Abstract
Purpose
The characteristics of patients with bilateral and unilateral breast cancer at the time of diagnosis or during follow-up have been compared, focusing on the differences in disease-free survival and overall survival between these groups.
Methods
A total of 1,947 patients diagnosed with invasive carcinoma were included in the study. 1876 (96.4%) of our patients had unilateral and 71 (3.6%) had bilateral breast cancer. Among the bilateral breast cancer patients n = 47 were metachronous, while n = 24 were synchronous.
Results
SBBC, which had the lowest OS duration, showed a statistically significant difference compared to MBCC, similar to that observed in unilateral breast cancer (p = 0.027).
Conclusion
The fact that SBBC has the lowest survival rate despite more aggressive treatments should be considered a poor prognostic factor for survival on its own.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Breast cancer screening programs with mammography have led to an increase not only in the incidence of unilateral breast cancer but also in bilateral breast cancer. This is evident from the trend in the literature showing a rise in bilateral breast cancer incidence over the years, ranging from 1.4% to 21% [1,2,3,4]. Bilateral breast cancer can present in two ways: as synchronous bilateral breast cancer (SBBC) when diagnosed at the same time as the first tumor or within 3, 6, or 12 months after the initial diagnosis, or as metachronous bilateral breast cancer (MBBC) if diagnosed after a longer period [5,6,7,8]. Different time criteria are reported in the literature on this subject. MBBC is more common than SBBC, the incidence of SBBC accounts for 1% to 3% of all breast cancer cases [2, 3].
Although there are studies in the literature investigating the clinicopathological features that predispose to bilateral breast cancer and the effects of these features on disease-free survival and overall survival, their numbers are quite limited [4, 6, 7]. There are also conflicting data regarding whether the development of bilateral breast cancer affects prognostic outcomes [2]. While some series have analyzed that bilaterality negatively impacts overall and disease-free survival [4,5,6,7,8], others have found no significant difference [3, 9, 10]. The effect of bilaterality on survival remains uncertain. Additionally, SBBC (synchronous bilateral breast cancer) is often associated with larger tumor sizes and worse histopathological features. However, some studies in literature report findings that contradict these associations, highlighting ongoing disagreements and inconsistencies in this area of research. However, the increase in the incidence of bilateral breast cancer in recent years will help to conduct more clinical studies on the subject, which will help to clarify its prognostic significance and treatment protocols [2,3,4, 10,11,12].
We aim to compare the prognostic factors and survival time of bilateral and unilateral breast cancer patients in our series. To achieve this, we used propensity score analysis to balance the groups in terms of number and prognosis. We also analyzed the prognostic impact of bilaterality and patient or treatment characteristics affecting prognosis.
Materials and methods
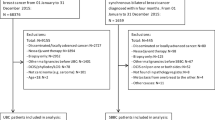
The Human Research Ethics Committee of Trakya University Faculty of Medicine approved the use of patients’ information for the study (TUTF-GOBAEK 2020/334). To use the relevant information, informed consent forms were obtained from patients or the relatives of deceased patients in accordance with the Helsinki Declaration [13]. Following the approval of the institutional review board, the records of 2,086 breast cancer patients who applied to the Departments of Radiation Oncology and Medical Oncology at Trakya University Faculty of Medicine between July 1999 and December 2019 were examined. Data from 127 patients with in situ ductal carcinoma and 12 men among the unilateral breast cancer patients were excluded. A total of 1,947 patients with a diagnosis of invasive carcinoma were included in the study. The following parameters were analyzed for these cases: gender, age, family history, menopausal status, histological types, the location of the tumor at the time of diagnosis, whether bilateral breast cancer was metachronous or synchronous, clinical and pathological TNM stages, grade mentioned in pathology reports, lymphovascular invasion, perineural invasion, skin involvement, surgical margin status, hormone receptors, Ki-67 index, status of extracapsular invasion, type and date of surgery, axillary approach, type of chemotherapy, use of hormone therapy, whether they received radiotherapy, and the type of radiotherapy received. The staging was performed according to AJCC 2017 guidelines [14].
The characteristics of patients with bilateral and unilateral breast cancer at the time of diagnosis or during follow-up have been compared, focusing on the differences in disease-free survival and overall survival between these groups. Prognostic factors contributing to this difference have been investigated.
Statistical analysis
The statistical analysis of the findings obtained in the study was performed using the SPSS (Statistical Package for Social Sciences) 20.0 program. Numerical results were expressed as mean ± standard deviation, while categorical results were presented as "n". Normality distribution of the numeric variables were tested by the Shapiro-Wilks test. The data were divided into two groups, unilateral and bilateral. Comparisons between the two groups were made using the Student’s T-test for continuous variables and the Pearson Chi-Square test or Fisher’s Exact test for categorical variables. A logistic regression model was used to analyze risk factors associated with bilateral breast cancer. Propensity score matching (PSM) was performed between the unilateral and bilateral breast cancer groups using an optimal matching algorithm (optimum, 1:1) with variables such as axillary nodal status, subgroup, ER status, HER-2 status, and surgical type [15, 16]. Variables that showed significant differences between the two groups or were clinically significant as determined by the Pearson Chi-Square test were used to create propensity scores. After PSM, DFS and OS were calculated using the Kaplan–Meier method. Survival curves were generated using the Kaplan–Meier method, and the significance of survival differences between selected variables was compared using the log-rank test [17]. Univariate Cox regression analysis was used to estimate hazard ratios. Then, multivariate Cox regression analysis with a backward stepwise method was performed to estimate hazard ratios and identify independent prognostic factors [18]. All reported p-values were two-sided, and p-values below 0.05 were considered significant.
Results
In our study, 1947 patients diagnosed with invasive breast cancer, who received treatment and had follow-up, were included. The median follow-up period was 77.3 months. Of the 1947 patients whose records we reviewed, 1876 (96.4%) had unilateral breast cancer, and 71 (3.6%) had bilateral breast cancer. Among the bilateral breast cancer patients, 66.2% (n = 47) were metachronous, and 33.8% (n = 24) were synchronous. There were no male patients in the bilateral breast cancer group. The average age in the unilateral group was 52 years, while in the bilateral group, it was 52.8 years. A family history of breast cancer was present in 31.5% (n = 595) of the patients in the unilateral group and 32.3% (n = 23) in the bilateral group (Table 1).
When disease-free survival (DFS) and overall survival (OS) times were calculated for breast cancer patients in our series based on whether they were in the unilateral or bilateral group, the DFS times were 220.25 ± 4.77 months in the unilateral group and 168.52 ± 13.92 months in the bilateral group (p = 0.022). The OS times were 228.37 ± 3.89 months in the unilateral group and 213.41 ± 11.80 months in the bilateral group (p = 0.859) (Fig. 1a, 1b).
When examining patient characteristics in Table 1, we recalculated the DFS and OS durations using propensity scoring to balance the statistically significant (p < 0.05) criteria identified between the two groups. The factors that showed statistical differences or were close to significance among the unilateral and bilateral breast cancer patients in our series were identified as axillary nodal status, subgroup, estrogen receptor (ER) status, Her-2 expression status, axillary surgery, and chemotherapy type. In the propensity score statistical analysis of our series, balance was achieved based on these six different characteristics. A statistical analysis was performed using data from 71 patients with bilateral breast cancer compared to 71 patients with unilateral breast cancer. After the propensity score analysis, the patient characteristics are again shown in Table 1.
After the propensity score analysis, the DFS and OS durations calculated using the Kaplan–Meier method for patients with unilateral and bilateral breast cancer were as follows: The DFS duration was 132.24 ± 13.86 months (105.07–159.42 months) in the unilateral group and 166.86 ± 13.70 months (140.0–193.72 months) in the bilateral group (HR: 0.769, 95% CI 0.418–1.413, p = 0.397). The OS duration was 170.48 ± 14.22 months (142.61–198.36 months) in the unilateral group and 211.57 ± 11.62 months (188.78–234.35 months) in the bilateral group (HR: 0.472, 95% CI 0.232–0.963, p = 0.039) (Fig. 2a, 2b).
After the propensity score balancing for patients with bilateral breast cancer, when examining the patient characteristics, we found that the following factors were statistically significantly different compared to unilateral breast cancer: a higher T stage of the tumor (p < 0.001), a greater proportion of skin involvement (p = 0.044), a lower rate of PR positivity (p = 0.011), a lower frequency of axillary surgery being axillary curettage (p < 0.001), a higher proportion of chemotherapy being adjuvant chemotherapy (p = 0.005), a greater use of FAC/FEC/TAC chemotherapy regimens (p = 0.018), a lower rate of tamoxifen usage (p = 0.039), and a higher frequency of radiotherapy application (p = 0.034) (Table 1). We separated patients with bilateral breast cancer into metachronous and synchronous groups to calculate their survival durations. The DFS durations were as follows: synchronous 132.77 ± 30.76 months (72.4–193.0 months), metachronous 170.74 ± 14.59 months (142.14–199.33 months) (p = 0.143). The overall survival durations were: synchronous 164.86 ± 30.65 months (104.78–224.94 months), metachronous 215.04 ± 10.66 months (194.13–235.94 months) (p = 0.027) (Fig. 3a, 3b).
We also examined the clinical, histopathological, and treatment characteristics of patients with metachronous and synchronous bilateral breast cancer (Table 2). Among patients with bilateral breast cancer, the number of patients with metachronous bilateral breast cancer was n = 47 (66.2%), while the number of patients with synchronous bilateral breast cancer was n = 24 (33.8%). In the bilateral breast cancer group, the most common pathology was invasive ductal carcinoma (IDC) at a rate of 61.4% (n = 43). When comparing the first tumors of metachronous and synchronous cases, there was a statistically significant difference in the N stage, with synchronous breast cancer having a more advanced N stage (p = 0.039) and a higher rate of positive surgical margins (p = 0.007). When comparing the second tumors of metachronous and synchronous cases, a statistically significant difference was found only in the higher rate of PR positivity in synchronous breast cancer (p = 0.009). Due to synchronous bilateral breast cancer having a lower survival duration within the bilateral breast cancer group, in the final step, we divided our series into three groups for comparison of survival durations: unilateral breast cancer, metachronous bilateral breast cancer, and synchronous bilateral breast cancer (Table 3). In our series, the DFS duration was 132.24 ± 13.86 months for unilateral breast cancer, 170.74 ± 14.59 months for metachronous bilateral breast cancer (p = 0.257), and 132.77 ± 30.76 months for synchronous bilateral breast cancer (p = 0.770). The OS duration was 170.48 ± 14.22 months for unilateral breast cancer, while a statistically significant difference was found in metachronous bilateral breast cancer at 215.04 ± 10.66 months (p = 0.015). When comparing unilateral breast cancer with synchronous bilateral breast cancer, the difference disappeared with a duration of 164.86 ± 30.65 months (p = 0.872) (Fig. 4a, 4b). The synchronous bilateral breast cancer (SBBC), which had the lowest OS duration, showed a statistically significant difference compared to metachronous bilateral breast cancer (MBCC), similar to that observed in unilateral breast cancer (p = 0.027) (Table 3).
When conducting univariate and multivariate Cox regression analyses to identify risk factors affecting DFS and OS, we identified 12 possible risk factors with p < 0.1 associated with DFS: being T4, N2 and N3 status, high-grade III (HG III), positive lymphovascular invasion (LVI), presence of skin infiltration, presence of extensive intraductal component (EIC), being ER and PR positive, type of surgery performed on the breast, timing of chemotherapy, and type of radiotherapy. In the multivariate backward stepwise Cox regression analysis considering these risk factors, the following were found to be significant for DFS: T4 stage (4.3 times), N3 stage (6.3 times), being PR positive (3.9 times).
In the univariate Cox regression analysis for OS, 10 potential risk factors (metachronous bilateral breast cancer, age, menopausal status, T4 stage, N2 and N3 stage, LVI positivity, positive skin infiltration, presence of EIC, PR positivity, type of surgery) were identified with p < 0.1. In the multivariate backward stepwise Cox regression analysis with these risk factors: N3 stage (8.1 times), presence of skin involvement (5.7 times), and ER status (2.3 times) were found to be statistically significant. When we focused on the primary objective of our study, which was the effect of bilaterality on survival, BL status did not affect disease-free survival. The presence of metachronous bilateral breast cancer reduced the risk of overall survival by 0.413 times (95% CI: 0.155 – 1.100) compared to unilateral breast cancer (p = 0.077). On the other hand, the presence of synchronous bilateral breast cancer increased the risk of OS by 2.458 times (95% CI: 0.745 – 8.110) (p = 0.140) (Table 4, Table 5).
Discussion
The rate of bilateral breast cancer in our series was 3.6%, with MBBC constituting 2.4% and SBBC 1.2%. Our incidence of bilateral breast cancer, particularly SBBC, was comparable to those reported in the Western series [7,8,9]. Regarding synchronous bilateral breast cancer, DFS duration was similar to MBBC and unilateral breast cancer, while it was the breast cancer subtype with the shortest overall survival time. In our study, patients with metachronous bilateral breast cancer (MBBC) had a statistically significantly longer survival time compared to those with unilateral breast cancer. The overall survival (OS) durations in our series ranked as follows: MBBC had the longest survival, followed by unilateral breast cancer, and then synchronous bilateral breast cancer (SBBC). Additionally, there was a statistically significant difference in survival time between SBBC and MBBC (p = 0.027). Consequently, our findings did not support previous reports suggesting that bilateral breast cancer has a similar or better survival time compared to unilateral breast cancer [2, 9].
In the histopathological, clinical, and treatment characteristics of the patients in our series, after propensity score analysis, it was found that unilateral BC had a more advanced tumor stage (p < 0.001), a higher rate of skin involvement (p = 0.044), leading to a higher rate of neoadjuvant chemotherapy (p = 0.005), and a higher rate of receiving radiotherapy (p = 0.034). In the bilateral BC group, the statistically significant differences included a higher rate of PR positivity (p = 0.011), a higher rate of axillary curettage (p < 0.001), and a greater use of tamoxifen (p = 0.039). In the comparison of the primary tumors of patients with BBC, categorized as MBBC and SBBC, we observed a more advanced N stage in metachronous BBC (p = 0.030), a higher rate of positive surgical margins in SBBC (p = 0.007), and a higher rate of progesterone receptor (PR) positivity in the secondary tumors of SBBC (p = 0.009). Despite the more advanced N stage in MBBC, the longest survival time was observed. At the same time, SBBC had the shortest survival time, which was notable for its association with positive surgical margins and higher PR positivity. It was particularly challenging to explain the lowest survival rate observed in SBBC based on patient and treatment characteristics. A study by Kwast et al. suggested that SBBC might have an undiscovered genetic mutation, which could be significant in the cancer’s different progression. They reported that SBBC’s tendency toward tertiary cancers outside the breast could indicate that it is a distinct subtype of cancer [19].
In the literature review, studies supporting the results of our series [4, 7, 8] also reported that the overall survival (OS) time in patients with SBBC is shorter. As Holm M. et al. stated in their meta-analysis, SBBC is associated with the lowest OS, and they propose that, even though these patients may have smaller and less aggressive tumors, the presence of SBBC should be considered a poor prognostic factor [7, 8]. Additionally, Jobsen JJ et al. noted that synchronous tumors are less frequently observed. While this rate may increase with modern imaging techniques, the preference of patients with unilateral BC for bilateral mastectomy as a surgical option may reduce this likelihood [8].
In our series, while the bilateral status did not affect DFS in the multivariate Cox regression analysis when bilateral cancers were collectively compared to unilateral breast cancer for OS, it had a risk-reducing effect (by 2.1 times). When the same analysis was conducted by comparing MBBC and unilateral BC, we observed that the risk of death was reduced by 2.8 times, which was close to statistical significance. However, when SBBC was compared to unilateral BC, the risk of death increased by 2.5 times, although this increase was not statistically significant. The much better survival observed in metachronous breast cancer compared to unilateral breast cancer can be attributed to the fact that these patients are already under follow-up and, with the diagnosis of the secondary tumor, they re-enter a strict follow-up process.
In our series, risk factors affecting DFS (T4 stage, N3 stage, being PR positive) and OS (N3 stage, skin involvement, being ER positive) were identified through multivariate Cox regression analysis. However, risk factors for BBC mentioned in the literature, such as a family history of breast cancer, young age at first diagnosis, and having lobular carcinoma, were not identified as risk factors in our series. Another risk factor mentioned in the literature is the increased risk of developing BBC in patients with germline mutations in the BRCA1 or BRCA2 genes [2, 7, 20]. In our series, a germline mutation in the BRCA1 or BRCA2 genes could not be statistically analyzed due to the limited number of patients tested for it. As noted by Mruthyunjayappa S. and colleagues, since the prevalence of BRCA1 and BRCA2 mutations is very low and the tendency for prophylactic bilateral mastectomy in healthy women and prophylactic contralateral mastectomy in women with UBC is increasing, most BBC patients do not carry these mutations [2, 9].
When examining Figs. 3a and 3b in our series, 75% of SBBC patients experienced recurrence/metastasis in a shorter period, and 62.5% of the patients were lost within the first 5 years. The finding that “bilaterality has no negative effect on survival.” As mentioned in studies where SBBC and MBBC are evaluated together [2, 9, 10, 19], was not surprising. Perhaps the fact that we are dealing with a subgroup with a lower incidence compared to unilateral breast cancer may have caused an imbalance in the comparisons. However, it is possible to say that propensity score analysis, a statistical method, is a golden statistical analysis method designed precisely for the evaluation of groups with numerical imbalances, such as in this case [15, 16].
There are currently no standard treatment guidelines for BBC treatment. Therefore, developing a personalized treatment plan is done by considering the patient’s age, histological subtype of the tumor, and tumor stage. Although the incidence of BBC differs between Western and Eastern countries, we see that treatment strategies, especially surgical approach options, are similar. Chen, JJ et al. [21, 22] reported that bilateral mastectomy was the primary surgical treatment option, particularly for SBBC patients, which was consistent with the findings in our series. Similarly, in the study by Beckmann et al., the number of patients who underwent mastectomy and ALND in BBC was higher, while the number of patients receiving RT was lower [23, 24]. In our study, while the surgical findings were similar, the number of patients receiving RT was higher.
In our study, the rate of axillary lymph node dissection (ALND) was 45.1% for unilateral breast cancer and 85.9% for bilateral breast cancer. More aggressive surgical approaches were preferred in the bilateral breast cancer group in terms of both local and axillary surgery. However, when comparing the T stage and N staging of unilateral breast cancer patients to bilateral breast cancer patients after propensity score analysis, the unilateral breast cancer group was found to be at a more advanced stage (Table 1). For this reason, the neoadjuvant chemotherapy option was more frequently preferred in patients with unilateral breast cancer (23.9% in unilateral breast cancer, 11.3% in bilateral breast cancer, p = 0.005).
In the series by Jia H. et al., the luminal B type was the most observed group in the bilateral breast cancer group [3]. In our series, Luminal B was also the most frequently seen subtype. Similarly, in the study by Kollias et al., which had results like ours, high-grade tumors constituted 29% of metachronous tumors, while they made up 45% and 54% of unilateral and synchronous tumors, respectively. In SBBC, tumors with a diameter ≥ 2 cm had lymph node involvement [12]. In our series, the rate of grade III was 39.4% for MBBC, 47.9% for unilateral BC, and 41.7% for SBBC. Notably, the rate of positive surgical margins in our series was higher in patients with synchronous breast cancer. This finding is significant because a considerable number of patients chose mastectomy, and both breasts were operated on simultaneously. This approach aimed to minimize surgical morbidity for both the surgeon and the patient, as well as to prevent delays in the initiation of adjuvant therapy.
Although international evidence regarding the prognostic significance of synchronous bilateral breast cancer is inconsistent, survival rates for these patients are generally found to be similar to or worse than those for unilateral breast cancer [7, 8, 23,24,25,26,27]. To better understand the reasons for the poor prognosis, researchers propose that undiscovered genetic factors may contribute, acknowledging that the low incidence rate of approximately 1% may prevent large-scale studies or randomized trials. Consequently, future research should focus on exploring these potential genetic factors [19].
Despite the small number of patients, when matched with propensity score analysis, the patient group with the lowest overall survival was the synchronous bilateral breast cancer group. Due to the wide variability in clinical and histopathological features and the time elapsed between the diagnosis of the first and second tumors [28], it has not been possible to conduct a risk analysis for synchronous bilateral breast cancer. Therefore, meticulous planning and modification of surgical and adjuvant treatment for patients with synchronous bilateral breast cancer is crucial. The discovery of genetic factors that may be identified in the coming years will also be particularly important for this patient group.
The limiting factors in our study were the retrospective nature of our series and the lack of information on BRCA-1 and BRCA-2 mutations in our patients. Although there are studies suggesting that survival outcomes for breast cancer patients with BRCA-1 or BRCA-2 mutations may be worse, similar, or better compared to non-carriers, none of the patients in our study had mutation status available for propensity score analysis, and therefore, we could not include this information in our analysis. In studies analyzing patients with BRCA1 and BRCA2 mutations, Lee et al. found that BRCA1 mutations decreased overall survival (OS) and progression-free survival (PFS), while BRCA2 mutations did not. On the other hand, Zhong et al. suggested that BRCA1 mutations were associated with worse OS, but not with PFS, and that BRCA2 mutations were not linked to either worse OS or PFS. They noted that the contradictory nature of these findings was likely due to limited statistical power [29,30,31]. However, in recent years, particularly with younger patients, we have been able to identify individuals with BRCA1 and BRCA2 mutations and have had the opportunity to provide genetic counseling, similar to the study by Liu et al. [32]. Therefore, we believe that future studies will allow us to share this information as well.
Conclusion
The fact that SBBC has the lowest survival rate despite more aggressive treatments should be considered a poor prognostic factor for survival on its own. Current treatment guidelines are insufficient for SBBC due to characteristics that remain unknown to us. Therefore, SBBC should be evaluated as a separate subgroup. The time elapsed from the initial diagnosis to the diagnosis of secondary breast cancer (0–12 months) needs clarification regarding which subgroup the BCC belongs to. This clarity in timing will facilitate determining the treatment and follow-up process for MBBC and SBCC. Since a common language will emerge in future studies, the similarities and differences of the series will be better understood.
Data Availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33
Mruthyunjayappa S, Zhang K, Zhang L, Eltoum IA, Siegal GP, Wei S (2019) Synchronous and metachronous bilateral breast cancer: clinicopathologic characteristics and prognostic outcomes. Hum Pathol 92:1–9
Jia H, Zheng Y, Wang P, Wei Z, Li X, Fu G et al (2022) A retrospective study on the clinicopathologic characteristics and outcomes of 179 cases of synchronous and metachronous bilateral breast cancer in China. Clin Breast Cancer 22(3):e341–e349
Pan B, Xu Y, Zhou YD, Yao R, Wu HW, Zhu QL, Wang CJ, Mao F, Lin Y, Shen SJ, Sun Q (2019) The prognostic comparison among unilateral, bilateral, synchronous bilateral, and metachronous bilateral breast cancer: A meta-analysis of studies from recent decade (2008–2018). Cancer Med 8(6):2908–2918. https://doi.org/10.1002/cam4.2198
Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO et al (2007) Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol 25(27):4210–4216
Shi YX, Xia Q, Peng RJ, Yuan ZY, Wang SS, An X et al (2012) Comparison of clinicopathological characteristics and prognoses between bilateral and unilateral breast cancer. J Cancer Res Clin Oncol 138(4):705–714
Holm M, Tjønneland A, Balslev E, Kroman N (2014) Prognosis of synchronous bilateral breast cancer: a review and meta-analysis of observational studies. Breast Cancer Res Treat 146(3):461–475. https://doi.org/10.1007/s10549-014-3045-0
Jobsen JJ, van der Palen J, Ong F, Riemersma S, Struikmans H (2015) Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res Treat 153(2):277–283
Verkooijen HM, Chatelain V, Fioretta G, Vlastos G, Rapiti E, Sappino AP et al (2007) Survival after bilateral breast cancer: results from a population-based study. Breast Cancer Res Treat 105(3):347–357
Ozturk A, Alco G, Sarsenov D, Ilgun S, Ordu C, Koksal U et al (2018) Synchronous and metachronous bilateral breast cancer: A long-term experience. J BUON 23(6):1591–1600
Beinart G, Gonzalez-Angulo AM, Broglio K, Mejia J, Ruggeri A, Mininberg E et al (2007) Clinical course of 771 patients with bilateral breast cancer: characteristics associated with overall and recurrence-free survival. Clin Breast Cancer 7(11):867–874
Kollias J, Ellis IO, Elston CW, Blamey RW (2001) Prognostic significance of synchronous and metachronous bilateral breast cancer. World J Surg 25(9):1117–1124
World Medical A (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Lee SB, Sohn G, Kim J, et al. (2018). A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging system for breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-018-4682-5.
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424
Staffa SJ, Zurakowski D (2018) Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth Analg 127(4):1066–1073. https://doi.org/10.1213/ANE.0000000000002787
Bewick V, Cheek L, Ball J (2004) Statistics review 12: survival analysis. Crit Care 8(5):389–394
Bradburn MJ, Clark TG, Love SB, Altman DG (2003) Survival analysis part II: multivariate data analysis–an introduction to concepts and methods. Br J Cancer 89(3):431–436
Kwast AB, Liu L, Roukema JA, Voogd AC, Jobsen JJ, Coebergh JW, Soerjomataram I, Siesling S (2012) Increased risks of third primary cancers of non-breast origin among women with bilateral breast cancer. Br J Cancer 107(3):549–555. https://doi.org/10.1038/bjc.2012.270
Haffty BG, Harrold E, Khan AJ et al (2002) Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 359:1471–1477
Chen J-j, Huang N-s, Xue J-y, Quan C-l, Tan Y-l, Liu G-y et al (2015) Surgical management for early-stage bilateral breast cancer patients in China. PLoS ONE 10(4):e0122692. https://doi.org/10.1371/journal.pone.0122692
Kuo WH, Yen AM, Lee PH, Hou MF, Chen SC, Chen KM et al (2006) Incidence and risk factors associated with bilateral breast cancer in area with early age diagnosis but low incidence of primary breast cancer: analysis of 10-year longitudinal cohort in Taiwan. Breast Cancer Res Treat 99(2):221–228
Beckmann KR, Buckingham J, Craft P, Dahlstrom JE, Zhang Y, Roder D et al (2011) Clinical characteristics and outcomes of bilateral breast cancer in an Australian cohort. Breast 20(2):158–164
Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH (2003) Synchronous, bilateral breast cancer: prognostic value and incidence. Breast 12(2):83–88
Carmichael AR, Bendall S, Lockerbie L, Prescott R, Bates T (2002) The long-term outcome of synchronous bilateral breast cancer is worse than metachronous or unilateral tumours. Eur J Surg Oncol 28(4):388–391
Wang T, Liu H, Chen KX, Xun P, Li HX, Tang SC (2011) The risk factors and prognosis of bilateral primary breast cancer: a comparative study with unilateral breast cancer. Oncol Res 19(3–4):171–178
Heron DE, Komarnicky LT, Hyslop T, Schwartz GF, Mansfield CM (2000) Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 88(12):2739–2750
Holmberg L, Adami HO, Ekbom A, Bergstrom R, Sandstrom A, Lindgren A (1988) Prognosis in bilateral breast cancer. Effects of time interval between first and second primary tumours. Br J Cancer 58(2):191–194
Zhu Y, Wu J, Zhang C, Sun S, Zhang J, Liu W, Huang J, Zhang Z (2016) BRCA mutations and survival in breast cancer an updated systematic review and meta-analysis. Oncotarget 7(43):70113–70127. https://doi.org/10.18632/oncotarget.12158
Lee E-H, Park SK, Park B, Kim S-W, Lee MH, Ahn SH, Son BH, Yoo K-Y, Kang D, Group KR (2010) Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival a systematic review and meta-analysis. Breast Cancer Res Treat 122:11–25
Zhong Q, Peng H-L, Zhao X, Zhang L, Hwang W-T (2015) Effects of BRCA1-and BRCA2-related mutations on ovarian and breast cancer survival. a meta-analysis. Clin Cancer Res 21:211–220
Liu J, Ho PJ, Tan THL, Yeoh YS, Chew YJ, Mohamed Riza NK, Khng AJ, Goh SA, Wang Y, Oh HB, et al. (2022) Chin CH, Kwek SC, Zhang ZP, Ong DLS, Quek ST, Tan CC, Wee HL, Li J, Iau PTC, Hartman M. BREAst screening Tailored for HEr (BREATHE)-A study protocol on personalised risk-based breast cancer screening programme. PLoS One. 17(3): e0265965. https://doi.org/10.1371/journal.pone.0265965.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
T.O and R.C. wrote the main manuscript text and worked as data collector, project administration. N.S. did formal analysis. S.A., M.H.D., Y.K., S.K, M.C, G.Y did data curation. N.T., M.B.H., E.T., S.T.,D.N. and S.P. contributed to the methodology.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozler, T., Cosar, R., Sut, N. et al. Comparison of survival between unilateral and bilateral breast cancers using propensity score matching: a retrospective single-center analysis. Breast Cancer Res Treat 210, 673–686 (2025). https://doi.org/10.1007/s10549-024-07606-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07606-1