Abstract
Cytological examination serves as a crucial diagnostic tool for pleural effusion, with its diagnostic efficacy influenced by variations in specimen processing and staining techniques. Cellular morphological analysis of pleural effusions was performed using Wright-Giemsa staining to assess its diagnostic accuracy and clinical utility in differentiating the various etiologies of exudative pleural effusion. A routine examination was conducted on 2305 cases of unexplained pleural effusion, followed by cellular classification and morphological analysis in 1376 cases identified as exudative effusion. Among the 479 patients with malignant tumors, cytomorphological examination identified malignant cells in 295 patients, resulting in a clinical diagnosis coincidence rate of 98.6%. Abnormal cells, including malignant and heterogeneous nuclear cells, were observed in 364 cases, yielding a detection rate of 76.0%. The proportion of positive malignant cells in the newly diagnosed patient group was significantly higher than that in the previously diagnosed group (P < 0.01). Cytological analysis revealed the presence of bacteria, fungi, and phagocytes in 51 out of 1376 cases. The positivity rate for multiple bacterial infections detected through cytology was significantly greater than that identified by culture (P < 0.01). Additionally, various special morphologies and pathogens, which are rare in clinical practice, were detected, including mixed metastasis of small cell lung carcinoma and adenocarcinoma cells, as well as concurrent infections with Talaromyces marneffei and Pneumocystis jirovecii. This method enables the rapid and comprehensive differentiation between malignant tumors, tuberculosis, pneumonia, and rare exudative pleural effusions resulting from specific clinical conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Clinically determining the nature of unexplained pleural effusion poses significant challenges. Pleural effusion can arise from benign conditions, malignant diseases, or even life-threatening critical illnesses [1]. Consequently, it is crucial to conduct a systematic and rapid assessment, followed by appropriate treatment based on the results of pleural fluid analysis, to alleviate respiratory distress and enhance the quality of life for patients [2]. Traditional laboratory examinations typically assess parameters such as specimen color, transparency, qualitative protein analysis, total white blood cell count, mononuclear cells, and multinuclear cells. These parameters aid in distinguishing transudate from exudate in conjunction with the Light criteria [3]. Common causes of transudate include heart failure, nephrotic syndrome, and liver cirrhosis. In contrast, the etiologies of exudate are more complex and diverse, necessitating further studies to address this unresolved issue [4]. Based on routine tests conducted on 2305 cases, we employed rapid cytomorphological tests on 1376 cases of pleural effusion classified as exudates to identify and analyze the types of benign and malignant cells present, as well as to calculate the percentages for each cell type. Additionally, abnormal components such as bacteria, fungi, crystals, and parasites were detected to identify exudates stemming from various etiologies, providing a diagnostic basis for cell morphology in clinical diagnosis.
Materials and methodology
Subjects
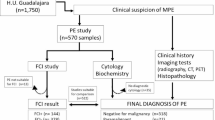
A retrospective analysis of pleural effusion samples was conducted on a cohort comprising 2305 patients, which included inpatients, outpatients, and emergency department patients, collected at Jinjiang Municipal Hospital from October 2015 to December 2023. The cohort comprised 1577 males and 728 females, yielding a male-to-female ratio of 2.166:1, with an age range of 0-102 years (Fig. 1). Patients provided informed consent for pleural puncture and for both routine and cytological examinations of pleural effusion. This analysis was approved by the Medical Ethics Committee of the Jinjiang Municipal Hospital (No. JJSYY2021N003S).
Methods
Handling of the pleural effusion samples
Routine examinations
The color, transparency, qualitative protein analysis, cell count, and presence of mononuclear and multinuclear cells were evaluated.
Cell collection
The exudate was collected in a specialized anticoagulant tube (10 mL), centrifuged at low speed (400 g) for 10 min, and the supernatant was subsequently discarded.
The slice preparation
The 'push piece' slicing method was employed. The sediment concentrated by centrifugation was mixed thoroughly, and 10 μL was placed on the right side of a clean slide. The slide was then pressed onto the sediment, allowing the sample to spread to an appropriate width along the edge of the slide, forming an angle of 25-35°. The deposit was quickly pushed to the other end of the slide, creating a shape similar to that of a blood smear. Two thin slides with tails, two thick slides with tails, and two thick slides without tails (Fig. 2) were prepared and allowed to dry naturally at room temperature before undergoing Wright-Giemsa staining or other staining techniques. The thin slides with tails facilitated the complete unfolding of cells, enhancing morphological observation, while the thick slides with tails increased cell yield, and the thick slides without tails created a concentration zone for cells at the tail end.
Wright–Giemsa staining
The dried slide was taken, and 0.5 mL of Wright-Giemsa stain solution (solution A) was added dropwise to ensure all cells were covered. Subsequently, 1.0 mL to 1.5 mL of phosphate buffer (pH 6.8) (solution B) was added dropwise to solution A and mixed thoroughly. The mixture was allowed to stain for 5 to 10 min, then rinsed with running water and left to dry.
Slice review and cell classification
Slice review
The entire slide was examined using a 10×10 magnification lens to observe cell distribution and arrangement, particularly focusing on the presence of larger cells, cells with a high nucleus-to-cytoplasm ratio, and clumps of cells at the ends, sides, and center of the slide, as well as to evaluate the staining quality. If any valuable cells or other formed elements were identified, they were further observed and classified using the 10×100 oil immersion objective lens.
Classification of nucleated cells
The area where the cells were evenly distributed at the junction between the head and tail was selected, and nucleated cells (including neutrophils, eosinophils, basophils, lymphocytes, reactive lymphocytes, plasma cells, monocytes-macrophages, and mesothelial cells) were counted according to cell type. The classification method was similar to that used for blood smears, and the results were reported as percentages. Tumor cells, nuclear heterogeneous cells, and other special components were morphologically described.
The four-level cytological description method was used to grade abnormal cells
① No malignant cells were observed: the smear contained only normal cells or general inflammatory cells. ② Nuclear heterogeneous cells were noted: these could indicate severe inflammatory degeneration, but the possibility of cancerous transformation could not be ruled out. ③ Suspicious malignant cells were identified: although the morphology of these cells generally met the criteria for malignant tumor cells, they were not highly characteristic, and the number of cells was limited, requiring further confirmation. ④ A large number of malignant cells with typical morphology were classified as malignant tumor cells. If it is determined through immunohistochemical staining or flow cytometry immunophenotyping that malignant cells originate from epithelium, they are reported as cancer cells. If it is confirmed that they originate from hematopoietic lymphoid tissue, they are classified as leukemia cells or lymphoma cells.
Identification of other morphological factors
Other specific cell forms identified in the smear included bacteria, fungi, phagocytes, and naive or abnormal cells from lymphohaematopoietic tissue. The spare smears were subjected to various staining techniques, including gram staining divides bacteria into positive (blue-purple) and negative (red); acid-fast staining positive bacteria (red); Prussian blue staining iron particles are blue-green; myeloperoxidase in blood cells is stained by POX to form blue-black particles, deposited in the cytoplasm; periodic acid-Schiff (PAS) staining red in the cytoplasm of blood cells is a positive reaction; argyrophilic nucleolar organizing region (AgNOR) staining can make the cyst wall of Pneumocystis yersinii black, trophozoites are not stained. The method of experimental staining referred to the reagent manual of Zhuhai Baso Biotechnology Co., Ltd. The advantage of this approach was that additional tests could be conducted without the need to recollect the specimen, thus providing timely and reliable diagnostic information for clinical practice.
Definitions
The Light criterion was proposed by American respiratory scientist Professor Richard W.Light in 1972. There are three main criteria, which can be diagnosed as exudate if any one of the three conditions is met: (1) pleural fluid/serum protein ratio > 0.5; (2) pleural fluid/serum lactate dehydrogenase (LDH) ratio > 0.6; (3) pleural fluid LDH level greater than serum LDH level greater than 2/3 of the normal upper limit of serum LDH. If none of the above three conditions is met, it is considered as leakage liquid.
Serum-pleural fluid albumin gradient standard: serum-pleural fluid albumin gradient > 12g/L, suggesting that the effusion is transudate.
Transudate is primarily observed in patients with congestive heart failure, liver cirrhosis, and nephrotic syndrome, based on clinical and laboratory data, along with negative cytology and microbiology results from pleural effusion.
Malignant pleural effusion (MPE) was diagnosed in the following cases: (1) A positive cytological examination of the pleural fluid or a positive histological examination of the pleural biopsy. (2) In patients with known malignant disease, pleural effusion was classified as MPE, considering the possibility of false-negative cytology, particularly in cases of recurrent pleural effusions after excluding other causes.
Tuberculous pleural effusion (TPE): Patients with pleural effusion, pleural biopsy, or respiratory specimens (sputum, bronchial washing fluid, and bronchoalveolar lavage fluid) exhibiting one or more positive Mycobacterium tuberculosis smears, cultures, or Mycobacterium tuberculosis DNA, and/or granulomas in pleural biopsy specimens can be diagnosed with TPE.
Parapneumonic pleural effusion (PPE) is generally secondary to bacterial pneumonia, lung abscess, or bronchiectasis associated with infection. It typically presents with symptoms and signs such as acute febrile illness and pleuritic pain, along with lung effusion observed on chest radiographs. Patients exhibiting lung infiltration without any other identifiable causes of pleural effusion may be diagnosed with PPE.
Eosinophilic pleural effusion (EPE) is diagnosed in patients with pleural effusion containing ≥ 10% eosinophils.
Post-traumatic exudate is diagnosed as bloody pleural effusion following chest trauma.
Pleural effusion associated with connective tissue diseases was diagnosed in patients with a known specific connective tissue disorder, following the exclusion of other potential causes of pleural effusion.
Miscellaneous pleural effusions refer to the classification of pleural effusions resulting from rare causes.
Statistical analysis
The measurement data of the system were entered into Excel, and statistical analysis was conducted following data conversion. Differences in dichotomous variables were assessed using the chi-square test and Mann-Whitney U test. The analysis was performed using SPSS 26.0 statistical software.
Results
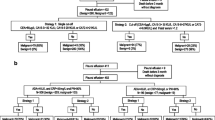
Among the 2305 cases of pleural effusion, 929 cases associated with heart failure, nephrotic syndrome, and liver cirrhosis were excluded, resulting in the identification of 1376 exudates based on clinical, cytological, and microbiological results of pleural fluid, the serum-pleural fluid albumin gradient standards, and the Light criteria [1].
This accounted for 59.7% of the total specimens. Among these, there were 479 cases of MPE from patients with malignant tumors, 460 cases of pleural effusion from TPE, including 30 concurrent tumor cases, 450 cases of PPE due to pneumonia, and 17 cases of exudative pleural effusion attributed to other etiologies. Overall, tumors, tuberculosis, and lung infections emerged as the primary causes of exudative pleural effusion clinically [2, 5,6,7].
Among the 1376 exudates, 479 cases were attributed to pleural effusion in patients with malignant tumors, comprising 282 newly diagnosed patients and 197 previously diagnosed patients. Cytomorphological examination revealed malignant cells in 295 cases. Of these, 291 patients received a clinical diagnosis of malignant tumors (excluding 4 patients who did not complete follow-up), resulting in a clinical diagnosis coincidence rate of 98.6%. Additionally, 91 cases exhibited severe nuclear heterogeneity and suspicious malignant cells, which were classified as 'heterogeneous nuclear cells.' Among these, 73 cases were clinically diagnosed as malignant tumors, yielding a clinical diagnosis coincidence rate of 80.2%. Totally, 364 out of 479 cases (76.0%) showed abnormal cells, including malignant cells and heterogeneous nuclear cells.
In the group of 282 newly diagnosed tumor patients, routine cytology identified 196 cases of malignant cells (69.5%), 37 cases of heterogeneous nuclear cells (13.12%), and 49 cases with no abnormal cells (17.38%). In contrast, among the 197 patients with previously diagnosed tumors, 95 exhibited malignant cells (48.2%), 36 had heterogeneous nuclear cells (18.27%), and 66 showed no abnormal cells (33.50%). The percentage of malignant cells in the newly diagnosed group was significantly higher than that in the previously diagnosed group (P < 0.01) (Table 1). Supplementary Figs. S1, S2, S3 illustrate malignant tumor cells, while Supplementary Fig. S4 depicts malignant lymphoma cells.
Bacteria, fungi, and phagocytes were identified in 51 out of 1367 patients (excluding Mycobacterium tuberculosis), confirming bacterial and fungal infectious effusions through culture. Among these patients, 27 were diagnosed with a single-species infection (52.94%), while 24 had multiple bacterial infections (47.06%). During the same period, the positivity rate for pleural fluid cultures was noted, yielding 90 strains from 2405 cases across 62 patients. The distribution of bacteria is illustrated in Fig. 3. Among these cases, 49 presented with a single-species infection (79.03%), and 13 had multiple bacterial infections (20.97%). Notably, the percentage of multiple bacterial infections detected by cytology was higher than that identified by culture, and the difference between these two groups was statistically significant (P < 0.01) (Table 2). Supplementary Fig. S5 displays images of the various bacterial infections.
Lymphocytes comprising ≥ 70% were detected in 563 out of 1376 patients. The percentage of lymphocytes in the effusion fluid was categorized into ten intervals, ranging from 0% ≤ X < 10% to 90% ≤ X < 100%. The incidence rates of MPE, TPE, and PPE were calculated for each interval. A positive correlation was observed between the lymphocyte percentage and the incidence of TPE, while a negative correlation was noted with the incidence of PPE. The correlation with MPE incidence was highest in the middle range and slightly decreased on both ends. Notably, when the lymphocyte proportion reached ≥ 70%, the correlation with TPE was enhanced (Fig. 4A-D).
The analysis result of lymphocyte percentage. A Relationship between the incidence of different types of PE and the lymphocyte percentage (L%). B Relationship between lymphocyte percentage (L%) and tuberculous pleural effusion (TPE). C Relationship between lymphocyte percentage (L%) and parapneumonic pleural effusion (PPE). D Mann-Whitney rank sum test of tuberculous pleural effusion (TPE) and lymphocyte percentage (L%), neutrophil percentage (N%)
A total of 272 out of 1376 patients exhibited a neutrophil proportion of ≥ 50%. The percentage of neutrophils in the effusion fluid was categorized into ten intervals, ranging from 0% ≤ X < 10% to 90% ≤ X < 100%. The incidences of MPE, TPE, and PPE were calculated for each interval. When the neutrophil count (N) was < 50%, the incidence rates of the various diseases remained relatively stable. However, when N reached ≥ 50%, the curves began to exhibit significantly different trends. The neutrophil percentage showed a positive correlation with the incidence of PPE and a negative correlation with the incidence of TPE, while the correlation with MPE incidence was greater in the middle range and slightly diminished on both ends (Fig. 5A-D).
The analysis result of neutrophil percentage. A Relationship between the incidence of different types of PE and the neutrophil percentage (N%). B Relationship between neutrophil percentage (N%) and parapneumonic pleural effusion (PPE). C Relationship between neutrophil percentage (N%) and tuberculous pleural effusion (TPE). D Mann-Whitney rank sum test of parapneumonic pleural effusion (PPE) and lymphocyte percentage (L%), neutrophil percentage (N%)
There were 60 out of 1376 patients with eosinophil proportions of ≥ 10%. Among the eosinophilic effusions, 31 cases were attributed to malignant tumors, while 17 cases were associated with emphysema (including 7 linked to tumors and 2 to tuberculosis). Additionally, there were 7 cases of hydropneumothorax (with 2 related to tumors), 9 cases of tuberculosis (including 3 with concurrent tumors), 7 cases of pleural infection, 2 cases treated surgically, and 1 case of parasitic infection.
The percentage of eosinophils in the effusion fluid was categorized into ten intervals, ranging from 0% ≤ X < 10% to 90% ≤ X < 100%. Thirty patients with tumors (one of whom had hydropneumothorax) exhibited eosinophil proportions ranging from 10 to 43%. Only 4 patients had eosinophil proportions between 50 and 100%, which included 3 patients with hydropneumothorax (one case associated with tumors) and one patient with a parasitic infection. The highest percentage of eosinophils in the effusion reached 94%. Additionally, Charcot-Leyden crystals were also detected (Supplementary Fig. S6).
Detection of rare morphologies and pathogens in clinical practice revealed several significant findings. One case demonstrated simultaneous metastasis of small cell lung cancer and adenocarcinoma to pleural effusion (Supplementary Fig. S7). Additionally, simultaneous infections with Talaromyces marneffei (Supplementary Fig. S8) and Pneumocystis jirovecii (Supplementary Fig. S9) were identified in another case. Bilirubin crystals were observed in 2 cases (Supplementary Fig. S10), while cholesterol crystals were detected in 2 cases (Supplementary Fig. S11). Orange hemoglobin crystals were found in 5 cases (Supplementary Fig. S12). Furthermore, 9 cases of Langhans giant cells (Supplementary Fig. S13) and 11 cases of caseous particles were identified (Supplementary Fig. S14). Seventeen cases of hemosiderin-containing cells were also detected (Supplementary Fig. S15), along with one case of Paragonimus infection.
Discussion
Pleural puncture is a clinical intervention aimed at alleviating dyspnea, which can effectively reduce pleural thickening and improve lung function to some extent.
The British Thoracic Society Pleural Disease Guideline 2010 recommends ultrasound-guided thoracentesis to minimize the incidence of pneumothorax and dry aspiration [4]. Consequently, obtaining pleural effusion fluid is generally easier than acquiring tissue biopsy specimens. The results from routine cytology and morphological examinations can aid clinical staff in narrowing down the diagnostic possibilities while reducing the risks associated with multiple invasive procedures [5].
MPE is a frequent manifestation of disseminated or advanced cancer, primarily associated with lung cancer, breast cancer, and lymphoma. It is estimated that 15% of lung cancer patients present with MPE at diagnosis, and up to 50% develop it during the disease course [2]. Wright-Giemsa staining, a fundamental technique in clinical laboratories, is extensively employed in hematological examinations. Compared to Papanicolaou and hematoxylin-eosin staining, Wright-Giemsa staining offers a faster and more practical method for evaluating pleural fluid morphology. It stains nuclei purplish-red, nucleoli blue, and cytoplasm in varying shades of blue, depending on cellular differentiation. This makes it effective for distinguishing benign from malignant cells and aids in diagnosing primary or metastatic malignancies.
In our analysis of 479 cases of pleural effusion from cancer patients (282 newly diagnosed and 197 previously diagnosed), malignant cells were identified in 295 cases. Of these, 291 cases were clinically confirmed as malignant, yielding a clinical diagnostic concordance rate of 98.6%. Additionally, 91 cases exhibited severe nuclear heterogeneity or suspicious malignant cells (classified as nuclear heterogeneous cells). Among these, 73 cases were clinically diagnosed as malignant, achieving an 80.2% concordance rate. Altogether, abnormal cells were detected in 364 out of 479 cases, resulting in a detection rate of 76.0%. These outcomes align with previously reported findings in the literature [5].
Further analysis of the MPE-newly diagnosed group and the MPE-confirmed group indicated that the percentage of malignant cells was significantly higher in the newly diagnosed group than in the confirmed group, with a statistically significant difference between the two (P < 0.01). Notably, in the MPE-confirmed group, the detection rate of malignant cells was only 6.7% (1/15) among cases complicated by bacterial infections, whereas none of the patients in the newly diagnosed group presented with concurrent bacterial infections.
Chemotherapy, tyrosine kinase inhibitor (TKI), and immunotherapy significantly reduce the number of malignant cells in malignant pleural effusions. Malignant cell morphology may change after treatment, such as nuclear pyknosis, cytoplasmic vacuolation, or apoptosis. These changes may be associated with treatment-induced tumor cell death, differentiation, or immune system activation [8,9,10,11]. Granulocyte infiltration and subsequent lytic activity can also damage tumor cells, contributing to a reduced detection rate of malignant cells. We compared cytological findings at onset and after treatment of MPE in 16 patients, significant changes were observed in the morphology of malignant cells. These included alterations in cell size, cytoplasmic content, nucleus-to-cytoplasm ratio, nuclear shape, nucleoli, chromatin distribution, and cell arrangement. Following treatment, some tumors lost their typical morphological characteristics (Supplementary Fig. S16), making morphological evaluation more challenging. This may account for the reduced positive detection rate observed in the previously confirmed group.
Pleural infection, a frequent complication of pneumonia, is associated with high mortality. Although the bacterial positivity rate in pleural effusion specimens is relatively low, its clinical significance is considerable. In the experimental group, bacteria, fungi, and phagocytes were detected in 3.7% of cases (51/1367) (excluding Mycobacterium tuberculosis). Our laboratory typically uses sterile syringes to inject pleural effusion directly into blood culture bottles for routine cultivation [12, 13]. The cultured bacteria were predominantly Streptococcus pharyngitis, Streptococcus constellatus, and Staphylococcus aureus.
According to research, patients with community-acquired pneumonia are more prone to infections by Streptococcus species and anaerobic bacteria (such as Proteus and Peptostreptococcus), whereas nosocomial infections are more often associated with methicillin-resistant Staphylococcus and gram-negative bacteria, including Enterobacteria [2, 14].
In this experimental group, the bacterial culture positivity rate was 2.6% (62/2405 patients). Although bacterial culture remains the 'gold standard' for identifying pathogens, factors such as the presence of anaerobic or fastidious bacteria, along with interactions between medications or bacterial species, can lower the detection rate. Some researchers recommend multiplex PCR for improved pathogen identification, although this technique is expensive [12, 13, 15].
In our experimental group, the rate of multiple bacterial infections detected by cytology was higher than that identified through culture, with a statistical difference between the two methods (P < 0.01). Compared to the direct smear method, centrifugation concentrates the sample, allowing for uniform distribution during slide preparation, which enhances observation. Wright-Giemsa staining, in addition to its utility for evaluating cell morphology, facilitates the identification of specific bacteria, such as P. jirovecii, which exhibits distinctive morphology with this stain.
Cell morphology test is less time-consuming and fast to report. Gram staining, acid-fast staining and other common laboratory stains can be added according to needs. It can be used as a beneficial supplement to sterile body fluid culture method to help select appropriate culture medium and culture conditions, which is conducive to improving the positive rate of culture. For example, anaerobic infection is inferred from bacterial morphology, additional anaerobic culture tests are recommended, when tuberculosis or non-tuberculosis mycobacteria infection is suspected, Roche medium and liquid medium are used, culture time is extended, and advanced methods such as DNA microarray chip and next generation sequencing are applied. The main task of bacterial culture is to identify bacterial species, and on this basis, further drug sensitivity test, clinical medication guidance. Our experimental results confirm the validity of this comprehensive diagnostic method.
The proportion of benign cells—such as neutrophils, lymphocytes, eosinophils, macrophages, and mesothelial cells—holds diagnostic value in various diseases. Tuberculosis is the most frequent cause of lymphocytic pleural effusion [6]. However, given the low positivity rates in smears and cultures, tuberculosis polymerase chain reaction (TB-PCR) offers high specificity but limited sensitivity [12, 16]. In patients with a history of tuberculosis who present with recurrent pleural effusion but no evidence of pleural tuberculosis infiltration, a lymphocyte ratio ≥ 70% can serve as a diagnostic clue [6, 13, 16, 17].
PPE is the leading cause of elevated neutrophil levels in pleural fluid, typically secondary to bacterial pneumonia, lung abscesses, or bronchiectasis with infection. However, when the neutrophil proportion is ≥ 50%, it not only points to PPE but may also indicate pneumonia or bacterial infections in patients with tumors, early-stage tuberculosis, or tuberculous empyema. In the MPE group, cytological analysis generally reveals a combination of lymphocytes, neutrophils, macrophages, and mesothelial cells.
The overall incidence of pleural infection is increasing, with bacterial pleural effusions accounting for about 5% to 10% of patients with pneumonia and empyema accounting for about 1% to 2% of patients with pneumonia, in a recent randomisedtrial 54% of patients with pleural infection had positive pleuralfluid cultures and 12% positive blood culture results [18]. In non-purulent parapneumonic effusions, the positive rate of microbiologically identified pathogenic organisms is approximately 25 percent [12]. We analyzed the cytological bacterial test results of 1376 patients with neutrophil percentages ranging from 0% ≤ X < 10% to 90% ≤ X < 100% (Table 3, Fig. 6A-B), which are consistent with the positive correlation between neutrophil counts and percentages and bacterial culture positivity rates in pleural effusion literature[19], In our experiment, neutrophil ratios ≥ 80% significantly increased bacterial detection rates.
EPE is commonly defined as pleural fluid containing ≥ 10% eosinophils [20, 21].
In our experimental group, we identified 60 cases of EPE out of 1376 cases of exudative pleural effusion, accounting for 4.4%. Among these, 30 patients had tumors (excluding one with hydropneumothorax) and exhibited eosinophil levels between 10 and 43%, which aligns with most cases of malignant eosinophilia and solid tumors documented in the literature [22,23,24]. Notably, the incidence of malignancy is significantly higher in patients with low eosinophil percentages (< 40%) in their pleural effusion [21, 25].
Additionally, among 4 cases with eosinophil levels ranging from 50 to 94%, 3 were associated with hydropneumothorax (one of whom also had tumors), while 1 patient had a parasitic infection [17, 26]. This finding suggests a potential link between the presence of air and EPE, which may be a critical factor contributing to the elevated eosinophil count [27].
We identified several rare morphologies and pathogens in our study. One case involved simultaneous metastasis of small-cell lung cancer and adenocarcinoma to the pleural effusion. Small cell lung carcinoma (SCLC) is a very aggressive, poorly differentiated, and high-grade neuroendocrine carcinoma representing approximately 13% of all lung carcinomas. The current sub-classification recognizes two subtypes: pure SCLC and combined SCLC (C-SCLC), the latter is determined by the presence of features of a different histological carcinoma, non-small cell lung carcinoma (NSCLC), variant or at least containing 10% of large cell carcinoma component [8, 28, 29]. That the diagnosis rate of C-SCLC is affected by the size and integrity of the biopsy specimen, and the number of pathological sections [30].
But, as a highly aggressive disease, SCLC is found disseminated in the initial clinical presentation in the majority of cases, and surgery is limited to a small subgroup of patients with confined disease [31]. Some studies have shown that up to 28% of surgically resected SCLC tumors are C-SCLC, approximately 1-3% of all lung cancer cases, and of these, 16% belong to the large cell carcinoma variant [32, 33].
NSCLC components in C-SCLC are mainly squamous cell carcinoma and adenocarcinoma, Cimen et al. reported 31 cases of C-SCLC confirmed by bronchoscopy biopsy, with 15 cases diagnosed as small cells + squamous cells, 9 cases diagnosed as large cells + small cells, and 7 cases diagnosed as small cells + adenocarcinoma [34]. The rarity of C-SCLC itself, the limited number of specimens after surgery and the incompleteness of the biopsy tissue of bronchoscope make the specific incidence rate data of C-SCLC lack large-scale research support. Due to limited cellular materials, the detection of C-SCLC in pleural effusion cytology is even rarer.
In another patient with newly diagnosed HIV, a concurrent infection with T. marneffei and P. jirovecii was detected. Cholesterol crystals were observed in 2 patients: one with TPE and the other with MPE [35]. The formation of cholesterol crystals in pleural effusions is often associated with long-standing pleural effusions (e.g., tuberculous pleurisy, rheumatoid pleurisy, or malignancy), and chronic pleural effusions and pleural inflammation may lead to pleural cell necrosis and cholesterol release, in which crystals form. The formation of bilirubin crystals is associated with elevated bilirubin levels due to hepatobiliary disorders, infections, tumors, or other chronic diseases [36], and their production is dependent on the action of enzymes (eg, the process by which hemoglobin is broken down into bilirubin). Bilirubin crystals are usually small, a few microns to a dozen microns, and appear in the form of elongated needles, bundles, or granules. Yellowish-brown or dark yellow, often in bundles or clusters, sometimes radially arranged. We identified bilirubin crystals in the pleural fluid of 2 patients with gastrointestinal perforation complicated by bacterial infection due to esophageal cancer.
Additionally, orange hemoglobin crystals were found in 5 patients, diamond-shaped, needle-shaped, or flaky, golden or orange-yellow, medium-sized, scattered, and sometimes variable in size or clustered. representing iron-free hemoglobin deposits formed due to old bleeding in anaerobic or hypoxic environments [37]. Langhans giant cells are multinucleated giant cells, and caseous necrosis is a form of necrotic tissue with a caseous appearance, both of which are commonly found in granulomatous diseases such as tuberculosis [38, 39]. Langhans giant cells were detected in 9 patients, and caseous particles were identified in 11 patients—both features associated with TPE fluid. Moreover, 17 patients had hemosiderin-laden cells, and one patient presented with a Paragonimus infection. Hemosiderin-laden macrophages are formed by macrophages that engulf and break down red blood cells and contain hemosiderin granules. The presence of hemosiderin cells in the pleural effusion suggests intrapleural bleeding, possibly due to trauma, surgery, vascular rupture, or malignancy such as pleural mesothelioma or lung cancer [10, 40]. Eosinophilia is a typical laboratory finding of lung fluke infection and may be associated with the parasite-induced immune and inflammatory responses [41].
Cytological and morphological tests of humoral fluids hold significant diagnostic value. Following centrifugation and concentration, these tests demonstrate high specificity and reliable performance in distinguishing benign from malignant cells through smear slides and Wright-Giemsa staining. The classification and proportion of benign cells in pleural effusion serve as essential diagnostic indicators for eosinophilic pleural effusion and help guide the diagnostic approach toward tuberculosis- or pneumonia-related effusions.
Spare smears are utilized for various staining techniques, including Gram, acid-fast, Prussian blue, POX, PAS, and AgNOR staining, to detect abnormal components such as bacteria, fungi, parasites, crystals, and atypical cells from lymphohematopoietic tissues. This type of analysis provides valuable guidance for further assessments through flow cytometry, bacterial culture, Pathological analysis, immunohistochemistry and molecular biology research. As a comprehensive, efficient, and cost-effective method, cytological and morphological testing offers numerous morphology-based diagnostic or reference indicators for clinical application.
Availability of data and material
All data generated or analyzed during this study are included in the published article and its supplementary information files. Further enquiries can be directed to the corresponding author.
Abbreviations
- MPE:
-
Malignant pleural effusion
- TPE:
-
Tuberculous pleural effusion
- PPE:
-
Parapneumonic pleural effusion
- EPE:
-
Eosinophilic pleural effusion
- TB-PCR:
-
Tuberculosis polymerase chain reaction
References
Beaudoin S, Gonzalez AV. Evaluation of the patient with pleural effusion. Can Med Assoc J. 2018;190(10):E291–5. https://doi.org/10.1503/cmaj.170420.
Feller-Kopman D, Ingelfinger JR, Light R. Pleural disease. N Engl J Med. 2018;378(8):740–51. https://doi.org/10.1056/NEJMra1403503.
Porcel JM, Light RW. Pleural fluid analysis. Clin Chest Med. 2021;42(4):599–609. https://doi.org/10.1016/j.ccm.2021.07.003.
Hooper C, Lee YCG, Maskell N. Investigation of a unilateral pleural effusion in adults: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii4–17. https://doi.org/10.1136/thx.2010.136978.
Kassirian S, et al. Diagnostic sensitivity of pleural fluid cytology in malignant pleural effusions: systematic review and meta-analysis. Thorax. 2023;78(1):32–40. https://doi.org/10.1136/thoraxjnl-2021-217959.
Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology. 2019;24(10):962–71. https://doi.org/10.1111/resp.13673.
Ferreiro L, San José ME, Valdés L. Manejo del derrame pleural paraneumónico en adultos. Arch Bronconeumol. 2015;51(12):637–46. https://doi.org/10.1016/j.arbres.2015.01.009.
Travis WD, et al. The 2015 world health organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–60. https://doi.org/10.1097/jto.0000000000000630.
Yano S, Zebrowski BK, Liu W, Hicklin DJ, Shaheen RM, Putnam JB Jr, Ellis LM. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res. 1999;5(11):3364–8.
Loddenkemper R, Antony VB, Astoul P, Boutin C, Goldstraw P, Hott J, Rodriguez Panadero F, Sahn SA. Management of malignant pleural effusions. Eur Respir J. 2001. https://doi.org/10.1183/09031936.01.00225601.
Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–26. https://doi.org/10.1038/nrclinonc.2012.71.
Jany B, Welte T. Pleural effusion in adults—etiology, diagnosis, and treatment. Dtsch Ärztebl Int. 2019. https://doi.org/10.3238/arztebl.2019.0377.
Botana Rial M, et al. Diagnosis and treatment of pleural effusion. Recommendations of the Spanish society of pulmonology and thoracic surgery. Update 2022. Arch Bronconeumol. 2023;59(1):27–35. https://doi.org/10.1016/j.arbres.2022.09.017.
Addala DN, Bedawi EO, Rahman NM. Parapneumonic effusion and empyema. Clin Chest Med. 2021;42(4):637–47. https://doi.org/10.1016/j.ccm.2021.08.001.
Franchetti L, Schumann DM, Tamm M, Jahn K, Stolz D. Multiplex bacterial polymerase chain reaction in a cohort of patients with pleural effusion. BMC Infect Dis. 2020. https://doi.org/10.1186/s12879-020-4793-6.
Shaw JA, Koegelenberg CFN. Pleural tuberculosis. Clin Chest Med. 2021;42(4):649–66. https://doi.org/10.1016/j.ccm.2021.08.002.
Ai L, Li J, Ye T, Wang W, Li Y. Exudative pleural effusion caused by lung fluke infection: case report. Int J Infect Dis. 2022;114:175–7. https://doi.org/10.1016/j.ijid.2021.11.005.
Davies HE, Davies RJ, Davies CW. Management of pleural infection in adults: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(2):41–53. https://doi.org/10.1136/thx.2010.137000.
Colice GL, Curtis A, Deslauriers J, Heffner J, Light R, Littenberg B, Weinstein RA, Yusen RD, Panel PE. Medical and surgical treatment of parapneumonic effusions. Chest. 2000;118:1158. https://doi.org/10.1378/chest.118.4.1158.
Mark Adelman MD. Diagnostic utility of pleural fluid eosinophilia. Am J Med. 1984;77:915. https://doi.org/10.1016/0002-9343(84)90542-4.
Takeuchi E, et al. Eosinophilic pleural effusion due to lung cancer has a better prognosis than non-eosinophilic malignant pleural effusion. Cancer Immunol Immunother. 2021;71(2):365–72. https://doi.org/10.1007/s00262-021-02994-5.
Kalomenidis I, Light RW. Eosinophilic pleural effusions. Curr Opin Pulm Med. 2003;9:254. https://doi.org/10.1097/00063198-200307000-00002.
Rubins JB, Rubins HB. Etiology and prognostic significance of eosinophilic pleural effusions: a prospective study. Chest. 1996;110(5):1271–4. https://doi.org/10.1378/chest.110.5.1271.
Oba Y, Abu-Salah T. The prevalence and diagnostic significance of eosinophilic pleural effusions: a meta-analysis and systematic review. Respiration. 2012;83(3):198–208. https://doi.org/10.1159/000327200.
Krenke R, et al. Incidence and aetiology of eosinophilic pleural effusion. Eur Respir J. 2009;34(5):1111–7. https://doi.org/10.1183/09031936.00197708.
Wang H, et al. Exudative pleural effusion caused by lung fluke infection: a practical diagnostic approach. Int J Infect Dis. 2023;135:8–11. https://doi.org/10.1016/j.ijid.2023.07.013.
Kalomenidis I, et al. Pneumothorax-associated pleural eosinophilia is tumour necrosis factor-alpha-dependent and attenuated by steroids. Respirology. 2007;13(1):73–8. https://doi.org/10.1111/j.1440-1843.2007.01153.x.
Metovic J, et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021;478(1):5–19. https://doi.org/10.1007/s00428-020-03015-z.
Raso MG, Bota-Rabassedas N, Wistuba II. Pathology and classification of SCLC. Cancers. 2021;13(4):820. https://doi.org/10.3390/cancers13040820.
Fraire AE, Johnson EH, Yesner R, Zhang XB, Spjut HJ, Greenberg SD. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol. 1992;23(5):520–8. https://doi.org/10.1016/0046-8177(92)90129-q.
Hamilton G, Rath B. Mesenchymal-epithelial transition and circulating tumor cells in small cell lung cancer. In: Isolation and molecular characterization of circulating tumor cells, (advances in experimental medicine and biology, chapter 12). Cham: Springer; 2017. p. 229–45.
Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, Falk R, Travis WD. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26(9):1184–97. https://doi.org/10.1097/00000478-200209000-00009.
Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25:S18–30. https://doi.org/10.1038/modpathol.2011.150.
Çimen F, Düzgün S, Atikcan S. Combined SCLC clinical and pathological aspects. Monaldi Arch Chest Dis. 2022. https://doi.org/10.4081/monaldi.2022.2226.
Braun CM, Ryu JH. Chylothorax and pseudochylothorax. Clin Chest Med. 2021;42(4):667–75. https://doi.org/10.1016/j.ccm.2021.08.003.
Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology. 2007;13(1):5–20. https://doi.org/10.1111/j.1440-1843.2007.01154.x.
Marver HS, Tenhunen R, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968. https://doi.org/10.1073/pnas.61.2.748.
Light RW. Pleural diseases. Curr Opin Pulm Med. 2003;9(4):251–3. https://doi.org/10.1097/00063198-200307000-00001.
Sharma SK, Mohan A. Tuberculosis: from an incurable scourge to a curable disease–journey over a millennium. Indian J Med Res. 2013;137(3):455–93.
Sahn SA. The differential diagnosis of pleural effusions. West J Med. 1982;137(2):99–108.
Martínez-García MA, Cases-Viedma E, Cordero-Rodríguez PJ, et al. Diagnostic utility of eosinophils in the pleural fluid. Eur Respir J. 2000;15(1):166–9. https://doi.org/10.1183/09031936.00.15116600.
Acknowledgements
We would like to express our gratitude to Daoyin Zhou and Chaoqun Liu for their proposed improvements of the manuscript.
Funding
This work was supported by the 2021 Guiding Science and Technology Program in the Medical and Health Field of the Quanzhou Municipal Health Commission, Quanzhou Science and Technology Bureau (No. 2021N043S).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Xiaoting Chen, Hongyan Wang Acquisition of data: Yongyu Li, Kaizhen Wen Analysis and interpretation of the data: Hongyan Wang, Yongyu Li Statistical analysis: Yongyu Li, Hongyan Wang Obtaining financing: Xiaoting Chen, Kaizhen Wen Writing of the manuscript: Xiaoting Chen, Hongyan Wang Critical revision of the manuscript for intellectual content: Kaizhen Wen, Xiaoting Chen All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki(as was revised in 2013). The study was approved by Ethics Committee of the Jinjiang Municipal Hospital (No.JJSYY2021N003S). Written informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Li, Y., Wang, H. et al. Evaluation of cytomorphological examination in the diagnosis of pleural effusion. Clin Exp Med 25, 112 (2025). https://doi.org/10.1007/s10238-025-01642-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-025-01642-x