Abstract
Astroglial Kir4.1 and AQP4 channels are pivotal regulators of potassium (K+) and water homeostasis in the brain, playing essential roles in maintaining neuronal stability, facilitating synaptic transmission, and supporting overall brain function. Kir4.1 channels promote the efficient uptake of K+ ions from the extracellular space, particularly during periods of high neuronal activity, thereby preventing excessive neuronal excitability—a condition linked to several neurological disorders, including Autism Spectrum Disorder (ASD). Meanwhile, AQP4 channels, predominantly expressed in the astrocytic end-feet at the blood–brain barrier, regulate water transport across cell membranes, ensuring osmotic balance that complements the function of Kir4.1 in K+ clearance. Recent studies have underscored the critical link between dysfunctions in these channels and the pathophysiology of ASD, a complex neurodevelopmental disorder characterized by a broad range of social, communicative, and behavioral impairments. Mutations or dysregulations in Kir4.1 and AQP4 channels can disrupt K+ and water homeostasis, exacerbating neuronal hyperexcitability and contributing to hallmark ASD symptoms, such as sensory processing abnormalities, social deficits, and an increased risk of seizures. This review synthesizes current findings, focusing on the molecular mechanisms of Kir4.1 and AQP4 channels, their role in astrocyte–neuron interactions, and their pathophysiological implications in ASD. It also provides a detailed discussion of potential therapeutic interventions targeting these channels to mitigate ASD symptoms.
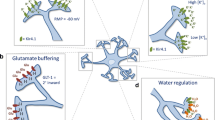
Graphical Abstract
Astroglial Kir4.1 and AQP4 Channels in ASD: disruptions in the activity of astroglial Kir4.1 and AQP4 channels can lead to imbalances in potassium (K⁺) and water homeostasis, resulting in neuronal excitability and dyshomeostasis. These changes contribute to the behavioral traits associated with Autism Spectrum Disorders (ASD).

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterized by a complex range of symptoms that vary in severity and presentation across individuals. These symptoms typically include challenges with social communication, repetitive behaviors, restricted interests, and sensory processing abnormalities (Joon et al. 2021). From a neurobiological perspective, ASD involves intricate disruptions in both neural connectivity and synaptic function. These disruptions manifest at both the gross anatomical and cellular levels, contributing to the broad heterogeneity of ASD symptomatology.
Recent research has highlighted the role of astrocytes—glial cells traditionally thought to support neurons in maintaining homeostasis—in the pathophysiology of ASD (Sauer et al. 2021). Based on data collected from multiple research centers, Ostrowski et al. (2024) estimate that the global prevalence of ASD ranges from 0.02 to 3.66%. Additionally, there is an overall trend of increasing recorded diagnoses of ASD (Underwood et al. 2022; Ostrowski et al. 2024). However, researchers suggest that this rise may be attributed to increased public awareness, improved diagnostic practices, and evidence-based research on ASD.
Astrocytes, as key regulators of brain homeostasis, play critical roles in maintaining the extracellular environment. They are involved in neurotransmitter uptake, synapse formation, and regulating ionic concentrations within the brain. Central to these functions are the Kir4.1 and AQP4 channels, which mediate K+ and water homeostasis, respectively. Kir4.1 is an inwardly rectifying potassium channel, facilitates the uptake of K+ from the extracellular space during neuronal activity, a function crucial for preventing neuronal hyperexcitability. AQP4, on the other hand, is a water channel that regulates water movement across astrocytic membranes, ensuring osmotic balance and supporting K+ clearance. Together, these channels form a dynamic system that protects neurons from excitotoxicity, a condition arising from excessive neuronal activation.
Astrocyte-derived factors are also crucial for the formation and maintenance of neural synapses and connections. An increased level of glial fibrillary acidic protein (GFAP), an intermediate filament produced by astrocytes in the CNS, is considered one of the main cellular mechanisms underlying ASD (Sloan and Barres 2014). Given that astrocytes contribute to memory consolidation and high executive functions in the brain, abnormalities in astrocyte function in ASD can lead to social and neural disabilities, in addition to memory impairments (Elahi-Mahani et al. 2018; Santello et al. 2019; Darvishmolla et al. 2022; Saeedi et al. 2022; Davoudi et al. 2023).
The involvement of these channels in ASD has garnered significant attention in recent years. Sauer et al. (2021) demonstrated that mutations or functional impairments in the genes encoding Kir4.1 and AQP4 are associated with a range of neurodevelopmental disorders, including ASD. Dysregulations in Kir4.1 impair the brain’s ability to maintain K+ homeostasis, leading to increased extracellular K+ levels, neuronal hyperexcitability, and a higher propensity for seizures (Sicca et al. 2011, 2016; Sauer et al. 2021), which are frequently observed in individuals with ASD (Fig. 1). Similarly, disruptions in AQP4-mediated water transport can lead to abnormal osmotic imbalances, contributing to neuroinflammatory responses and further exacerbating neuronal dysfunction (Salman et al. 2022).
Astroglia AQP4 and Kir401 channels in brain homeostasis—Control vs. autism. This figure illustrates the essential roles of astrocytic AQP4 channels and Kir4.1 channels in preserving blood–brain barrier (BBB) integrity and regulating water homeostasis, osmotic balance, and potassium (K+) equilibrium under normal physiological conditions. In contrast, the figure highlights the dysregulation of these channels in autism, where disturbances—resulting from gain of loss of function, altered distribution, decreased expression, or impaired glymphatic clearance—lead to K+ and water imbalance and neuroinflammation. These disruptions contribute to neuronal excitability and dyshomeostasis, which are linked to sensory processing abnormalities, social deficits, heightened seizure susceptibility, neuronal hyperexcitability, and synaptic dysfunction—ultimately shaping the behavioral phenotypes characteristic of autism spectrum disorder (ASD)
While most studies investigate the physiological effects of water and electrolytes in the human body, it is worthwhile to consider the psychological effects of imbalances in the body’s electrolytes and homeostasis. The imbalances of ions and electrolytes in ASD are influenced by abnormalities in astroglia activity. Specifically, Kir channels and AQP4 regulate the flow of K+ and water between the CNS neurons and extracellular space, which can alter neuronal excitation and inhibition patterns. Disruptions of AQP4 activity in ASD are associated with neuronal hyperexcitability due to its effects on K+ homeostasis (Gzielo and Nikiforuk 2021).
Furthermore, the implications of abnormalities in Kir and AQP4 activities can be observed in their interactions with other channels and receptors. Findings by Shinohe et al. (2006) suggest a statistically significant increase in serum levels of glutamate in ASD, which can be related to the experiment conducted by Gzielo and Nikiforuk (2021) (Shinohe et al. 2006; Gzielo and Nikiforuk 2021). Their study indicated that the knockout of Kir4.1 and its subsequent depolarization can inhibit glutamate and K+ uptake in glial cells. These interconnected networks of protein activities highlight the nuances of cellular and biochemical pathways in our brains and the potential of studying cellular proteins to design targeted interventions for various neuropsychiatric disorders, not limited to ASD.
This review explores the structure and function of Kir4.1 and AQP4 channels, the mechanisms through which they regulate K+ and water homeostasis, and their implications for synaptic function and neuronal health. By synthesizing current research on these channels, this review aims to elucidate their role in ASD pathophysiology and to explore potential therapeutic strategies targeting astrocytic dysfunction to alleviate the cognitive and behavioral symptoms associated with ASD.
The Inwardly Rectifying Kir4.1 and AQP4 Channels: Structural Characteristics and Functional Mechanisms
Kir4.1 Channels: Structure and Function
Kir4.1 is a member of the inwardly rectifying potassium channel family, primarily expressed in astrocytes located in various regions of the brain, including the thalamus, hippocampus, and brainstem. Its structure consists of four identical subunits that form a channel through which K+ ions pass. Unlike other potassium channels, Kir4.1 allows the more efficient influx of K+ into the cell rather than efflux, hence the term “inwardly rectifying.” This inward flow of K+ is crucial for maintaining the resting membrane potential of neurons, especially during periods of high synaptic activity when extracellular K+ concentration increases. By facilitating the uptake of excess K+ from the extracellular space, Kir4.1 channels prevent the accumulation of K+ that could otherwise lead to neuronal hyperexcitability and excitotoxicity (Weaver and Denton 2021).
Kir4.1 also plays a key role in a process known as spatial buffering, where astrocytes redistribute K+ from regions of high concentration to regions of lower concentration through their extensive cellular networks (Nwaobi et al. 2016). This process is essential for maintaining the electrochemical gradients necessary for normal synaptic function and neuronal signaling. Disruptions in Kir4.1 function can have profound implications for brain homeostasis. Mutations in the KCNJ10 gene, which encodes Kir4.1, have been linked to a range of neurological disorders, including ASD, epilepsy, and ataxia (Morin et al. 2020; Lo et al. 2022). Studies in animal models have shown that a loss of Kir4.1 function leads to increased extracellular K+ levels, resulting in neuronal hyperexcitability (Fig. 2), increased seizure susceptibility, and altered synaptic transmission—features consistent with the neurodevelopmental abnormalities observed in ASD (Sicca et al. 2016).
Glutamate accumulation-induced neuronal excitability and ASD-related neurocognitive and behavioral deficits. This figure illustrates the pathological impact of excessive glutamate accumulation at the synapse, leading to neuronal hyperexcitability and contributing to neurocognitive and behavioral deficits characteristic of autism spectrum disorder (ASD). The tripartite synapse includes pre- and postsynaptic neurons along with an astrocyte. On the postsynaptic neuron, AMPA and NMDA receptors mediate excitatory neurotransmission, further exacerbated by abnormal glutamate levels. The reactive astrocyte exhibits disrupted spatial K⁺ buffering, Kir4.1 dysfunction, and AQP4 impairment at the perivascular end-feet, leading to ionic imbalance, altered homeostasis, and neuroinflammation. These disruptions collectively contribute to heightened neuronal excitability and dysregulated synaptic function, underscoring their role in ASD pathophysiology
Gain-of-function defects in Kir4.1 channels may be a primary cause of ASD and epilepsy, as Kir4.1 is crucial for maintaining the resting membrane potential and regulating extracellular potassium levels to prevent neuronal overexcitement (Kinboshi et al. 2020). Kir4.1 facilitates the astroglial transport of excess potassium ions, thereby preventing K+ accumulation and reducing the risk of high potassium load. However, unlike the strong inward rectifier Kir2.1, Kir4.1 exhibits weak rectification, potentially rendering it more vulnerable to pathological conditions. During neuronal excitation, elevated extracellular potassium levels can cause neuronal damage by initiating harmful secondary cascades (Li et al. 2021). These findings underscore the importance of Kir4.1 in safeguarding proper neuronal functioning.
Recently, we have highlighted the critical role of astroglial Kir4.1 channels in modulating neuronal excitability and behavioral abnormalities in a rat model of autism induced by prenatal exposure to valproic acid (VPA). Using whole-cell patch-clamp recordings from hippocampal CA1 pyramidal neurons, we revealed that inhibition of Kir4.1 channels in control neurons led to electrophysiological changes indicative of neuronal hyperexcitability, similar to those observed in VPA-exposed neurons. These changes included increased input resistance and voltage threshold, along with decreased time constant and rheobase. Behavioral assessments following intrahippocampal administration of PA6 further demonstrated significant social withdrawal, heightened anxiety, reduced exploration, and impaired recognition memory, reflecting the behavioral deficits linked to autism. These findings underscore the importance of astroglial Kir4.1 channels in regulating neuronal excitability and associated behavioral impairments, suggesting that targeting these channels could be a promising therapeutic strategy for autism (Davoudi et al. 2025).
Cucchiara et al. (2020) investigated the electrophysiological features of sleep in children with Kir4.1 channel mutations and their association with an autism-epilepsy phenotype. Their preliminary study revealed distinct abnormalities in sleep architecture and brain electrical activity in these children. Specifically, the study found that Kir4.1 channel mutations, which disrupt potassium ion homeostasis in astrocytes, lead to alterations in sleep patterns, including modified sleep spindle activity and reduced slow-wave sleep. These electrophysiological changes are closely linked to the co-occurrence of autism and epilepsy, highlighting how these genetic mutations impact both sleep regulation and the broader neurodevelopmental and epileptic spectrum. The study emphasizes the critical role of Kir4.1 channels in maintaining normal sleep physiology and its implications for understanding the complex interplay between genetic mutations, sleep disturbances, and neurodevelopmental disorders in affected children (Cucchiara et al. 2020).
AQP4 Channels: Structure and Function
Aquaporin-4 (AQP4) is a water channel protein primarily expressed in the astrocytic end-feet at the blood–brain barrier, where it facilitates the bidirectional transport of water across cell membranes. Structurally, AQP4 forms orthogonal arrays of particles (OAPs), allowing for the rapid movement of water in response to osmotic gradients. Functionally, AQP4 plays a critical role in maintaining water homeostasis in the brain, particularly in regions of high neuronal activity where K+ clearance is essential for synaptic function. As neurons release K+ during synaptic transmission, astrocytes uptake the excess K+ through Kir4.1 channels, while AQP4 regulates water transport to balance the accompanying osmotic changes (Ohno et al. 2021).
In addition to its role in water homeostasis, Wang et al. (2022) suggest that AQP4 is involved in regulating cell migration, neuroinflammation, and the glymphatic clearance of metabolic waste products from the brain. This latter function is particularly important for maintaining overall brain health, as impaired AQP4 function has been linked to the accumulation of neurotoxic proteins, such as amyloid-beta, which is implicated in neurodegenerative diseases like Alzheimer’s disease (AD). In the context of AD, AQP4 dysfunction may contribute to altered water balance, leading to changes in neuronal signaling and increased vulnerability to neuroinflammation—a hallmark feature of the disorder (Wang et al. 2022).
As Ohno et al. (2021) explained, the coupling of Kir4.1 and AQP4 is essential for maintaining K+ and water homeostasis in the brain. Disruptions in this coupling, whether due to genetic mutations or environmental factors, can result in significant impairments in synaptic function and neuronal health (Ohno et al. 2021), contributing to the pathophysiology of ASD. Studies have shown that individuals with ASD often exhibit mutations in genes associated with ion channel function, including Kir4.1 and AQP4, further underscoring the importance of these channels in maintaining normal brain function (Cucchiara et al. 2020; Davoudi et al. 2023).
Astrocyte–Neuron Interplay: Mechanisms and Implications
Astrocytes are crucial for maintaining the homeostasis of the extracellular environment in the brain, and their interactions with neurons are essential for normal synaptic function. Through the expression of Kir4.1 and AQP4 channels, astrocytes regulate the ionic and osmotic balance in the brain, ensuring that neurons can fire and transmit without becoming hyperexcitable or dysfunctional. This regulation is particularly important in regions of the brain where neuronal activity is high, such as the hippocampus and cortex, which are critical for learning, memory, and social behaviors—functions that are often impaired in individuals with ASD (Li et al. 2021).
As Kinboshi et al. (2020) explored, one of the key ways in which astrocytes support neuronal activity is by modulating synaptic transmission. During neuronal depolarization, K+ ions are released into the extracellular space, which, if left unchecked, can lead to an accumulation of K+ and increased neuronal excitability. Astrocytes, via Kir4.1 channels, take up the excess K+ and redistribute it to other regions of the brain, thereby preventing K+ buildup and maintaining the resting membrane potential of neurons. At the same time, AQP4 channels facilitate the movement of water to balance the osmotic changes that occur with K+ uptake (Fig. 1), ensuring that astrocytes can efficiently clear K+ without disrupting the extracellular environment (Kinboshi et al. 2020).
Astrocyte Contributions to Neurotransmitter Regulation and Neuroinflammation in Autism Spectrum Disorders
In addition to their role in K+ and water homeostasis, astrocytes also contribute to synaptic plasticity by regulating neurotransmitter levels in the extracellular space (Kinboshi et al. 2020). Astrocytes express high levels of glutamate transporters, which allow them to take up excess glutamate released during synaptic transmission, preventing excitotoxicity and maintaining the excitatory/inhibitory balance in the brain. This process is particularly important in ASD, where disruptions in glutamate signaling are thought to contribute to the sensory processing abnormalities and cognitive impairments characteristic of the disorder (Fig. 2). Moreover, astrocytes provide metabolic support to neurons, supplying them with the necessary nutrients and energy substrates to maintain synaptic function and prevent neuronal degeneration.
Astrocytes play a significant role in autism spectrum disorders by affecting neurotransmitter regulation and synaptic function. Research has highlighted their involvement in autism-linked conditions, such as Fragile X syndrome. Studies of mice with the FMR1 gene deleted specifically in astrocytes reveal traits akin to Fragile X syndrome, including social difficulties and learning impairments. These traits are attributed to reduced astrocyte expression of GLT-1, a protein essential for clearing glutamate from synapses. When GLT-1 expression is compromised, glutamate levels remain elevated, leading to neuronal hyperexcitability. Additionally, disrupted expression of metabotropic glutamate receptor 5 (MGLAR5) in astrocytes, rather than in neurons, further complicates this mechanism. MGLAR5 is crucial during developmental stages and its dysregulation in astrocytes underscores the importance of astrocyte function in neurodevelopmental disorders (Schenkman 2022).
Neuroinflammation is another critical aspect of astrocyte–neuron interactions in the context of ASD. Under normal conditions, astrocytes help regulate the brain’s immune response, preventing excessive inflammation that can damage neurons and disrupt synaptic function. However, in individuals with ASD, astrocytes often exhibit a reactive phenotype, characterized by increased expression of pro-inflammatory cytokines and other immune mediators. This chronic neuroinflammatory state can exacerbate the synaptic and neuronal dysfunctions associated with ASD, leading to further impairments in cognitive and behavioral function (Bataveljic et al. 2024).
Microglia and astrocytes play important roles in mediating neuroinflammation and synaptic susceptibility in ASD. Xiong et al. (2023) highlight that these glial cells are central to the pathophysiology of ASD through their involvement in neuroinflammatory processes and synaptic alterations. Microglia, the resident immune cells of the central nervous system, become activated in ASD, leading to the release of pro-inflammatory cytokines and reactive oxygen species, which contribute to neuroinflammation and synaptic dysfunction. Concurrently, astrocytes, which regulate neurotransmitter homeostasis and synaptic function, also exhibit aberrant activation in ASD. This dysregulation includes altered expression of glutamate transporters and inflammatory mediators, further exacerbating synaptic excitability and neuronal connectivity issues. The interplay between hyperactive microglia and dysfunctional astrocytes disrupts the neuroinflammatory balance and impairs synaptic plasticity, contributing to the cognitive and behavioral abnormalities observed in ASD (Xiong et al. 2023).
The Roles of Kir4.1 and AQP4 in Autism Spectrum Disorders: Implications for Understanding Neurodevelopmental Mechanisms
The link between astrocytic dysfunction and ASD has become increasingly evident, particularly concerning the roles of Kir4.1 and AQP4 channels in maintaining brain homeostasis. Genetic studies have identified mutations in the KCNJ10 gene, which encodes Kir4.1, in individuals with ASD, suggesting that impaired K+ homeostasis may play a key role in the development of the disorder. Mutations or dysregulations in AQP4 have been linked to altered water balance in the brain, further contributing to the neurodevelopmental abnormalities observed in ASD (Li et al. 2021).
At the pathophysiological level, disruptions in Kir4.1 and AQP4 function can lead to a range of neurological impairments that are characteristic of ASD. Impaired K+ buffering by Kir4.1 results in elevated extracellular K+ levels, which increase neuronal excitability and predispose individuals to seizures, a common comorbidity in ASD. Furthermore, the increased excitability of neurons can result in abnormal synaptic transmission, contributing to the sensory processing deficits and cognitive impairments seen in individuals with ASD (Shinohe et al. 2006).
In addition to neuronal hyperexcitability, astrocytic dysfunction may also contribute to neuroinflammation, which has been increasingly recognized as a key feature of ASD. Studies have shown that individuals with ASD often exhibit elevated levels of pro-inflammatory cytokines in the brain, suggesting that chronic neuroinflammation may exacerbate the synaptic and neuronal dysfunctions associated with the disorder. The role of AQP4 in regulating the brain’s immune response is particularly important in this context, as disruptions in AQP4-mediated water transport can lead to increased neuroinflammation and further impairments in neuronal function (Shinohe et al. 2006).
Clinical observations have provided additional evidence of the connection between astrocytic dysfunction and ASD. Individuals with mutations in Kir4.1 often exhibit not only cognitive and behavioral impairments but also a heightened risk for seizures, underscoring the importance of K+ homeostasis in brain function. Similarly, alterations in AQP4 expression have been associated with abnormal water retention in the brain, which may contribute to the neurodevelopmental deficits seen in ASD (Banker et al. 2021). Table 1 provides a detailed summary of the primary functions, expression sites, physiological roles, and pathological implications of Kir4.1 and AQP4 dysfunction in the brain.
The Therapeutic Potential of Kir4.1 and AQP4 in Autism Spectrum Disorders
Current treatments for ASD are primarily symptomatic, focusing on managing behavioral and cognitive symptoms rather than addressing the underlying molecular dysfunctions that contribute to the disorder. However, emerging research suggests that targeting astrocytic dysfunction—particularly through the modulation of Kir4.1 and AQP4 channels—may offer new therapeutic opportunities for treating ASD (Li et al. 2021).
One potential therapeutic approach involves using pharmacological agents that enhance the function of Kir4.1 channels, thereby restoring K+ homeostasis and reducing neuronal hyperexcitability. Several experimental compounds have been developed to increase the activity of Kir4.1 channels, and early studies in animal models have shown promising results in reducing seizure susceptibility and improving synaptic function. These findings suggest that pharmacological modulation of Kir4.1 may be an effective strategy for managing the cognitive and behavioral symptoms associated with ASD (Kinboshi et al. 2020).
Similarly, therapies targeting AQP4 function could provide new avenues for treating ASD by restoring water homeostasis in the brain. AQP4 is involved in various processes, including cell migration, neuroinflammation, and the clearance of metabolic waste products from the brain. Modulating AQP4 activity may help mitigate the neuroinflammatory processes that contribute to synaptic dysfunction in ASD, as well as enhance the brain’s ability to clear neurotoxic proteins that may exacerbate neurodevelopmental deficits (Kinboshi et al. 2020).
Gene therapy represents another promising approach for addressing astrocytic dysfunction in ASD. Advances in gene editing technologies, such as CRISPR-Cas9, have made it possible to target specific genes involved in ion channel function, including Kir4.1 and AQP4. By correcting the genetic mutations that impair the function of these channels, gene therapy could potentially restore K+ and water homeostasis, thereby alleviating the cognitive and behavioral impairments associated with ASD.
In addition to pharmacological and gene-based therapies, Bataveljic et al. (2024) suggest that targeting these channels could have broader therapeutic applications beyond ASD. For instance, disruptions in Kir4.1 and AQP4 function have been implicated in various neurological disorders, including epilepsy, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS). Understanding the role of these channels in maintaining brain homeostasis across different disorders could provide valuable insights into developing targeted therapies that address the underlying molecular dysfunctions common to these conditions (Bataveljic et al. 2024).
Conclusion
Kir4.1 and AQP4 channels are essential for maintaining K+ and water homeostasis in the brain. They play critical roles in regulating synaptic transmission, preventing neuronal hyperexcitability, and protecting neurons from excitotoxicity. Dysfunction in these channels has increasingly been recognized as a contributing factor in the pathophysiology of Autism Spectrum Disorder (ASD), with mutations in these channels linked to the cognitive, behavioral, and sensory processing abnormalities characteristic of the disorder.
Understanding the precise mechanisms through which Kir4.1 and AQP4 dysfunction lead to these abnormalities provides valuable insights into developing novel therapeutic approaches. Targeting these channels through pharmacological agents, gene therapy, or other interventions holds the potential to restore K+ and water homeostasis, improve synaptic function, and mitigate the cognitive and behavioral symptoms associated with ASD. Future research should focus on elucidating the complex interactions between these channels and other homeostatic mechanisms in the brain, with the goal of developing targeted treatments that not only manage the symptoms of ASD but also address its underlying molecular dysfunctions.
Potential Limitations
-
(1)
Scope of Research the review may not encompass all the existing studies on Kir4.1 and AQP4 channels in relation to ASD, potentially overlooking some relevant findings.
-
(2)
Biased Selection the selection of studies included in the review might have an inherent bias, leading to an overemphasis on certain aspects while underrepresenting others.
-
(3)
Heterogeneity of Studies the included studies might have methodological differences and varied sample sizes, making it difficult to draw definitive conclusions.
-
(4)
Emerging Research new discoveries and ongoing research may alter the current understanding of Kir4.1 and AQP4 channels in ASD, making some of the review’s conclusions subject to change.
-
(5)
Therapeutic Implications the potential therapeutic interventions discussed might not be comprehensive and could benefit from further experimental validation.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- ALS:
-
Amyotrophic Lateral Sclerosis
- AQP4:
-
Aquaporin-4
- ASD:
-
Autism Spectrum Disorder
- BBB:
-
Blood–Brain Barrier
- CNS:
-
Central Nervous System
- GFAP:
-
Glial Fibrillary Acidic Protein
- GLT-1:
-
Glutamate Transporter 1
- OAPs:
-
Orthogonal Arrays of Particles
- MGLAR5:
-
Metabotropic Glutamate Receptor 5
- K+ :
-
Potassium
- Kir4.1:
-
Potassium Inwardly Rectifying Channel subtype 4.1
- KCNJ10:
-
Potassium Inwardly Rectifying Channel Subfamily J Member 10
- PA6:
-
Pentamidin Analog 6
- VPA:
-
Valproic acid
References
Banker SM, Gu X, Schiller D, Foss-Feig JH (2021) Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci 44(10):793–807. https://doi.org/10.1016/j.tins.2021.08.005
Bataveljic D, Pivonkova H, de Concini V, Hébert B, Ezan P, Briault S, Bemelmans AP, Pichon J, Menuet A, Rouach N (2024) Astroglial Kir4.1 potassium channel deficit drives neuronal hyperexcitability and behavioral defects in Fragile X syndrome mouse model. Nat Commun 15(1):3583. https://doi.org/10.1038/s41467-024-47681-y
Cucchiara F, Frumento P, Banfi T, Sesso G, Di Galante M, D’Ascanio P, Valvo G, Sicca F, Faraguna U (2020) Electrophysiological features of sleep in children with Kir4.1 channel mutations and Autism–Epilepsy phenotype: a preliminary study. Sleep. https://doi.org/10.1093/sleep/zsz255
Darvishmolla M, Heysieattalab S, Saeedi N, Hosseinmardi N, Janahmadi M (2022) Involvement of hippocampal astrocytic connexin-43 in morphine dependence. Physiol Behav 247:113710. https://doi.org/10.1016/j.physbeh.2022.113710
Davoudi S, Rahdar M, Hosseinmardi N, Behzadi G, Janahmadi M (2023) Chronic inhibition of astrocytic aquaporin-4 induces autistic-like behavior in control rat offspring similar to maternal exposure to valproic acid. Physiol Behav 269:114286. https://doi.org/10.1016/j.physbeh.2023.114286
Davoudi S, Rahdar M, Borjkhani M, Alavi-Majd H, Hosseinmardi N, Behzadi G, Janahmadi M (2025) The impact of astroglia Kir4.1 channel dysfunction on neuronal activity and autism-related behavioral abnormalities. Glia. https://doi.org/10.1002/glia.24676. (Epub ahead of print)
Elahi-Mahani A, Heysieattalab S, Hosseinmardi N, Janahmadi M, Seyedaghamiri F, Khoshbouei H (2018) Glial cells modulate hippocampal synaptic plasticity in morphine dependent rats. Brain Res Bull 140:97–106. https://doi.org/10.1016/j.brainresbull.2018.04.006
Gzielo K, Nikiforuk A (2021) Astroglia in autism spectrum disorder. Int J Mol Sci. https://doi.org/10.3390/ijms222111544
Joon P, Kumar A, Parle M (2021) What is autism? Pharmacol Rep 73(5):1255–1264. https://doi.org/10.1007/s43440-021-00244-0
Kinboshi M, Ikeda A, Ohno Y (2020) Role of astrocytic inwardly rectifying potassium (Kir) 4.1 channels in epileptogenesis. Front Neurol 11:626658. https://doi.org/10.3389/fneur.2020.626658
Li X, Lv J, Li J, Ren X (2021) Kir4.1 may represent a novel therapeutic target for diabetic retinopathy (Review). Exp Ther Med 22(3):1021. https://doi.org/10.3892/etm.2021.10453
Lo J, Forst AL, Warth R, Zdebik AA (2022) EAST/SeSAME syndrome and beyond: the spectrum of Kir4.1- and Kir5.1-associated channelopathies. Front Physiol 13:852674. https://doi.org/10.3389/fphys.2022.852674
Morin M, Forst AL, Pérez-Torre P, Jiménez-Escrig A, Barca-Tierno V, García-Galloway E, Warth R, Lopez-Sendón Moreno JL, Moreno-Pelayo MA (2020) Novel mutations in the KCNJ10 gene associated to a distinctive ataxia, sensorineural hearing loss and spasticity clinical phenotype. Neurogenetics 21(2):135–143. https://doi.org/10.1007/s10048-020-00605-6
Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML (2016) The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132(1):1–21. https://doi.org/10.1007/s00401-016-1553-1
Ohno Y, Kunisawa N, Shimizu S (2021) Emerging roles of astrocyte Kir4.1 channels in the pathogenesis and treatment of brain diseases. Int J Mol Sci. https://doi.org/10.3390/ijms221910236
Ostrowski J, Religioni U, Gellert B, Sytnik-Czetwertyński J, Pinkas J (2024) Autism spectrum disorders: etiology, epidemiology, and challenges for public health. Med Sci Monit 30:e944161. https://doi.org/10.12659/msm.944161
Saeedi N, Heysieattalab S, Janahmadi M, Hosseinmardi N (2022) The role of glial glutamate transporter in the baseline synaptic response and short-term synaptic plasticity of CA1 area of the hippocampus in male Wistar rat. Med J Tabriz Univ Med Sci 44(5):380–389. https://doi.org/10.34172/mj.2022.044
Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ, Bill RM (2022) Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 145(1):64–75. https://doi.org/10.1093/brain/awab311
Santello M, Toni N, Volterra A (2019) Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22(2):154–166. https://doi.org/10.1038/s41593-018-0325-8
Sauer AK, Stanton JE, Hans S, Grabrucker AM (2021) Chapter 1: autism spectrum disorders: etiology and pathology. In: Grabrucker AM (ed) Autism spectrum disorders. Exon Publications, Brisbane (AU). https://doi.org/10.36255/exonpublications.autismspectrumdisorders.2021.etiology
Schenkman L (2022) Portrait of a research field: astrocytes in autism. Transmitter. Retrieved 14 July 2022 from https://doi.org/10.53053/zjbk3878
Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N (2006) Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry 30(8):1472–1477. https://doi.org/10.1016/j.pnpbp.2006.06.013
Sicca F, Imbrici P, D’Adamo MC, Moro F, Bonatti F, Brovedani P, Grottesi A, Guerrini R, Masi G, Santorelli FM, Pessia M (2011) Autism with seizures and intellectual disability: possible causative role of gain-of-function of the inwardly-rectifying K+ channel Kir4.1. Neurobiol Dis 43(1):239–247. https://doi.org/10.1016/j.nbd.2011.03.016
Sicca F, Ambrosini E, Marchese M, Sforna L, Servettini I, Valvo G, Brignone MS, Lanciotti A, Moro F, Grottesi A, Catacuzzeno L, Baldini S, Hasan S, D’Adamo MC, Franciolini F, Molinari P, Santorelli FM, Pessia M (2016) Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci Rep 6:34325. https://doi.org/10.1038/srep34325
Sloan SA, Barres BA (2014) Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol 27:75–81. https://doi.org/10.1016/j.conb.2014.03.005
Underwood JF, DelPozo-Banos M, Frizzati A, John A, Hall J (2022) Evidence of increasing recorded diagnosis of autism spectrum disorders in Wales, UK: an e-cohort study. Autism 26(6):1499–1508. https://doi.org/10.1177/13623613211059674
Wang S, Wang B, Shang D, Zhang K, Yan X, Zhang X (2022) Ion channel dysfunction in astrocytes in neurodegenerative diseases. Front Physiol 13:814285. https://doi.org/10.3389/fphys.2022.814285
Weaver CD, Denton JS (2021) Next-generation inward rectifier potassium channel modulators: discovery and molecular pharmacology. Am J Physiol Cell Physiol 320(6):C1125-c1140. https://doi.org/10.1152/ajpcell.00548.2020
Xiong Y, Chen J, Li Y (2023) Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Front Neurosci 17:1125428. https://doi.org/10.3389/fnins.2023.1125428
Acknowledgements
This publication was supported by Professor Mahyar Janahmadi and Neurophysiology Research Center, Institute of Neuroscience and Cognition, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Funding
No funding was received to support the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Vesal Abbasian contributed to methodology, investigation, and writing—original draft. Shima Davoudi contributed to conceptualization, methodology, and review & editing. Amin Vahabzadeh contributed to methodology and investigation. Mohammad Javad Maftoon-Azad contributed to methodology and investigation. Mahyar Janahmadi contributed to conceptualization, supervision, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
We would like to declare that none of the authors have any conflicts of interest related to the content of this article.
Declaration of Generative AI in Scientific Writing
AI assistance was utilized solely for proofreading and grammatical corrections in the preparation of this manuscript, in order to maintain ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abbasian, V., Davoudi, S., Vahabzadeh, A. et al. Astroglial Kir4.1 and AQP4 Channels: Key Regulators of Potassium Homeostasis and Their Implications in Autism Spectrum Disorders. Cell Mol Neurobiol 45, 56 (2025). https://doi.org/10.1007/s10571-025-01574-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-025-01574-w