 2017;17(1):459.
Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–53.
Wang J, Mittendorf EA, Sahin AA, et al. Outcomes of sentinel lymph node dissection alone vs. axillary lymph node dissection in early stage invasive lobular carcinoma: a retrospective study of the surveillance, epidemiology and end results (SEER) database. PLoS ONE. 2014;9(2):89778.
Gou Z, Lu X, He M, et al. Trends in axillary surgery and clinical outcomes among breast cancer patients with sentinel node metastasis. Breast. 2022;63:9–15.
Zheng Q, Luo H, Xia W, et al. Long-term survival after sentinel lymph node biopsy or axillary lymph node dissection in pN0 breast cancer patients: a population-based study. Breast Cancer Res Treat. 2022;196(3):613–22.
Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4). Ann Oncol. 2020;31(6):674–96.
Cardoso F, Loibl S, Pagani O, et al. The European society of breast cancer specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355–77.
Ye FG, Xia C, Ma D, et al. Nomogram for predicting preoperative lymph node involvement in patients with invasive micropapillary carcinoma of breast: a SEER population-based study. BMC Cancer. 2018;18(1):1085.
Zhu K, Sui Y, Zhu M, et al. Development and validation of a nomogram for predicting lymph node metastasis in ductal carcinoma in situ with microinvasion: a SEER population-based study. PLoS ONE. 2024;19(4):e0301057.
Zhang M, Wang B, Liu N, et al. Nomogram for predicting preoperative regional lymph nodes metastasis in patients with metaplastic breast cancer: a SEER population-based study. BMC Cancer. 2021;21(1):565.
Cui X, Zhu H, Huang J. Nomogram for predicting lymph node involvement in triple-negative breast cancer. Front Oncol. 2020;10:608334.
Zhao YX, Liu YR, Xie S, et al. A nomogram predicting lymph node metastasis in T1 breast cancer based on the surveillance, epidemiology, and end results program. J Cancer. 2019;10(11):2443–9.
Min Y, Wei X, Chen H, et al. Identifying clinicopathological risk factors of the regional lymph node metastasis in patients with T1–2 mucinous breast cancer: a population-based study. J Oncol. 2021;2021:3866907.
Gao X, Luo W, He L, et al. Nomogram models for stratified prediction of axillary lymph node metastasis in breast cancer patients (cN0). Front Endocrinol (Lausanne). 2022;13:967062.
Wu SL, Gai JD, Yu XM, et al. A novel nomogram and risk classification system for predicting lymph node metastasis of breast mucinous carcinoma: a SEER-based study. Cancer Med. 2022;11(24):4767–83.
Anderson WF, Chu KC, Chang S, et al. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1128–35.
Wong SM, Freedman RA, Sagara Y, et al. The effect of paget disease on axillary lymph node metastases and survival in invasive ductal carcinoma. Cancer. 2015;121(24):4333–40.
Jiang C, Xiu Y, Qiao K, et al. Prediction of lymph node metastasis in patients with breast invasive micropapillary carcinoma based on machine learning and SHapley additive explanations framework. Front Oncol. 2022;12:981059.
Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–73.
Rivadeneira DE, Simmons RM, Christos PJ, et al. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6.
Gou ZC, Liu XY, Xiao Y, et al. Decreased survival in patients with carcinoma of axillary tail versus upper outer quadrant breast cancers: a SEER population-based study. Cancer Manag Res. 2018;10:1133–41.
Siotos C, McColl M, Psoter K, et al. Tumor site and breast cancer prognosis. Clin Breast Cancer. 2018;18:e1045–52.
Reyal F, Rouzier R, Depont-Hazelzet B, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS ONE. 2011;6:e20297.
Mattes MD, Bhatia JK, Metzger D, et al. Breast Cancer subtype as a predictor of lymph node metastasis according to the SEER registry. J Breast Cancer. 2015;18(2):143–8.
James TA, Palis B, McCabe R, et al. Evaluating the role of sentinel lymph node biopsy in patients with DCIS treated with breast conserving surgery. Am J Surg. 2020;220(3):654–9.
Johnson MK, Cortina CS, Aldakkak M, et al. The use of sentinel lymph node biopsy in patients undergoing mastectomy for DCIS. Clin Breast Cancer. 2024;24(7):611–9.
Zhou W, He Z, Xue J, et al. Molecular subtype classification is a determinant of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph nodes. PLoS ONE. 2012;7:e35881.
Lauria R, Perrone F, Carlomagno C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer. 1995;76:1772–8.
Reimer T, Stachs A, Veselinovic K, INSEMA Investigators, et al. Patient-reported outcomes for the Intergroup Sentinel Mamma study (INSEMA): a randomised trial with persistent impact of axillary surgery on arm and breast symptoms in patients with early breast cancer. EClinicalMedicine. 2022;55:101756.
Acknowledgements
This study was supported by National Natural Science Foundation of China (62476285). We are grateful to all the individuals who participated in this study. We would also like to thank the highly qualified English speaking editors from AJE who edited the English language, grammar, punctuation, spelling, and overall style of this manuscript.
Funding
National Natural Science Foundation of China, 62476285, 62476285, 62476285, 62476285, 62476285, 62476285, 62476285, 62476285, 62476285, 62476285.
Author information
Authors and Affiliations
Contributions
J.D.W contributed to conceptualization, methodology, supervision, editing the manuscript, and funding acquisition. L.Z contributed to conceptualization, methodology, supervision, and editing the manuscript. Q.Y.L contributed to investigation, data analysis, validation, visualization, and writing the manuscript. H.X contributed to data analysis, validation, and writing the manuscript. B.S.B, Y.J.X, S.Q.G, Z.F.G, S.Y.C, and J.H.S contributed to investigation and data analysis. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
All the procedures followed were in accordance with the Helsinki Declaration and its later amendments. The data released by the SEER database were publicly available and does not require informed patient consent because cancer is a reportable disease in every state in the USA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hu Xu should be considered joint first author
Li Zhu should be considered joint corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Xu, H., Bao, B. et al. Predicting sentinel lymph node metastasis in breast cancer: a study based on the SEER database. Clin Exp Med 25, 82 (2025). https://doi.org/10.1007/s10238-025-01591-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-025-01591-5