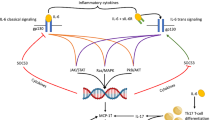
Abstract
The mechanisms underlying the formation and rupture of intracranial aneurysms remain unclear. Rupture of the aneurysmal wall causes subarachnoid hemorrhage, with a mortality rate of 35–50%. Literature suggests that rupture is associated with the remodeling of the aneurysmal wall, including endothelial cell damage, smooth muscle cells (SMCs) proliferation, and inflammatory cell infiltration, particularly macrophages. Transforming growth factor β (TGF-β) is a multifunctional factor that plays a diverse role in cell growth and differentiation. It is crucial for strengthening vessel walls during angiogenesis and also regulates the proliferation of SMCs, indicating the potential involvement of TGF-β signaling in the pathogenesis and development of cerebral aneurysms. This review examines the complex role of TGF-β, its receptors, and signaling pathways in cerebral aneurysm formation and progression. Understanding the molecular mechanisms of TGF-β signaling in aneurysm development is vital for identifying potential therapeutic targets to prevent aneurysm rupture. Further research is necessary to fully elucidate the role of TGF-β in aneurysm pathophysiology, which could lead to the development of novel therapeutic strategies for aneurysm prevention and management, particularly in preventing subarachnoid hemorrhage.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The walls of intracranial arteries differ structurally from other arteries and are additionally immersed in cerebrospinal fluid, which may make them more prone to aneurysm formation (Etminan and Rinkel 2016). Brain aneurysms most commonly form in the anterior part of the circle of Willis, at the branching points of arteries in the cerebral circulation. At these locations, there is a change in blood flow and an increase in hemodynamic stress; additionally, the vessel walls are naturally thinner and less elastic (Hashimoto et al. 2006; Signorelli et al. 2018). Rupture of a cerebral aneurysm wall causes a subarachnoid hemorrhage, which leads to death in 35–50% of cases (Østbye et al. 1997; Ingall et al. 2000). Frösen et al. (2004) demonstrated that aneurysm rupture is associated with remodeling of its wall, resulting from endothelial cells (ECs) damage, smooth muscle cells (SMCs) proliferation, and infiltration of inflammatory cells, especially macrophages (Frösen et al. 2006). Macrophages secrete metalloproteinases (MMPs), such as MMP-2 and MMP-9, which degrade the extracellular matrix in the wall of the intracranial aneurysm, potentially leading to its rupture. Additionally, they may release cytokines and chemokines that recruit other inflammatory cells, such as lymphocytes, neutrophils, and mast cells, thereby intensifying the chronic inflammatory response (Chalouhi et al. 2013; Signorelli et al. 2018). Proliferation and migration of cells lead to myointimal hyperplasia, which is a repair and adaptation mechanism of healthy arterial walls (Intengan and Schiffrin 2001). However, in the case of aneurysms, this process contributes to their formation (Frösen et al. 2006).
In recent years, the TGF-β signaling pathway has gained particular attention because it regulates a wide range of cellular processes (Moustakas et al. 2002; Morikawa et al. 2016). TGF-β is essential for maintaining the integrity and function of blood vessels and plays a key role as a molecular factor fortifying the vessel wall during angiogenesis (Li et al. 2006; Xu et al. 2019). In pathological conditions, its significance has been shown in atherosclerosis and heart attack (Hanna and Frangogiannis 2019), in the development of inflammation and cancers (Batlle and Massagué 2019), in immunological diseases (Stalińska and Ferenc 2005). It has also been associated with diseases of the central nervous system, particularly with injuries (Mattson et al. 1997; Yun et al. 2002; Fee et al. 2004), as well as Alzheimer’s disease, schizophrenia, depression, and multiple sclerosis (Caraci et al. 2011; Diniz et al. 2019; He et al. 2021; Pan et al. 2022; Su et al. 2023; Esmaeilzadeh et al. 2023). Research, particularly in the last two decades, suggests that TGF-β, as a multifunctional growth factor, may also be involved in the formation and development of brain aneurysms (Frösen et al. 2006; Tsai et al. 2009; Vincze et al. 2010; Xu et al. 2019).
Since the pathomechanism of aneurysm formation is not yet fully understood, the aim of our study was to conduct a literature review on the role of TGF-β in brain aneurysm formation.
Search Strategy
We searched the PubMed database for articles on the involvement of TGF-β in the vascular pathology of brain aneurysms, published around the past 25 years. During the search, we limited ourselves to works published in English and Polish, the native language of the authors. Our review includes 104 references published between 1997 and 2024. We used the following keywords and terms: transforming growth factor, transforming growth factor beta, TGF-β, TGF-β pathway, TGF-β and intracranial aneurysms, TGF-beta and brain aneurysms, TGF-beta and subarachnoid hemorrhage, TGF-beta and vascular smooth muscle cell, TGF-beta and saccular cerebral artery aneurysms, TGF-beta and human brain vascular smooth muscle cells, TGF-beta and brain aneurysm and therapeutic targets. Although this study does not include experimental validation, the review of available research provides valuable insights into the role of TGF-β in brain aneurysm formation and development. Therefore, the findings should be interpreted as a summary of existing knowledge rather than newly generated evidence.
Transforming Growth Factors (TGFs) Superfamily
In humans, the TGF-β superfamily consists of 33 members, which can be further categorized into several subfamilies (Hanna and Frangogiannis 2019). Transforming growth factor β (TGF-β) exists in several isoforms, with three recognized as prototypical: TGF-β1, TGF-β2, and TGF-β3. In humans, all three isoforms are located on different chromosomes: 19q13.2, 1q41, and 14q24, respectively (Govinden and Bhoola 2003). All types of TGF-β factors share nearly 70% homology in their amino acid sequences (Stalińska and Ferenc 2005). This family also includes other polypeptides, such as activin A, B, and AB; inhibin A and B; bone morphogenetic proteins (BMPs); growth differentiation factors (GDFs); left–right asymmetry determination factors (nodal, lefty-1, and lefty-2); and Müllerian-inhibiting substance/anti-Müllerian hormone (MIS/AMH) (Fig. 1) (Shi and Massagué 2003).
TGF-β molecules are homodimeric, with a molecular mass of 25 kDa, consisting of two subunits, each weighing 12.5 kDa, and connected by disulfide bridges (Stalińska and Ferenc 2005). Transforming growth factor is synthesized as a prepropeptide. Through the action of proteases in the Golgi apparatus, the precursor is cleaved to remove a C-terminal peptide of 112 amino acids, resulting in the formation of the mature protein form, known as the latency-associated protein (LAP), which remains bound to the homodimer of the mature TGF-β. The complex of the mature form and LAP is called the small latent complex (SLC). The SLC then binds to the latent TGF-β binding protein (LTBP), forming the large latent complex (LLC). LTBP interacts with extracellular matrix proteins and stores TGF-β until it is activated. TGF-β, present in both the small and large latent complexes, is unable to bind to receptors and must undergo activation. Once activated, it functions in an autocrine or paracrine manner as a soluble factor (Robertson et al. 2015; Zi 2019). Some members of TGF-β superfamily can be secreted in their active form and are locally inhibited by antagonists (de Caestecker 2004; Weiss and Attisano 2013).
TGF-β Receptors
TGF-β receptors are present on nearly all cell types. Members of the TGF-β superfamily transmit signals through serine/threonine kinases associated with transmembrane receptors, which have a cysteine-rich extracellular N-terminal domain, a single transmembrane domain, and an intracellular C-terminal serine/threonine kinase domain. There are three main types of membrane receptors: TβR-I, -II, and -III (Table 1), with their respective coding genes located on chromosomes 9q22.33, 3p24.1, and 1p22 (Derynck and Feng 1997; Chang et al. 2002; Massagué and Gomis 2006).
Each member of the family selectively binds and transmits signals through its own combination of receptors. Signal transduction primarily involves the TβR-I and TβR-II receptors, as well as Smad proteins (similar to mother against decapentaplegic) (Hata and Chen 2016). Type I and type II receptors are glycoproteins with molecular weights of approximately 55 kDa and 70 kDa, respectively. The extracellular regions of these receptors contain about 150 amino acids, including 10 or more cysteines, which determine the folding of this region. A unique feature of type I receptors is a 30-amino acid intracellular region located just before the kinase domain, known as the GS domain (GS region), which contains repetitive sequences of glycine and serine. This GS domain is critical because its phosphorylation by a type II receptor is essential for signal transduction (Chang et al. 2002). In human cells, seven type I receptors have been identified, collectively referred to as ALKs (ALK1-ALK7) or activin receptor-like kinases. For type II receptors, identified proteins include ActRII (activin receptor type II), ActRIIB, BMPRII (bone morphogenetic protein receptor), MISRII/AMHRII (Müllerian-inhibiting substance receptor/anti-Müllerian hormone receptor), and TGFβRII (TGFβ receptor II). Type II receptors appear to be the most critical among receptor types, as they are often the first to bind to the TGF-β ligand, initiating the signaling cascade. The type I receptor then joins the ligand-type II receptor complex, facilitated by the previously mentioned phosphorylation of the GS domain. This phosphorylation subsequently activates the type I receptor kinase, which then phosphorylates cytoplasmic effectors to initiate intracellular signaling cascades involving Smad proteins. The Smad proteins migrate to the cell nucleus, where they regulate the expression of target genes, leading to changes in cellular processes such as proliferation, differentiation, apoptosis, and others. Certain isoforms within the TGF-β family can bind to more than one type of receptor (Govinden and Bhoola 2003). Research into the mechanisms of TGF-β-induced signaling has revealed that, in addition to the roles of type I and type II receptors, certain proteins located on the cell membrane—referred to as auxiliary or accessory receptors, also known as type III receptors—play a significant role in this process (Rotzer et al. 2001). These receptors have a significantly higher molecular weight than type I and type II receptors. They are either transmembrane proteins or are anchored to the cell membrane via glycolipids. With an extensively developed extracellular domain capable of binding ligands, they lack a functional signaling domain. Three main type III receptors have been identified: betaglycan (TGFβRIII), endoglin (CD105 antigen), and CD109 antigen. Endoglin and betaglycan share segments with similar nucleotide sequences, suggesting functional similarities between them. Type III receptors primarily modulate signal intensity by forming complexes with ligands and presenting them to the appropriate receptors. Betaglycan, a transmembrane proteoglycan with a molecular weight of 280–330 kDa, forms dimers stabilized by non-covalent bonds. About two-thirds of its mass is contributed by glycosaminoglycans (GAGs), including heparan sulfate and chondroitin sulfate. In human cells, betaglycan has an affinity for all three TGF-β isoforms and interacts with molecules such as inhibins, BMP2, BMP4, BMP7, and GDF-5. It has been shown that, in addition to binding TGF-β ligands, betaglycan also interacts with the type II receptor (TGF-βRII), to which it likely presents a specific ligand. It is speculated that betaglycan may be crucial for effective TGF-β2 signal transduction due to the low affinity of this isoform for its TGF-βRII receptor. In this context, betaglycan acts as a co-receptor. Endoglin is structurally related to betaglycan, but its monomers are covalently linked by disulfide bonds. Endoglin is primarily found in endothelial cells. The endoglin molecule has the ability to bind TGF-β1 and TGF-β3 isoforms, as well as activin A, BMP-2, and BMP-7. Notably, all ligands, except for TGF-β1 and TGF-β3, bind to endoglin only in the presence of their corresponding type II receptor (Derynck and Feng 1997; Rotzer et al. 2001; Chang et al. 2002; Nickel et al. 2018).
Smad Proteins
The Smad protein family is divided into three types: receptor-activated Smads (R-Smads), which include Smad-1, -2, -3, -5, and -8; common mediator Smads (Co-Smads), with Smad-4 as the sole representative; and inhibitory Smads (I-Smads), which include Smad-6 and -7. Signaling through Smad proteins occurs in several stages. First, the ligand (e.g., TGF-β) binds to the type II receptor, which then phosphorylates the type I receptor. The activated type I receptor phosphorylates the R-Smad proteins, which subsequently form a complex with Smad-4 (Co-Smad). This complex translocates to the cell nucleus, where it interacts with other regulatory proteins and transcription factors, initiating the activation and expression of specific target genes. Inhibitory Smad proteins, Smad-6 and Smad-7, bind to receptors or R-Smads, preventing their phosphorylation and thereby inhibiting signaling at the receptor level (Ruiz-Ortega et al. 2007).
Canonical (Smad-Dependent) and Non-canonical (Smad-Independent) Cascade of TGF-ß
TGF-β signaling pathways are crucial for regulating numerous cellular processes. TGF-β signaling occurs through two main pathways: the canonical (Smad-dependent) pathway and the non-canonical (Smad-independent) pathway. Each pathway leads to different cellular responses and involves distinct signaling proteins. The canonical TGF-β signaling pathway involves the activation of Smad proteins, as previously discussed. In brief, this process begins with the binding of the ligand to the type II receptor, followed by phosphorylation of the type I receptor. This activates R-Smad proteins, which then bind to Smad-4, forming a complex that translocates to the cell nucleus. This complex triggers specific cellular responses. Key functions of the canonical pathway include regulating the immune response, controlling cell differentiation, and inhibiting cell proliferation. The non-canonical TGF-β pathway involves alternative signaling pathways activated by TGF-β receptors, without the involvement of Smad proteins. Although both pathways can be activated independently, they can also function simultaneously. The mechanisms of the non-canonical pathway include signaling through MAPK (mitogen-activated protein kinase) pathways, such as ERK (extracellular signal-regulated kinase), the PI3k/Akt pathway, the RhoA/ROCK (Rho-associated kinase) pathway, the TAK1/NF-κB (TGF-β-Activated Kinase 1) pathway, as well as the p38 and JNK pathways. Non-canonical pathways associated with MAPK and RhoA/ROCK are crucial for cell mobility and cytoskeletal changes. The PI3K/Akt pathway plays a key role in enhancing cell resistance to apoptosis, while the activation of p38 and JNK kinases is involved in the cellular stress response. Both the canonical and non-canonical pathways are essential for the proper functioning of cells (Derynck and Zhang 2003; Rahimi and Leof 2007; Guo and Wang 2009; Stipursky et al. 2012; Weiss and Attisano 2013; Diniz et al. 2019).
Transforming Growth Factor β (TGF-β)
TGF-β is produced by a wide range of cell types, including T lymphocytes, macrophages, epithelial cells, fibroblasts, keratinocytes, and platelets (Shi and Massagué 2003). In the central nervous system, TGF-β is primarily synthesized by astrocytes (Diniz et al. 2019). The role of TGF-β is multifaceted and depends on the specific cells it affects, as illustrated in Fig. 2 (Li et al. 2006; Travis and Sheppard 2014; Liu et al. 2019; Larson et al. 2020; Kim et al. 2021; Stolfi et al. 2021; Tie et al. 2022). The TGF-β superfamily members are essential for regulating cell survival, differentiation, proliferation, and function. Moreover, it has been linked to the control of inflammatory and reparative responses (Agrawal 2013; Frangogiannis 2017; Hanna and Frangogiannis 2019). TGF-β promotes the growth, differentiation, inhibition, and downregulation of vascular smooth muscle cells (SMCs) (Agrawal 2013). TGF-β has dual properties: it can stimulate the division of mesenchymal cells, like fibroblasts, as well as inhibit cell division in other types, such as endothelial cells (ECs) (Chen et al. 2002; Siegel and Massagué 2003; Stalińska and Ferenc 2005). TGF-β induces the expression of genes responsible for producing fibrillar collagen and elastin. Furthermore, TGF-β upregulates the expression of connective tissue growth factor (CTGF), which in turn initiates various cellular processes, including cell proliferation, adhesion, migration, and extracellular matrix synthesis (Shih et al. 2003; Agrawal 2013). It also regulates immune responses and directs cells into the apoptotic pathway (Stalińska and Ferenc 2005). TGF-β plays a crucial role in angiogenesis by stimulating extracellular matrix synthesis and supporting tissue repair and wound healing processes (Shah et al. 1999; Stalińska and Ferenc 2005; Li et al. 2006; Morikawa et al. 2016; Larson et al. 2020; Tie et al. 2022). TGF-β1 can exhibit both pro-angiogenic and anti-angiogenic effects, depending heavily on the context. Low concentrations of TGF-β1 promote endothelial cell (EC) proliferation and migration, whereas high concentrations inhibit these processes by increasing the expression of angiogenesis inhibitors, such as plasminogen activator inhibitor-1 (PAI-1), a potent inhibitor of EC migration (Viñals and Pouysségur 2001; Derynck and Zhang 2003).
The pleiotropic effects of TGF-β on diverse cell populations. ECM extracellular matrix, ECs endothelial cells, EMT epithelial-mesenchymal transition, IgA immunoglobulin A, N1 neutrophils N1 type, N2 neutrophils N2 type, NK cells natural killer cells, SMCs smooth muscle cells, TGF-β transforming growth factor β
Normal Cerebral Arteries
Intracranial arteries are composed of three layers: the inner, middle, and outer layers, the latter known as the adventitia. The inner layer faces the lumen of the vessel and is in direct contact with the flowing blood. It consists of a single layer of endothelial cells (ECs), subendothelial connective tissue, and the internal elastic lamina (IEL). In a normal intracranial artery, the internal elastic lamina is well-preserved and uniform. It is primarily composed of elastin, but also contains collagen, proteoglycans, and glycoproteins. The middle layer of the vessel is mainly made up of smooth muscle cells (SMCs) that produce the extracellular matrix (ECM), with type III collagen as its main component. The adventitia is the outermost layer, containing a complex network of type I collagen fibers, elastin, nerves, and fibroblasts, which form the external elastic lamina (EEL). Additionally, intracranial arteries are surrounded by cerebrospinal fluid. It is thought that the structural characteristics of cerebral vessels, along with their immersion in this fluid, may increase the susceptibility of cerebral arteries to aneurysm formation (Fig. 3A) (Etminan and Rinkel 2016).
Aneurysmal Cerebral Arteries
Studies based on animal models and clinical research have identified several pathological changes in the structure of arterial vessels that may lead to aneurysms formation and rupture. Three types of cells are involved in this process, playing a key role: endothelial cells, vascular smooth muscle cells, and leukocytes. Literature data suggest that the formation of an aneurysm is a distinct process from the mechanisms that lead to its rupture. The morphological changes in the vessel wall differ between unruptured and ruptured saccular cerebral artery aneurysms (SCAAs). The wall of an intact SCAAs can remain undamaged for many years, underscoring the presence of robust maintenance and repair mechanisms. However, before rupture, the arterial vessel wall becomes unstable, undergoing morphological changes (Frösen et al. 2004). Studies on aneurysm walls have shown that thicker, intima-like walls are generally associated with unruptured aneurysms, especially in younger patients, with more efficient repair mechanisms. In contrast, thinner, degenerated walls containing hyaline deposits are more prone to rupture. The histology of unruptured saccular intracranial aneurysm walls often resembles myointimal or neointimal hyperplasia (Kataoka et al. 1999). Hemodynamic stress causes damage to endothelial cells, which initiate an inflammatory response through NF-κB signaling (Liu et al. 2019; Kamińska et al. 2022b). This leads to fragmentation, partial degradation, and even complete loss of the internal elastic lamina (Krings et al. 2011). Additionally, the luminal surface of the intima in an aneurysmal vessel shows numerous indentations and may even have small gaps at the junctions between endothelial cells and the vessel lumen (Drăghia et al. 2008). The degradation or loss of the internal elastic lamina enables smooth muscle cells from the medial layer of the aneurysmal vessel to migrate into the intima. SMCs undergo a "phenotypic switch" from a contractile to a synthetic type. Intimal SMCs in the synthetic phenotype secrete matrix metalloproteinases (MMPs), which degrade components of the extracellular matrix, potentially weakening the vessel wall through structural remodeling. On the other hand, these SMCs also proliferate and contribute to the production of collagen and other matrix components, which may reinforce the vessel wall. The SMCs of synthetic phenotype in the wall are likely to contribute with their proliferation and matrix synthesis to the adaptation and repair processes that try to maintain sufficient strength of the wall to resist hemodynamic stress. Myointimal hyperplasia is often regarded as a “wound-healing response” of the vascular wall (Thyberg 1998; Frösen et al. 2004; Frösen 2014; Fennell et al. 2016).
The loss of mural cells, extracellular matrix degeneration, inflammatory cell infiltration, and activation of the humoral immune system impart a pro-inflammatory and pro-remodeling character to the wall, which are hallmark features of ruptured aneurysm wall (Kataoka et al. 1999; Frösen et al. 2004; Tulamo et al. 2006; Frösen 2014). The appearance of smooth muscle cells in the aneurysmal vessel changes from spindle-shaped to a more spider-like form, leading to the loosening of smooth muscle cell bands (Chalouhi et al. 2012; Song et al. 2018). Additionally, in the necks and domes of cerebral aneurysms, there may be increased degeneration, apoptosis, and necrosis of smooth muscle cells. Necroptosis, a newly described type of cell death, like apoptosis can occur in a programmed manner and has been associated with aortic aneurysms due to the death of smooth muscle cells (Wang et al. 2015). The irregular surface of the intimal lumen promotes the migration of leukocytes, including macrophages, T and B lymphocytes, neutrophils, mast cells, and platelets into the vessel lumen. This response is associated with the inflammation. An increased polarization of pro-inflammatory M1 macrophages is observed, which play a crucial role in the pathological remodeling of the vessel. These macrophages excessively secrete enzymes that degrade the extracellular matrix, as well as cytokines and chemokines that activate and recruit other inflammatory cells, thereby driving and sustaining chronic inflammation in the aneurysmal vessel. The increased inflammatory state in endothelial cells, compromised cell integrity, ongoing infiltration of inflammatory cells, disintegration, and increased necrosis/apoptosis/necroptosis of vascular smooth muscle cells, along with the degradation of the extracellular matrix, ultimately lead to the rupture of the aneurysmal vessel. Frösen et al., in their studies, identified four histological types of SCAA aneurysm wall types: 1) an endothelial wall with linearly organized smooth muscle cells; 2) a thickened wall with disorganized smooth muscle cells; 3) a hypocellular wall with miointimal hyperplasia or organizing thrombosis; 4) an extremely thin hypocellular wall lined with thrombosis, which reflect consecutive stages of wall degeneration. Loss of SMCs and absence of intimal hyperplasia in vessels exposed to pro-inflammatory, proteolytic conditions and hemodynamic stress are critical factors in the development of cerebral aneurysms. These conditions surpass the repair capacity of the damaged extracellular matrix within the arterial wall, ultimately leading to rupture. Studies suggest that the wall of ruptured aneurysms is fragile due to loss of SMCs and degradation of matrix proteins, which is likely due to infiltration of inflammatory cells into the aneurysm wall. The factors distinguishing unruptured from ruptured SCAAs include decellularization, apoptosis, degeneration of the wall matrix, de-endothelialization, thrombus organization, and inflammatory cells infiltration (Fig. 3B) (Chyatte et al. 1999; Intengan and Schiffrin 2001; Frösen et al. 2004, 2006, 2012; Chalouhi et al. 2012; Frösen 2014).
Role of the TGF-β Pathway in the Formation and Progression of Cerebral Aneurysms
In various cell types within cerebral vessels, the TGF-β pathway plays a pleiotropic role. TGF-β1 is the most extensively studied member of the TGF-β superfamily in the context of cerebral aneurysms. The significance of TGF-β in vascular pathology is evidenced by Loeys-Dietz syndrome, a condition associated with mutations in TGF-β pathway genes (primarily TGFBR1, TGFBR2, SMAD3, and TGFB2). These mutations result in severe vascular phenotypes in patients, often leading to bleeding and, in many cases, aneurysm dissection. Approximately one-third of deaths in patients with Loeys-Dietz syndrome are due to cerebral hemorrhage, with an overall prevalence of cerebral aneurysms as high as 28% in this population (Loeys et al. 2006; Rodrigues et al. 2009; Vanakker et al. 2011; Kim et al. 2016). In studies on mouse models, Itoh et al. and Crist et al. (Itoh et al. 2012; Crist et al. 2018) demonstrated that the loss of TGF-β pathway genes in endothelial cells deactivates Smad2/3 or Smad4, leading to vascular abnormalities. These findings highlight the essential role of TGF-β in the proper functioning of endothelial cells (Itoh et al. 2012; Crist et al. 2018). In familial cases of cerebral aneurysms, Santiago-Sim et al. (Santiago-Sim et al. 2009) identified rare variants in type III receptors, specifically in endoglin (ENG variant p.A60E) and betaglycan (TGFBR3 variant p.W112R). The authors suggest that, although these polymorphisms are unlikely to represent primary susceptibility genes for cerebral aneurysm formation, they may contribute to the pathogenesis of aneurysm development within the context of the TGF-β signaling pathway (Santiago-Sim et al. 2009).
High wall shear stress (WSS) and a positive WSS gradient are factors initiating aneurysm formation, as they are linked to the disruption of the internal elastic lamina and degeneration of SMCs layer. Notably, studies using animal models have confirmed that aneurysm initiation occurs in regions of high WSS (Meng et al. 2007). Smooth muscle cells involved in the remodeling of the aneurysmal vascular wall express receptors for growth factors such as TGF-β and platelet-derived growth factor B (PDGF-B). These growth factors are secreted by infiltrating leukocytes, particularly macrophages, which drive SMC proliferation and matrix synthesis. Continuous macrophage activation not only stimulates SMCs but also induces the secretion of matrix metalloproteinases, inflammatory cytokines (e.g., IL-6 and IL-8), and chemokines, such as macrophage chemotactic protein-1 (MCP-1), thereby promoting wall expansion and inflammation (Kamińska et al. 2020, 2021, 2022a). Once initiated, this activation is sustained through an autocrine loop, in which prostaglandin E2 (PGE2), via cyclooxygenase-2 (COX-2), activates nuclear factor kappa B (NF-κB), leading to increased MCP-1 and COX-2 expression and the recruitment of additional macrophages (Kamińska et al. 2022b, 2024). This feedback cycle may explain why aneurysm wall remodeling persists even after pathological flow has normalized (Frösen et al. 2004, 2019; Frösen 2014). Therefore, the changes in shear stress that contribute to the formation of cerebral aneurysms can lead to an increase in TGF-β1 expression (Nanjo et al. 2006). When the internal elastic lamina is lost due to the infiltration of inflammatory cells, and the collagen matrix is damaged to the point where the primary aneurysm can bulge, the aneurysm may begin to enlarge. While prolonged proteolytic damage by infiltrating macrophages, without concurrent remodeling of the collagen matrix, does not lead to significant aneurysm growth but may result in rupture, even of a small aneurysm (Frösen et al. 2019).
Darsaut et al. demonstrated that even a modest increase in TGF-β1 expression at the aneurysm neck following stent placement may provide a molecular explanation for the enhanced neointimal formation observed in aneurysm pathology (Darsaut et al. 2006). Studies in animal models and human tissue have shown that canonical TGF-β/Smad3 signaling is upregulated following arterial injury, converting TGF-β1 into a stimulator of smooth muscle cell proliferation (Edlin et al. 2009; Tsai et al. 2009). DiRenzo et al. (DiRenzo et al. 2016) proposed a new mechanism in which elevated TGF-β1/Smad3 signaling stimulates the secretion of canonical Wnt proteins (Wnt2b, 4, 5a, and 9a), which in turn increases smooth muscle cell proliferation through β-catenin stabilization. The authors emphasize that this interaction between the canonical TGF-β1/Smad3 and Wnt/β-catenin pathways promotes smooth muscle cell proliferation and is associated with intimal hyperplasia in the vessel wall (DiRenzo et al. 2016). In contrast, studies by Xu et al. (Xu et al. 2018), provide different findings. The authors observed inhibition of smooth muscle cell proliferation in response to increased TGF-β1 expression regulated by hypoxia-inducible factor 1α-antisense RNA (HIF1A-AS1) in patients with intracranial aneurysms (DiRenzo et al. 2016). They observed a significant correlation between increased TGF-β1 expression and HIF1A-AS1 in patients with aneurysms, with no such association in the control group, suggesting the existence of an intracranial aneurysm-specific interaction between TGF-β1 and HIF1A-AS1 (Xu et al. 2018). Studies by other authors (Liu et al. 2016; Tan et al. 2020) emphasize the role of the TGF-β1 pathway in the development of cerebral aneurysms in the context of increased expression of SPARC (secreted protein acidic and rich in cysteine). The authors demonstrated high SPARC expression, particularly in smooth muscle cells of the aneurysmal vessel. They suggest that this may contribute to damage of the medial layer and the internal elastic lamina (Liu et al. 2016; Tan et al. 2020). Smooth muscle cells and their extracellular matrix provide essential structural support to cerebral arteries, and their dysfunction plays a critical role in aneurysm formation and progression. In a human brain vascular smooth muscle cell (HBVSMC) model, Tan et al. demonstrated that SPARC protein enhances NOX4 expression through the TGF-β1-dependent signaling pathway. This process leads to increased oxidative stress, a pro-inflammatory matrix, and apoptosis of HBVSMCs. The study further showed that SPARC induces phenotypic transformation and apoptosis in HBVSMCs via the TGF-β1-NOX4-ROS pathway, contributing to the development of intracranial aneurysms (Tan et al. 2020). The TGF-β serves as the primary positive regulator of NOX4 expression, with its stimulation intensity correlating with elevated production of reactive oxygen species (ROS) (Martin-Garrido et al. 2011; Xu et al. 2014; Liu et al. 2016). Excessive oxidative stress is recognized as a key driver of SMCs apoptosis and inflammatory phenotypic changes, leading to the weakening of the arterial wall. This process results in vascular wall degradation through the secretion of MMPs and inflammation mediated by the MCP-1 of leukocytes, particularly macrophages. The subsequent release of additional MMPs further intensifies the inflammatory response (Laaksamo et al. 2013; Frösen 2014). Apoptosis, as a form of programmed cell death in human intracranial aneurysms, was first evaluated by Sakaki et al. (Sakaki et al. 1997). The loss of vascular SMCs reduces the mechanical strength of blood vessel walls and diminishes collagen synthesis, impairing the wall’s self-repair capacity to the point of allowing the outward bulging of the initial aneurysm, the aneurysm may start to enlarge and eventual rupture. (Sakaki et al. 1997; Kataoka et al. 1999; Frösen et al. 2004; Laaksamo et al. 2013). Pentimalli et al. demonstrated a significantly higher level of apoptosis in ruptured aneurysms compared to unruptured lesions. The authors suggest that rupture is more closely associated with the increased level of apoptosis than with the size of the aneurysm (Pentimalli et al. 2004). Long-term proteolytic damage by infiltrating macrophages in the absence of concomitant loss of SMCs and collagen matrix remodeling is unlikely to result in significant aneurysm growth and may instead lead to rupture of smaller aneurysms (Frösen et al. 2019).
TGF-β receptors (TβR-II and TβR-III) are upregulated in the damaged arterial wall, forming a heterodimer that enhances fibrosis by increasing extracellular matrix synthesis upon activation by TGF-β1 (Wells et al. 1997). Blocking TGF-β1 signaling with a soluble TβR-II receptor leads to enlargement of the damaged vessel due to reduced matrix synthesis (Ryan et al. 2003). Frösen et al. (Frösen et al. 2006) emphasize that in aneurysmal vessels, the upregulation of TβR-III and TβR-II receptors occurs in response to risk factors for aneurysm rupture. Interestingly, the authors found that, unlike damaged extracranial arteries, TβR-I receptor was not present in the walls of intracranial aneurysms. Immunohistochemical studies revealed increased expression of growth factor receptors in cells infiltrating the aneurysmal lumen, forming clots, as well as in polymorphonuclear inflammatory cells recruited to the vessel wall. The TβR-II receptor was significantly associated with the rupture of saccular cerebral artery aneurysms, while TβR-III was linked to the remodeling of the aneurysmal wall (Frösen et al. 2006).
Therapeutic Potential of the TGF-β in Brain Aneurysms
Aneurysm growth and rupture are closely associated with the breakdown of the extracellular matrix and the loss of smooth muscle cells within cerebral vessels (Miyata et al. 2020). Research on various cardiovascular diseases has demonstrated that local activation of TGF-β signaling pathway may serve as a therapeutic target by stabilizing plaque phenotypes (Lutgens et al. 2002; Miyata et al. 2020). TGF-β, recognized as a multipotent growth factor, stimulates the expression of genes encoding fibrillar collagen and elastin. It plays a pivotal role in vascular collagen remodeling by promoting collagen synthesis and inhibiting its degradation via the TGF-β1-Smad2/3 signaling pathway (Miyata et al. 2020). Furthermore, TGF-β contributes to vascular smooth muscle cell growth and differentiation while simultaneously suppressing inflammation and attenuating inflammatory responses (Agrawal 2013).
Frösen et al. investigated the remodeling and rupture of the SCAA wall in relation to the expression of 12 growth factor receptors that regulate vascular wall remodeling. Their findings suggested that TGF-β, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor receptors could be potential targets for bioactive endovascular implants or pharmacological interventions aimed at reinforcing the SCAA wall. They emphasized that these growth factors might stimulate myointimal hyperplasia while suppressing degenerative remodeling, ultimately reducing the risk of rupture [7].
Agrawal, utilizing an experimental rat aneurysm model, further highlighted the therapeutic potential of growth factors in aneurysm treatment. The study demonstrated that coils coated with TGF-β and VEGF enhanced clot organization and cellular proliferation, significantly improving the closure of large experimental aneurysms. The authors emphasized that TGF-β released from the coils played a pivotal role in inducing fibrosis, thereby contributing to the stabilization of vascular structures (Agrawal 2013). Another study demonstrated that platinum coils coated with TGF-β1 provided early cellular coverage within experimental aneurysms in rabbits following endovascular treatment (de Gast et al. 2001).
Intriguingly, in an animal model of intracranial aneurysms, the inhibition of Ets-1, a regulator known to strongly suppress TGF-β1-induced type-I collagen expression, was shown to prevent IA progression (Czuwara-Ladykowska et al. 2002). Similarly, another study highlighted the potential for minimally invasive molecular therapy targeting the inhibition of NF-κB and Ets-1 for IAs in humans (Aoki et al. 2012).
Moreover, a recent study utilizing an animal model and human vascular wall tissue specimens demonstrated that the administration of osteoprotegerin (OPG) suppressed the progression of intracranial aneurysms through a unique mechanism involving the activation of collagen biosynthesis and vascular SMCs proliferation via TGF-β1, without altering pro-inflammatory gene expression. The authors reported the colocalization of OPG and TGF-β1 expression in human unruptured intracranial aneurysms. They revealed that endogenous OPG in these unruptured intracranial aneurysms promotes the proliferation of SMCs and their abundant distribution through TGF-β1-Smad2/3 signaling (Miyata et al. 2020; Hashimoto et al. 2024).
Supriya et al. (Supriya et al. 2022) observed that significantly reduced expression of selected miRNAs (miR-26b, miR-199a, miR-497, miR-365) can notably modulate several genes within the TGF-β and MAPK signaling cascades. This modulation may influence inflammatory processes, extracellular matrix degradation, and smooth muscle cell apoptosis, ultimately leading to damage and rupture of the cerebral vessel wall (Supriya et al. 2022).
Taking the above into account, TGF-β and its associated pathways demonstrate significant therapeutic potential in the treatment of intracranial aneurysms by promoting vascular remodeling, enhancing collagen biosynthesis, and stabilizing vascular structures, while also serving as promising targets for innovative molecular therapies and bioactive implants.
Future Research Directions
Recognizing that the mechanisms behind aneurysm formation and rupture differ, a significant challenge for future studies is the development of accurate and easily accessible diagnostic tools to distinguish patients requiring intervention from those who can be safely monitored. Another crucial focus should be further studies, particularly those based on human tissue, to precisely determine the role of TGF-β in the formation, progression, and rupture of intracranial aneurysms. Understanding these mechanisms is essential for designing targeted therapies aimed at preventing aneurysm growth and rupture in the context of TGF-β. It is highly probable that different pharmaceutical or biological interventions will be necessary to effectively prevent the formation and rupture of intracranial aneurysms.
Conclusion
In conclusion, the role of TGF-β, its receptors, and signaling pathways in the pathogenesis and progression of cerebral aneurysms is complex and remains not fully elucidated. Further research is essential, particularly regarding the potential of TGF-β as a therapeutic target to prevent the most severe complication of cerebral aneurysms, subarachnoid hemorrhage. A comprehensive understanding of the molecular mechanisms underlying aneurysm formation, especially in the context of TGF-β signaling, could significantly contribute to the development of effective strategies for aneurysm prevention and treatment. This review emphasizes the importance of continued investigation in this area and provides a foundation for future studies aimed at exploring TGF-β as a potential target for therapeutic intervention (summarized in Fig. 4).
Impact of the transforming growth factor β (TGF-β) on brain aneurysm formation and development. HIF1A-AS1 hypoxia-inducible factor-1α—antisense RNA 1, HIF-1α hypoxia-inducible factor-1α, MAPK mitogen-activated protein kinase, miR microRNA, NOX4 NADPH, oxidase 4; RNA ribonucleic acid, SMAD3 SMAD3 gene, SMCs smooth muscle cells, SPARC secreted protein acidic and rich in cysteine, TGFB2 transforming growth factor beta 2 gene, TGFBR1 transforming growth factor beta receptor 1 gene, TGFBR2 transforming growth factor beta receptor 2 gene, TGF-β transforming growth factor β, TβR transforming growth factor beta receptor. All figures were created with BioRender.com
Data Availability
No datasets were generated or analysed during the current study.
References
Agrawal SK (2013) Aneurysm embolization with biologically active coils: an animal study. Neurol Res 35:37–43. https://doi.org/10.1179/1743132812Y.0000000110
Aoki T, Kataoka H, Nishimura M et al (2012) Regression of intracranial aneurysms by simultaneous inhibition of nuclear factor-κB and Ets with chimeric decoy oligodeoxynucleotide treatment. Neurosurgery 70:1534–1543. https://doi.org/10.1227/NEU.0b013e318246a390
Batlle E, Massagué J (2019) Transforming growth factor-β signaling in immunity and cancer. Immunity 50:924–940. https://doi.org/10.1016/j.immuni.2019.03.024
Caraci F, Battaglia G, Bruno V et al (2011) TGF-β1 pathway as a new target for neuroprotection in Alzheimer’s disease. CNS Neurosci Ther 17:237–249. https://doi.org/10.1111/j.1755-5949.2009.00115.x
Chalouhi N, Ali MS, Jabbour PM et al (2012) Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32:1659–1676. https://doi.org/10.1038/jcbfm.2012.84
Chalouhi N, Hoh BL, Hasan D (2013) Review of cerebral aneurysm formation, growth, and rupture. Stroke 44:3613–3622. https://doi.org/10.1161/STROKEAHA.113.002390
Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23:787–823. https://doi.org/10.1210/er.2002-0003
Chen C-R, Kang Y, Siegel PM, Massagué J (2002) E2F4/5 and p107 as Smad cofactors linking the TGFβ Receptor to c-myc repression. Cell 110:19–32. https://doi.org/10.1016/S0092-8674(02)00801-2
Chyatte D, Bruno G, Desai S, Todor DR (1999) Inflammation and intracranial aneurysms. Neurosurgery 45:1137–1147. https://doi.org/10.1097/00006123-199911000-00024
Crist AM, Lee AR, Patel NR et al (2018) Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of hereditary hemorrhagic telangiectasia. Angiogenesis 21:363–380. https://doi.org/10.1007/s10456-018-9602-0
Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M (2002) Ets1 is an effector of the transforming growth factor β (TGF-β) signaling pathway and an antagonist of the profibrotic effects of TGF-β. J Biol Chem 277:20399–20408. https://doi.org/10.1074/jbc.M200206200
Darsaut T, Salazkin I, Ogoudikpe C et al (2006) Effects of stenting the parent artery on aneurysm filling and gene expression of various potential factors involved in healing of experimental aneurysms. Interv Neuroradiol 12:289–302. https://doi.org/10.1177/159101990601200401
de Caestecker M (2004) The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev 15:1–11. https://doi.org/10.1016/j.cytogfr.2003.10.004
de Gast AN, Altes TA, Marx WF et al (2001) Transforming growth factor β-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 49:690–696. https://doi.org/10.1097/00006123-200109000-00030
Derynck R, Feng X-H (1997) TGF-β receptor signaling. Biochim Biophys Acta - Rev Cancer 1333:F105–F150. https://doi.org/10.1016/S0304-419X(97)00017-6
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584. https://doi.org/10.1038/nature02006
Diniz LP, Matias I, Siqueira M et al (2019) Astrocytes and the TGF-β1 pathway in the healthy and diseased brain: a double-edged sword. Mol Neurobiol 56:4653–4679. https://doi.org/10.1007/s12035-018-1396-y
DiRenzo DM, Chaudhary MA, Shi X et al (2016) A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell Signal 28:498–505. https://doi.org/10.1016/j.cellsig.2016.02.011
Drăghia F, Drăghia AC, Onicescu D (2008) Electron microscopic study of the arterial wall in the cerebral aneurysms. Rom J Morphol Embryol 49:101–103
Edlin RS, Tsai S, Yamanouchi D et al (2009) Characterization of primary and restenotic atherosclerotic plaque from the superficial femoral artery: potential role of Smad3 in regulation of SMC proliferation. J Vasc Surg 49:1289–1295. https://doi.org/10.1016/j.jvs.2008.11.096
Esmaeilzadeh A, Mohammadi V, Elahi R (2023) Transforming growth factor β (TGF-β) pathway in the immunopathogenesis of multiple sclerosis (MS); molecular approaches. Mol Biol Rep 50:6121–6131. https://doi.org/10.1007/s11033-023-08419-z
Etminan N, Rinkel GJ (2016) Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol 12:699–713. https://doi.org/10.1038/nrneurol.2016.150
Fee DB, Sewell DL, Andresen K et al (2004) Traumatic brain injury increases TGFβRII expression on endothelial cells. Brain Res 1012:52–59. https://doi.org/10.1016/j.brainres.2004.03.028
Fennell VS, Kalani MYS, Atwal G et al (2016) Biology of saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg. https://doi.org/10.3389/fsurg.2016.00043
Frangogiannis NG (2017) The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis 9:S52–S63. https://doi.org/10.21037/jtd.2016.11.19
Frösen J (2014) Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall-a review of current pathophysiological knowledge. Transl Stroke Res 5:347–356
Frösen J, Piippo A, Paetau A et al (2004) Remodeling of saccular cerebral artery aneurysm wall is associated with rupture. Stroke 35:2287–2293. https://doi.org/10.1161/01.STR.0000140636.30204.da
Frösen J, Piippo A, Paetau A et al (2006) Growth factor receptor expression and remodeling of saccular cerebral artery aneurysm walls: implications for biological therapy preventing rupture. Neurosurgery 58:534–541. https://doi.org/10.1227/01.NEU.0000197332.55054.C8
Frösen J, Tulamo R, Paetau A et al (2012) Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol 123:773–786. https://doi.org/10.1007/s00401-011-0939-3
Frösen J, Cebral J, Robertson AM, Aoki T (2019) Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus 47:1–23. https://doi.org/10.3171/2019.5.FOCUS19234
Govinden R, Bhoola KD (2003) Genealogy, expression, and cellular function of transforming growth factor-β. Pharmacol Ther 98:257–265. https://doi.org/10.1016/S0163-7258(03)00035-4
Guo X, Wang X-F (2009) Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res 19:71–88. https://doi.org/10.1038/cr.2008.302
Hanna A, Frangogiannis NG (2019) The role of the TGF-β superfamily in myocardial infarction. Front Cardiovasc Med 6:1–15. https://doi.org/10.3389/fcvm.2019.00140
Hashimoto T, Meng H, Young WL (2006) Intracranial aneurysms: links among inflammation, hemodynamics and vascular remodeling. Neurol Res 28:372–380. https://doi.org/10.1179/016164106X14973
Hashimoto Y, Karasaki K, Hara T et al (2024) Distribution of osteoprotegerin in unruptured intracranial aneurysms in humans: association with aneurysm wall protective remodeling. J Neurosurg 140:1745–1752. https://doi.org/10.3171/2023.10.JNS231410
Hata A, Chen YG (2016) TGF-β signaling from receptors to smads. Cold Spring Harb Perspect Biol 8:1–32. https://doi.org/10.1101/cshperspect.a022061
He S, Chen X-X, Ge W et al (2021) Are Anti-Inflammatory cytokines associated with cognitive impairment in patients with insomnia comorbid with depression? A pilot study. Nat Sci Sleep 13:989–1000. https://doi.org/10.2147/NSS.S312272
Ingall T, Asplund K, Mähönen M, Bonita R (2000) A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 31:1054–1061. https://doi.org/10.1161/01.STR.31.5.1054
Intengan HD, Schiffrin EL (2001) Vascular remodeling in hypertension. Hypertension 38:581–587. https://doi.org/10.1161/hy09t1.096249
Itoh F, Itoh S, Adachi T et al (2012) Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 119:5320–5328. https://doi.org/10.1182/blood-2011-12-395772
Kamińska J, Lyson T, Chrzanowski R et al (2020) Ratio of IL-8 in CSF versus serum is elevated in patients with unruptured brain aneurysm. J Clin Med 9:1761. https://doi.org/10.3390/jcm9061761
Kamińska J, Dymicka-Piekarska V, Chrzanowski R et al (2021) IL-6 quotient (the ratio of cerebrospinal fluid IL-6 to serum IL-6) as a biomarker of an unruptured intracranial aneurysm. J Inflamm Res 14:6103–6114. https://doi.org/10.2147/JIR.S335618
Kamińska J, Maciejczyk M, Ćwiklińska A et al (2022a) Pro-Inflammatory and anti-inflammatory cytokines levels are significantly altered in cerebrospinal fluid of unruptured intracranial aneurysm (UIA) patients. J Inflamm Res 15:6245–6261. https://doi.org/10.2147/JIR.S380524
Kamińska J, Tylicka M, Dymicka-Piekarska V et al (2022b) Canonical NF-κB signaling pathway and GRO-α/CXCR2 axis are activated in unruptured intracranial aneurysm patients. Sci Rep 12:21375. https://doi.org/10.1038/s41598-022-25855-2
Kamińska J, Tylicka M, Sutkowska K et al (2024) The preliminary study suggests an association between NF-ĸB pathway activation and increased plasma 20S proteasome activity in intracranial aneurysm patients. Sci Rep 14:3941. https://doi.org/10.1038/s41598-024-54692-8
Kataoka K, Taneda M, Asai T et al (1999) Structural fragility and inflammatory response of ruptured cerebral aneurysms. Stroke 30:1396–1401. https://doi.org/10.1161/01.STR.30.7.1396
Kim ST, Brinjikji W, Kallmes DF (2016) Prevalence of intracranial aneurysms in patients with connective tissue diseases: a retrospective study. Am J Neuroradiol 37:1422–1426. https://doi.org/10.3174/ajnr.A4718
Kim BG, Malek E, Choi SH et al (2021) Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol 14:1–20. https://doi.org/10.1186/s13045-021-01053-x
Krings T, Mandell DM, Kiehl T-R et al (2011) Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol 7:547–559. https://doi.org/10.1038/nrneurol.2011.136
Laaksamo E, Tulamo R, Liiman A et al (2013) Oxidative stress is associated with cell death, wall degradation, and increased risk of rupture of the intracranial aneurysm wall. Neurosurgery 72:109–117. https://doi.org/10.1227/NEU.0b013e3182770e8c
Larson C, Oronsky B, Carter CA et al (2020) TGF-beta: a master immune regulator. Expert Opin Ther Targets 24:427–438. https://doi.org/10.1080/14728222.2020.1744568
Li MO, Wan YY, Sanjabi S et al (2006) Transforming growth factor-β regulation of immune responses. Annu Rev Immunol 24:99–146. https://doi.org/10.1146/annurev.immunol.24.021605.090737
Liu X-H, Zhang Q-Y, Pan L-L et al (2016) NADPH oxidase 4 contributes to connective tissue growth factor expression through Smad3-dependent signaling pathway. Free Radic Biol Med 94:174–184. https://doi.org/10.1016/j.freeradbiomed.2016.02.031
Liu Z, Ajimu K, Yalikun N et al (2019) Potential therapeutic strategies for intracranial aneurysms targeting aneurysm pathogenesis. Front Neurosci 13:1–10. https://doi.org/10.3389/fnins.2019.01238
Loeys BL, Schwarze U, Holm T et al (2006) Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 355:788–798. https://doi.org/10.1056/NEJMoa055695
Lutgens E, Gijbels M, Smook M et al (2002) Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol 22:975–982. https://doi.org/10.1161/01.ATV.0000019729.39500.2F
Martin-Garrido A, Brown DI, Lyle AN et al (2011) NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic Biol Med 50:354–362. https://doi.org/10.1016/j.freeradbiomed.2010.11.007
Massagué J, Gomis RR (2006) The logic of TGFβ signaling. FEBS Lett 580:2811–2820. https://doi.org/10.1016/j.febslet.2006.04.033
Mattson MP, Barger SW, Furukawa K et al (1997) Cellular signaling roles of TGFβ, TNFα and βAPP in brain injury responses and Alzheimer’s disease. Brain Res Rev 23:47–61. https://doi.org/10.1016/S0165-0173(96)00014-8
Meng H, Wang Z, Hoi Y et al (2007) Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38:1924–1931. https://doi.org/10.1161/STROKEAHA.106.481234
Miyata T, Minami M, Kataoka H et al (2020) Osteoprotegerin prevents intracranial aneurysm progression by promoting collagen biosynthesis and vascular smooth muscle cell proliferation. J Am Heart Assoc. https://doi.org/10.1161/JAHA.119.015731
Morikawa M, Derynck R, Miyazono K (2016) TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8:a021873. https://doi.org/10.1101/cshperspect.a021873
Moustakas A, Pardali K, Gaal A, Heldin C-H (2002) Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett 82:85–91. https://doi.org/10.1016/S0165-2478(02)00023-8
Nanjo H, Sho E, Komatsu M et al (2006) Intermittent short-duration exposure to low wall shear stress induces intimal thickening in arteries exposed to chronic high shear stress. Exp Mol Pathol 80:38–45. https://doi.org/10.1016/j.yexmp.2005.04.005
Nickel J, Ten Dijke P, Mueller TD (2018) TGF-β family co-receptor function and signaling. Acta Biochim Biophys Sin (Shanghai) 50:12–36. https://doi.org/10.1093/abbs/gmx126
Østbye T, Levy AR, Mayo NE (1997) Hospitalization and case-fatality rates for subarachnoid Hemorrhage in Canada from 1982 through 1991. Stroke 28:793–798. https://doi.org/10.1161/01.STR.28.4.793
Pan S, Zhou Y, Yan L et al (2022) TGF-β1 is associated with deficits in cognition and cerebral cortical thickness in first-episode schizophrenia. J Psychiatry Neurosci 47:E86–E98. https://doi.org/10.1503/jpn.210121
Pentimalli L, Modesti A, Vignati A et al (2004) Role of apoptosis in intracranial aneurysm rupture. J Neurosurg 101:1018–1025. https://doi.org/10.3171/jns.2004.101.6.1018
Rahimi RA, Leof EB (2007) TGF-β signaling: a tale of two responses. J Cell Biochem 102:593–608. https://doi.org/10.1002/jcb.21501
Robertson IB, Horiguchi M, Zilberberg L et al (2015) Latent TGF-β-binding proteins. Matrix Biol 47:44–53. https://doi.org/10.1016/j.matbio.2015.05.005
Rodrigues VJ, Elsayed S, Loeys BL et al (2009) Neuroradiologic manifestations of Loeys-Dietz syndrome Type 1. Am J Neuroradiol 30:1614–1619. https://doi.org/10.3174/ajnr.A1651
Rotzer D, Roth M, Lutz M et al (2001) Type III TGF-β receptor-independent signalling of TGFβ2 via TβRII-B, an alternatively spliced TGF-β type II receptor. EMBO J 20:480–490. https://doi.org/10.1093/emboj/20.3.480
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E et al (2007) TGF-β signaling in vascular fibrosis. Cardiovasc Res 74:196–206. https://doi.org/10.1016/j.cardiores.2007.02.008
Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V (2003) Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res 40:37–46. https://doi.org/10.1159/000068937
Sakaki T, Kohmura E, Kishiguchi T et al (1997) Loss and apoptosis of smooth muscle cells in intracranial aneurysms studies with in situ DNA end labeling and antibody against single-stranded DNA. Acta Neurochir (Wien) 139:469–475. https://doi.org/10.1007/BF01808885
Santiago-Sim T, Mathew-Joseph S, Pannu H et al (2009) Sequencing of TGF-β pathway genes in familial cases of intracranial aneurysm. Stroke 40:1604–1611. https://doi.org/10.1161/STROKEAHA.108.540245
Shah M, Revis D, Herrick S et al (1999) Role of elevated plasma transforming growth factor-β1 levels in wound healing. Am J Pathol 154:1115–1124. https://doi.org/10.1016/S0002-9440(10)65364-3
Shi Y, Massagué J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700. https://doi.org/10.1016/S0092-8674(03)00432-X
Shih S-C, Ju M, Liu N et al (2003) Transforming growth factor β1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci 100:15859–15864. https://doi.org/10.1073/pnas.2136855100
Siegel PM, Massagué J (2003) Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer 3:807–820. https://doi.org/10.1038/nrc1208
Signorelli F, Sela S, Gesualdo L et al (2018) Hemodynamic stress, inflammation, and intracranial aneurysm development and rupture: a systematic review. World Neurosurg 115:234–244. https://doi.org/10.1016/j.wneu.2018.04.143
Song Y, Liu P, Li Z et al (2018) The effect of myosin light chain kinase on the occurrence and development of intracranial aneurysm. Front Cell Neurosci. https://doi.org/10.3389/fncel.2018.00416
Stalińska L, Ferenc T (2005) Rola TGF-b w regulacji cyklu komórkowego * The role of TGF-b in cell cycle regulation. Postep Hig Med Dosw Online pp 441–449
Stipursky J, Francis D, Gomes FCA (2012) Activation of MAPK/PI3K/SMAD pathways by TGF-β1 controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci 34:68–81. https://doi.org/10.1159/000338108
Stolfi C, Troncone E, Marafini I, Monteleone G (2021) Role of tgf-beta and smad7 in gut inflammation, fibrosis and cancer. Biomolecules 11:1–15. https://doi.org/10.3390/biom11010017
Su C, Miao J, Guo J (2023) The relationship between TGF-β1 and cognitive function in the brain. Brain Res Bull 205:110820. https://doi.org/10.1016/j.brainresbull.2023.110820
Supriya M, Christopher R, Devi BI et al (2022) Altered MicroRNA expression in intracranial aneurysmal tissues: possible role in TGF-β signaling pathway. Cell Mol Neurobiol 42:2393–2405. https://doi.org/10.1007/s10571-021-01121-3
Tan X, Li T, Zhu S et al (2020) Induction of SPARC on oxidative stress, inflammatory phenotype transformation, and apoptosis of human brain smooth muscle cells via TGF-β1-NOX4 pathway. J Mol Neurosci 70:1728–1741. https://doi.org/10.1007/s12031-020-01566-z
Thyberg J (1998) Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol Histopathol 13:871–891. https://doi.org/10.14670/HH-13.871
Tie Y, Tang F, Peng D et al (2022) TGF-beta signal transduction: biology, function and therapy for diseases. Mol Biomed. https://doi.org/10.1186/s43556-022-00109-9
Travis MA, Sheppard D (2014) TGF-β activation and function in immunity. Annu Rev Immunol 32:51–82. https://doi.org/10.1146/annurev-immunol-032713-120257
Tsai S, Hollenbeck ST, Ryer EJ et al (2009) TGF-β through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Circ Physiol 297:H540–H549. https://doi.org/10.1152/ajpheart.91478.2007
Tulamo R, Frösen J, Junnikkala S et al (2006) Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery 59:1069–1077. https://doi.org/10.1227/01.NEU.0000245598.84698.26
Vanakker OM, Hemelsoet D, De Paepe A (2011) Hereditary connective tissue diseases in young adult stroke: a comprehensive synthesis. Stroke Res Treat 2011:1–18. https://doi.org/10.4061/2011/712903
Viñals F, Pouysségur J (2001) Transforming growth factor β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signaling. Mol Cell Biol 21:7218–7230. https://doi.org/10.1128/MCB.21.21.7218-7230.2001
Vincze C, Pál G, Wappler EA et al (2010) Distribution of mRNAs encoding transforming growth factors-β1, -2, and -3 in the intact rat brain and after experimentally induced focal ischemia. J Comp Neurol 518:3752–3770. https://doi.org/10.1002/cne.22422
Wang Q, Liu Z, Ren J et al (2015) Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res 116:600–611. https://doi.org/10.1161/CIRCRESAHA.116.304899
Weiss A, Attisano L (2013) The TGFbeta superfamily signaling pathway. Wires Dev Biol 2:47–63. https://doi.org/10.1002/wdev.86
Wells RG, Yankelev H, Lin HY, Lodish HF (1997) Biosynthesis of the Type I and Type II TGF-β receptors. J Biol Chem 272:11444–11451. https://doi.org/10.1074/jbc.272.17.11444
Xu S, Chamseddine AH, Carrell S, Miller FJ (2014) Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol 2:642–650. https://doi.org/10.1016/j.redox.2014.04.004
Xu J, Zhang Y, Chu L et al (2018) Long non-coding RNA HIF1A-AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF-β1. Exp Ther Med. https://doi.org/10.3892/etm.2018.7144
Xu N, Meng H, Liu T et al (2019) Stent-jailing technique reduces aneurysm recurrence more than Stent-Jack technique by causing less mechanical forces and angiogenesis and inhibiting TGF-β/Smad2,3,4 signaling pathway in intracranial aneurysm patients. Front Physiol. https://doi.org/10.3389/fphys.2018.01862
Yun S-J, Kim M-O, Kim SO et al (2002) Induction of TGF-β-inducible gene-h3 (βig-h3) by TGF-β1 in astrocytes: implications for astrocyte response to brain injury. Mol Brain Res 107:57–64. https://doi.org/10.1016/S0169-328X(02)00447-3
Zi Z (2019) Molecular engineering of the TGF-β signaling pathway. J Mol Biol 431:2644–2654. https://doi.org/10.1016/j.jmb.2019.05.022
Acknowledgements
We are grateful to Martin Lenkiewicz, MSc for his language assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by the Medical University of Bialystok (statutory work: B.SUB.24.325).
Author information
Authors and Affiliations
Contributions
Conceptualization: Joanna Kamińska; Investigation: Kinga Sutkowska, Olga Martyna Koper-Lenkiewicz, Joanna Matowicka-Karna, Joanna Kamińska; Supervision: Joanna Kamińska, Olga Martyna Koper-Lenkiewicz; Visualization: Kinga Sutkowska, Joanna Kamińska, Olga Martyna Koper-Lenkiewicz; Funding acquisition: Joanna Kamińska; Writing – Original Draft Preparation: Kinga Sutkowska, Joanna Kamińska; Writing – Review & Editing: Kinga Sutkowska, Olga Martyna Koper-Lenkiewicz, Joanna Matowicka-Karna, Joanna Kamińska. All authors have read and agreed to the published the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
None.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
AI-assisted copy editing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sutkowska, K., Koper-Lenkiewicz, O.M., Matowicka-Karna, J. et al. Impact of the Transforming Growth Factor β (TGF-β) on Brain Aneurysm Formation and Development: A Literature Review. Cell Mol Neurobiol 45, 46 (2025). https://doi.org/10.1007/s10571-025-01572-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-025-01572-y