Abstract
To model the dynamics of neuron membrane excitability many models can be considered, from the most biophysically detailed to the highest level of phenomenological description. Recent works at the single neuron level have shown the importance of taking into account the evolution of slow variables such as ionic concentration. A reduction of such a model to models of the integrate-and-fire family is interesting to then go to large network models. In this paper, we introduce a way to consider the impairment of ionic regulation by adding a third, slow, variable to the adaptive Exponential integrate-and-fire model (AdEx). We then implement and simulate a network including this model. We find that this network was able to generate normal and epileptic discharges. This model should be useful for the design of network simulations of normal and pathological states.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
In the study of seizure dynamics at the single neuron level, experiments have shown the importance of ionic regulation. Indeed, elevation of external ionic potassium concentration leads to seizure-like behaviors of the cells. Biophysical models have shown underlying mechanisms responsible for such activities (Depannemaecker et al., 2022b; Bazhenov et al., 2004).
Detailed biophysical network models have been developed to study seizure mechanisms (Rodrigues et al., 2015; Tejada et al., 2014; Santhakumar et al., 2005). However, these models are complex with many parameters and variables, making their dynamics difficult to analyze. While seizures are typically seen as network events, similar dynamics can also be observed at the single-cell level in biophysical models (Bikson et al., 2003; Bragin et al., 1997; Chizhov et al., 2018; Cressman et al., 2009). Phenomenological models, characterized by their minimal number of parameters and variables, enable comprehensive dynamical analyses and replicate many of the activities observed in experimental settings (Jirsa et al., 2014). Some works integrate both approaches by reducing a detailed biophysical model to a lower-dimensional form, preserving the benefits of a generic model (Depannemaecker et al., 2022b).
However, these models are based on the Hodgkin-Huxley formalism (Hodgkin & Huxley, 1952), where the action potential occurs thanks to the nonlinear dynamics of the gating variables. The stiffness of these gating variables requires to use of very small time steps in order to simulate them accurately. This limits the use of such models as building blocks of large networks for long simulations. To address this issue we propose to reduce some aspects of the dynamics captured by the biophysical detailed model by using a formalism based on an integrate-and-fire type model. The adaptive exponential integrate-and-fire (AdEx) (Brette & Gerstner, 2005; Naud et al., 2008) model extends the classical integrate-and-fire (IF) model by incorporating two additional mechanisms: spike threshold adaptation through an exponential term and adaptation with an additional variable. In the AdEx model, the exponential term captures the subthreshold behavior of the neuron’s membrane potential. Specifically, the exponential term reflects the voltage-dependent conductance of the neuron’s membrane, which is a function of the difference between the neuron’s membrane potential and its threshold potential. The adaptation mechanism is modeled by an additional current term that reflects the neuron’s ability to adjust its firing threshold in response to the recent history of input. Specifically, this current term represents the activation of voltage-dependent potassium channels that contribute to the membrane’s afterhyperpolarization following a spike. The strength and time course of the adaptation current can be adjusted by the model’s parameters in order to reproduce a variety of observed neuronal responses. This adaptation variable enters as a current in the membrane voltage equation, which can create unrealistic values of individual membrane potential (Górski et al., 2021), but may not affect qualitatively the global dynamics at network levels (Depannemaecker et al., 2022a). We thus introduce a third variable, which accounts for the effect of ionic impairment by modulating parameters that are conceptually associated with, or may be affected by ionic changes (e.g. reversal potential, spiking threshold).
Such a model can then become the basis to build large networks (Depannemaecker et al., 2022a), and hence study not only seizure propagation but also the emergence of such pathological patterns at the network level.
The article is organized as follows. In Section 2, we present both the single-neuron and the network model that we are going to analyze. Then, in Section 3, we show a correspondence with another model based on the Hodgkin-Huxley formalism and study different possible network configurations leading to the emergence of different patterns associated with epilepsy. We show how the severity of the local impairment or the size of the cell population concerned, leads to different patterns and propagation profiles. Finally, in Section 4, we summarise our findings and propose a few perspectives for future work.
2 Methods
We present here the single-neuron model. Subsequently, we explain how such a model is used for large network simulations.
2.1 Single-neuron models
The model builds on the AdEx (Brette & Gerstner, 2005; Naud et al., 2008). The AdEx model captures single neuron dynamics with two key equations: the membrane potential \( V \) and the adaptation current \( w \). The parameters are the capacitance \( C \), leak conductance \( g_L \), resting potential \( E_L \), threshold of sodium channels activation \( V_T \), sharpness of the exponential rise \( \Delta _T \), adaptation time constant \( \tau _w \), subthreshold adaptation \( a \), and input current \( I_s \). The equations are:
together with the following after-spike reset mechanism:
The membrane potential is reset to \( V_R \) and the adaptation variable is increase by a current \(b\).
Based on this formalism, we added a third variable z with a slow timescale (with associated time constant \(\varepsilon \)) compared to the two other variables V and w. This variable z will phenomenologically aggregate the different impairments that may affect the ionic concentration regulation and thus the excitability of the neuronal membrane. Thus, this variable z modulates parameters (\(E_L\), \(V_T\)), which enter into the other variables’ equations. The \(E_L\) parameter relates to the hyperpolarizing mechanism while the parameter \(V_T\) relates to the opening of sodium channels (Brette & Gerstner, 2005). Hence, \(V_T\) may not be affected in the same way as \(E_L\), and it is thus scaled by parameter \(\beta \). Furthermore, a counteractive term \(-g_p z\) models phenomenologically the effects of the aggregate of biophysical mechanisms that are “working against the impairment” (e.g., the Na/K-pump, co-transporters, exchangers, etc...). Parameter \(g_p\) is the global equivalent conductance resulting from all these mechanisms. Finally, parameter \(Z_0\) corresponds to the potential to which the system will be attracted (depolarized) due to the pathological impairment. Therefore, the extended AdEx model that will study takes the form:
with still the after-spike reset mechanism:
Parameter values are given in Table 1.
2.2 Network model
The network we will be studying is made up of inhibitory fast-spiking cells (FS) and excitatory regular spiking cells (RS). It contains 10,000 cells, 80% of which are excitatory and 20% inhibitory, connected through a random and sparse (Erdős-Rényi type) architecture with a probability of 5%. A conductance-based model of synapses enables connections between cells, it typically takes the form of Eq. (5) below:
where \(E_E=0 \ \textrm{mV}\) and \(E_I=-80 \ \textrm{mV}\) are the reversal potential of excitatory synapses and of inhibitory synapses, respectively. Parameters \(g_{E}\) and \(g_{I}\) are the excitatory and inhibitory conductances, respectively. They are governed by Eq. (6), and they are increased by a quantity \(Q_E=1.5 \ \textrm{nS}\) and \(Q_I=5 \ \textrm{nS}\) for each excitatory and inhibitory incoming spike, respectively. The timescale of synaptic conductance is determined by \(\tau _{syn} = 5 \ \textrm{ms}\).
3 Results
Next, we present our findings in two distinct parts. First, we explore the behavior of our single-neuron model Eq. (3) in detail. This part will provide insights into how individual neurons function and react under various conditions, with a particular focus on the role of ionic regulation. In the second part, we delve into the dynamics of a large-scale neural network composed of 10,000 individual neurons. This investigation will help us understand how the characteristics of the extended AdEx model Eq. (3), discussed in the first part, influence the collective behavior and patterns that emerge within the network. This second part gives some insights into complex pathological phenomena such as seizure propagation.
3.1 Single neuron model
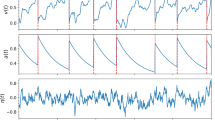
The single-neuron model exhibits different patterns of spontaneous pathological activities upon an increase of the value of \(Z_0\), consistent with what is observed in biophysical descriptions (Depannemaecker et al., 2022b). However, the integrate-and-fire type model proposed here captures the after-spike repolarization via a reset mechanism. Thus, this after-threshold reset does not allow for the existence of a fixed point for values corresponding to depolarized membrane potential. Therefore, it is impossible to capture events with depolarization blocks. In this model, spontaneous activity appears for values of \(Z_0>48\) mV, and an increase of frequency is observed at the onset of a sustained tonic spiking pattern. While increasing \(Z_0\) to values higher than 40 mV, the model exhibits a pathological bursting pattern that can be associated with seizures (Depannemaecker et al., 2022b). Finally, for \(Z_0>20\) mV the model dynamics corresponds to sustained ictal activity associated with status epilepticus and consisting of a constant high-frequency spiking pattern. After intense spiking activity, the current w reaches very high values leading to a strong negative input in the variable V that may take non-realistic values. This problem has been solved previously (Górski et al., 2021); however, it may not affect the global network dynamics, specifically in the case of seizures as shown in a previous work (Depannemaecker et al., 2022a). We distinguished the tonic firing pattern from the sustain ictal activities with two main observations. First these patterns appear for two different range of value of \(Z_0\) and are separated by the bursting pattern. Secondly, in the tonic firing pattern, the spiking activity reaches a steady state in interplay with the adaptation, while in sustain ictal activity pattern, the system quickly go to its maximal possible firing rate and the adaptation variable \(w\) saturates (Fig. 1).
Spontaneous spiking patterns: (a) tonic spiking for \(Z_0=-45\) mV, (b) pathological bursting pattern for \(Z_0=-40\) mV (c) Sustain ictal activity for \(Z_0=-20\) mV. The three simulations start from the same initial conditions, from which the (V, w) subsystem evolves to the specific pattern, driven by the effect of the slow variable z
In order to understand the different dynamics observed in system Eq. (3), we considered z as being the slowest variable, enabling the loss of stability that leads to spiking activity. We then consider z as a parameter—which amounts to taking the limit \(\varepsilon =0\) in system Eq. (3) and obtaining its so-called fast subsystem— and we investigate the bifurcation structure of the resulting 2D system (with reset). The Jacobian matrix Eq. (7) of the fast subsystem is given by:
Hence, the trace of \(\textbf{J}\) takes the form:
and its determinant reads:
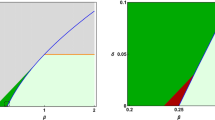
Thus, based on the trace and determinant, we can study the loss of stability in the \(V-w\) subsystem considering z as a parameter. In the Fig. 2(a), we show the evolution of the two fixed points of the sub-system \(V-w\) (similar to the classical AdEx Naud et al., 2008; Brette & Gerstner, 2005). With the evolution of z, the spiking activity appears through a loss of stability by crossing first an Andronov-Hopf bifurcation ; unstable sub-threshold oscillations appear and the corresponding branch disappear through a saddle-homoclinic bifurcation, as the unstable cycle collides with the saddle equilibrium of the system. Then, the two fixed points rapidly collapse and disappear through a saddle-node bifurcation. The spiking pattern appears for a lower value of z through another saddle-homoclinic bifurcation involving a homoclinic connection with possibly many resets, and followed in parameter space by a complex sequence of additional bifurcations, for which numerical evidence suggest that they are period-doubling bifurcations and whose analysis goes beyond the scope of the present work; see Desroches et al. (2021, 2024) for more precise details on such bifurcation sequences in similar models. Note that the actual z value of this homoclinic bifurcation does not correspond to the sketch shown in Fig. 2(a), this is only for convenience and to grasp the full bifurcation sequence. In the case of the pathological bursting pattern, the oscillation emerges from the interaction between the three variables V, w, and z (see Fig. 2(b)). We thus separate two mechanisms leading to healthy or pathological bursts. Indeed, we should mention that the AdEx model is capable of generating bursting activity, as shown in detail in previous work by Naud et al. (2008). The original AdEx model requires an external input to spike (or specific parametrization that would lead to permanent steady-state spiking activity). In the model presented here, the spiking patterns appear through the interaction with the slow variable z.
Bifurcations and trajectories: (a) Bifurcation diagram of the fast subsystem with respect to parameter z. The computed curve of equilibria is represented in black, the solid (resp. dashed) line corresponding to stable (resp. unstable) equilibria. Bifurcations along this family are an Andronov-Hopf (AH), a saddle-node (SN) and a saddle homoclinic (red Hom). The red curve is a sketch (i.e., not computed) of the branch of limit cycles (which exist due to reset). It appears through another, yet more complicated, saddle-homoclinic bifurcation (purple Hom). (b) Pathological bursting pattern simulation plotted in the variable spaces, the interaction between the three variables leads to this pattern. (c) Phase-space representation of another bursting pattern \(\Gamma \) (in blue), together with the Nullsurface of v (\(S^0\), in green, folded along the grey curve \(\textsf{F}\)), its intersection with the Nullsurface of w (\(\mathscr {S}^0\), in black), and the reset plane \(\textsf{R}\). The associated parameter values are: \( Z_0 = -51.2 \), \( C = 100 \), \( g_L = 10 \), \( E_L = -65 \), \( \alpha = 1 \), \( a = 1 \), \( b = 0.06 \), \( V_D = -40 \), \( V_R = -54 \), \( I_s = 0 \), \( V_T = -55 \), \({\Delta _T} = 2 \), \( \tau _W = 200 \), \( g_p = 1 \), and \( {\varepsilon = 0.001} \)
To understand the role of the slow variables we can plot the NullSurface into the phase space, and, project one trajectory of a simulation where the system exhibits bursting activity (see Fig. 2(c)). We thus visualise the structure enabling the emergence of the bursting pattern. The identification of different emerging electrophysiological patterns can be achieved through bifurcation analysis (Izhikevich, 2006). This method is also applicable to pathological patterns (Jirsa et al., 2014; Wei et al., 2014; Depannemaecker et al., 2022b), providing a common framework to understand the dynamics of both biophysical and phenomenological models of seizures observed clinically or experimentally (Jirsa et al., 2014; Depannemaecker et al., 2023; Hashemi et al., 2024). Thus, bursting patterns can be classified based on the type of bifurcation occurring at the onset and offset of the event.
The loop for bursting emerges with a concomitant effect of w and z, as shown by the trajectory in Fig. 2(c), that exists in a surface in the diagonal on the \(w-z\) plan. The loss of stability at the onset of the burst occurs through a saddle-node bifurcation at the folding of the v-nullsurface (\(S^0\), in green in Fig. 2(c)). The offset of the burst occurs through a (nonsmooth) homoclinic bursting, where the reset surface (grey) and the v Nullsurface (green) meet. The intersection of the v- and the w-nullsurfaces forms a curve \(\mathscr {S}_0\) traced on \(S^0\), which the bursting dynamics would follow if z was the only slow process in the model. However, Fig. 2(c) shows that the bursting pattern rather follows the surface \(S^0\) and much less the curve \(\mathscr {S}^0\). This indicates that w should also be considered as a slow process. Further analysis would be required to follow up on such considerations, which are an interesting topic for future work. For more elements about slow-fast bursting dynamics in IF models, we refer the reader to Desroches et al. (2021, 2024).
I- Propagation of pathological activity in a network model. a)-c) Maximum firing rates of the impaired cells (a), inhibitory cells (b), and excitatory cells (c) as a function of \(Z_0\) and \(N_{SC}\) (defining the severity of local impairment and number of impaired cells respectively. d)-f) Examples of different neuronal dynamics illustrate the effect of an increasing number of impaired cells \(N_{SC}\) leading to qualitative changes in the propagation of the pathological activity. For each case, the corresponding raster plot and average firing rate of each cell population is shown (impaired cells in blue, inhibitory cells in red, and excitatory cells in green). The plots correspond to the parameters \(Z_0=-50mV\) and \(N_{SC}=500, 100\), and 4000 respectively (the corresponding parameters are indicated with green rectangles in panels a,b,c).II- Examples of different activity patterns for the propagation of pathological activity. The network model exhibits a large repertoire of patterns of activity dependent of specific system parameters. We show in the figure the average dynamics obtained for three different sets of parameters: \(Z_0=-10mV\), \(N_{SC}=4000\) (a); \(Z_0=-10mV\), \(N_{SC}=7500\) (b) and \(Z_0=-40mV\), \(N_{SC}=7500\) (c). The color code is the same as in I
In this model, the variable z and the parameter \(Z_0\) serve to abstract and encapsulate the biophysical effects of ionic dysregulation, particularly during the elevation of extracellular potassium (\([K^+]_o\)) described in Depannemaecker et al. (2022b). The potassium elevation affects the neuronal membrane’s excitability by altering the Nernst potentials. The variable z, depending on \(Z_0\), captures these slow, cumulative changes in the ionic environment, reflecting the build-up of conditions leading to pathological seizures-like states. Thus, as \(Z_0\) increases, it pushes the neuronal dynamics towards states associated with seizure activity, effectively bridging the detailed biophysical processes of ionic regulation impairment (Depannemaecker et al., 2022b) with the neuronal excitability of AdEx model (Brette & Gerstner, 2005).
3.2 Network model
In this section, we detail how the severity of local impairment or the size of the affected cell population influences the emergence of distinctive patterns and propagation profiles in the context of epilepsy. The network consists of 2,000 inhibitory fast-spiking interneurons and 8,000 excitatory regular-spiking neurons, among which \(N_{SC}\) cells have values of \(Z_0\) that lead to pathological seizure-like patterns. To implement this analysis we performed a large parameter exploration, with a focus on the parameter \(Z_0\), which determines the level of local impairment, and the parameter \(N_{SC}\) which defines the number of impaired cells within the system. The results of the analysis are presented in Fig. 3 I. In panels a) to c) we show the maximum firing rates obtained during an 8-second simulation of the network as a function of \(Z_0\) and \(N_{SC}\). In panels d) to f) we show examples of the different dynamics obtained from the network. We see from this figure that the system’s behavior can be generally divided into three regions. In the first region (high \(Z_0\) and low \(N_{SC}\)) the activity of the system remains in an asynchronous irregular (AI) regime with the impaired cells firing at a higher rate compared to healthy excitatory neurons but remaining within physiologically functional values. In the second region (intermediate values of \(Z_0\) and \(N_{SC}\)), the firing rates of impaired cells exhibit already pathological dynamics with an oscillatory activity of large amplitude (up to 150Hz). In this second regime, the activity of the inhibitory cells follows the abnormal activity of the impaired cells, with the emergence of high-amplitude oscillations. This response of the inhibitory cells is enough to prevent the propagation of the pathological activity towards the healthy excitatory cells, which remain within normal values of firing rates with only moderate alteration in their dynamics. Finally, in the third region (low \(Z_0\) and high \(N_{SC}\)), the pathological activity also propagates to the healthy excitatory population. For the example shown in panel f) of the figure we see that in the third regime, the entire system exhibits an alternation of periods of normal dynamics and periods of global increases in the firing rates. We notice that the pattern of activity observed in the third regime depends on the specific values of \(Z_0\) and \(N_{SC}\) and that the network model exhibits a large repertoire of possible patterns and dynamics. For completeness, we show in Fig. 3 II three different patterns obtained from different combinations of parameters.
These network dynamics can be related to experimental observation (Wenzel et al., 2019). Indeed, the propagation of seizure-like activities from one population of impaired cells to the rest of the network, follows a dynamics which resembles previous experimental observation. In Fig. 3 we observe a threshold effect, where the propagation to healthy population is comparable to the phenomenon observable shown in Fig. 6 of the work of Wenzel et al. (2019).
4 Discussion
Finally, this article has explored the dynamics of single neurons with an additional variable to account for ionic regulation impairment leading to pathological behavior. Experiments have highlighted the critical role of ionic regulation at the single neuron level, particularly in inducing seizure-like behaviors through elevated external ionic potassium concentrations. Biophysical models, although informative, have their limitations due to the Hodgkin-Huxley formalism, which necessitates small time steps and restricts their applicability in large network simulations (Cressman et al., 2009; Depannemaecker et al., 2022b). To address this challenge, we have proposed an approach that simplifies certain aspects of the biophysical model using the adaptive exponential integrate-and-fire (AdEx) formalism, which includes subthreshold behavior modeling and adaptation mechanisms. This approach enables to capture different patterns emerging spontaneously from ionic changes. We show how the model captures these dynamics and their impact on neuronal responses. Furthermore, our exploration extends to the study of network configurations and their associations with epilepsy patterns, demonstrating how local impairment severity and cell population size influence patterns and propagation profiles.
If it is a simple model for seizure generation, it has some limitations in terms of dynamical repertoire. Indeed, a pattern associated with sustained depolarization (depolarization block, or permanent depolarization) can not be captured by this model due to the reset mechanism and thus the impossibility of the existence of a stable fixed point for high values (depolarized) of the membrane potential variable. In case of interest in these specific patterns, other models are more appropriate (Depannemaecker et al., 2023). Such behaviors appear in mean-field approximation (Bandyopadhyay et al., 2021) of biophysically neuron models (Depannemaecker et al., 2022b).
Our approach proposed here is interesting to model large network dynamics and to capture the overall emergent dynamics. This simple model is thus a good candidate for building a mean-field model (Carlu et al., 2020; Alexandersen et al., 2024; Stenroos et al., 2024) capturing epileptic focus dynamics and which can be integrated into large-scale models of pathological brain states (Wang et al., 2024).
Data Availability
No datasets were generated or analysed during the current study.
References
Alexandersen, C. G., Duprat, C., Ezzati, A., Houzelstein, P., Ledoux, A., Liu, Y., Saghir, S., Destexhe, A., Tesler, F., & Depannemaecker, D. (2024). A mean field to capture asynchronous irregular dynamics of conductance-based networks of adaptive quadratic integrate-and-fire neuron models. Neural Computation, 36(7), 1433–1448.
Bandyopadhyay, A., Rabuffo, G., Calabrese, C., Gudibanda, K., Depannemaecker, D., Ivanov, A., Bernard, C., Jirsa, V. K., & Petkoski, S. (2021). Mean-field approximation of network of biophysical neurons driven by conductance-based ion exchange. bioRxiv, 2021–2010.
Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T. J. (2004). Potassium model for slow (2–3 hz) in vivo neocortical paroxysmal oscillations. Journal of Neurophysiology, 92(2), 1116–1132.
Bikson, M., Hahn, P. J., Fox, J. E., & Jefferys, J. G. R. (2003). Depolarization block of neurons during maintenance of electrographic seizures. Journal of Neurophysiology, 90(4), 2402–2408.
Bragin, A., Penttonen, M., & Buzsáki, G. (1997). Termination of epileptic afterdischarge in the hippocampus. The Journal of Neuroscience, 17(7), 2567–2579.
Brette, R., & Gerstner, W. (2005). Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. Journal of Neurophysiology, 94(5), 3637–3642.
Carlu, M., Chehab, O., Porta, L. D., Depannemaecker, D., Héricé, C., Jedynak, M., Ersöz, E. K., Muratore, P., Souihel, S., Capone, C., Zerlaut, Y., Destexhe, A., & di Volo, M. (2020). A mean-field approach to the dynamics of networks of complex neurons, from nonlinear integrate-and-fire to hodgkin-huxley models. Journal of Neurophysiology, 123(3), 1042–1051.
Chizhov, A. V., Zefirov, A. V., Amakhin, D. V., Smirnova, E. Y., & Zaitsev, A. V. (2018). Minimal model of interictal and ictal discharges “epileptor-2’’. PLoS Computational Biology, 14(5), e1006186.
Cressman, J. R., Ullah, G., Ziburkus, J., Schiff, S. J., & Barreto, E. (2009). The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. single neuron dynamics. Journal of Computational Neuroscience, 26(2), 159–170.
Depannemaecker, D., Ivanov, A., Lillo, D., Spek, L., Bernard, C., & Jirsa, V. (2022b). A unified physiological framework of transitions between seizures, sustained ictal activity and depolarization block at the single neuron level. Journal of Computational Neuroscience, 1–17.
Depannemaecker, D., Carlu, M., Bouté, J., & Destexhe, A. (2022a). A model for the propagation of seizure activity in normal brain tissue. Eneuro, 9(6), ENEURO.0234-21.2022.
Depannemaecker, D., Ezzati, A., Wang, H. E., Jirsa, V., & Bernard, C. (2023). From phenomenological to biophysical models of seizures. Neurobiology of Disease, 182, 106131.
Desroches, M., Kowalczyk, P., & Rodrigues, S. (2024). Discontinuity-induced dynamics in the conductance-based adaptive exponential integrate-and-fire model. HAL e-print 04665937. https://inria.hal.science/hal-04665937
Desroches, M., Kowalczyk, P., & Rodrigues, S. (2021). Spike-adding and reset-induced canard cycles in adaptive integrate and fire models. Nonlinear Dynamics, 104(3), 2451–2470.
Górski, T., Depannemaecker, D., & Destexhe, A. (2021). Conductance-based adaptive exponential integrate-and-fire model. Neural Computation, 33(1), 41–66.
Hashemi, M., Depannemaecker, D., Saggio, M., Triebkorn, P., Rabuffo, G., Fousek, J., Ziaeemehr, A., Sip, V., Athanasiadis, A., Breyton, M., Woodman, M., Wang, H., Petkoski, S., Sorrentino, P., & Jirsa, V. (2024). Principles and operation of virtual brain twins.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500–544.
Izhikevich, E. M. (2006). Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting. The MIT Press.
Jirsa, V. K., Stacey, W. C., Quilichini, P. P., Ivanov, A. I., & Bernard, C. (2014). On the nature of seizure dynamics. Brain: A Journal of Neurology, 137(Pt 8), 2210–2230.
Naud, R., Marcille, N., Clopath, C., & Gerstner, W. (2008). Firing patterns in the adaptive exponential integrate-and-fire model. Biological Cybernetics, 99(4–5), 335.
Rodrigues, A. M., Santos, L. E. C., Covolan, L., Hamani, C., & Almeida, A.-C. G. (2015). pH during non-synaptic epileptiform activity–computational simulations. Physical Biology, 12(5), 056007.
Santhakumar, V., Aradi, I., & Soltesz, I. (2005). Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: A network model of the dentate gyrus incorporating cell types and axonal topography. Journal of Neurophysiology, 93(1), 437–453.
Stenroos, P., Guillemain, I., Tesler, F., Montigon, O., Collomb, N., Stupar, V., Destexhe, A., Coizet, V., David, O., & Barbier, E. L. (2024). Eeg-fmri in awake rat and whole-brain simulations show decreased brain responsiveness to sensory stimulations during absence seizures. eLife, 12, RP90318.
Tejada, J., Garcia-Cairasco, N., & Roque, A. C. (2014). Combined role of seizure-induced dendritic morphology alterations and spine loss in newborn granule cells with mossy fiber sprouting on the hyperexcitability of a computer model of the dentate gyrus. PLoS Computational Biology, 10(5), e1003601.
Wang, H. E., Triebkorn, P., Breyton, M., Dollomaja, B., Lemarechal, J.-D., Petkoski, S., Sorrentino, P., Depannemaecker, D., Hashemi, M., & Jirsa, V. K. (2024). Virtual brain twins: From basic neuroscience to clinical use. National Science Review, 11(5).
Wei, Y., Ullah, G., & Schiff, S. J. (2014). Unification of neuronal spikes, seizures, and spreading depression. The Journal of Neuroscience, 34(35), 11733–11743.
Wenzel, M., Hamm, J. P., Peterka, D. S., & Yuste, R. (2019). Acute focal seizures start as local synchronizations of neuronal ensembles. The Journal of Neuroscience, 39(43), 8562–8575.
Acknowledgements
This research is supported by grants from the European Union, including the Human Brain Project H2020-94553 and the European Union’s Horizon Europe Programme under Specific Grant Agreement No. 101137289 (Virtual Brain Twin Project) and NSF-ANR (ImpactCom project). Additionally, funding has been received from the European Union’s Horizon Europe Programme under Specific Grant Agreement No. 101147319 (EBRAINS 2.0 Project). This work has also benefited from a government grant managed by the Agence Nationale de la Recherche (ANR) under the France 2030 program (PEPR) reference ANR-22-PESN-0012.
Funding
Open access funding provided by Université Paris-Saclay.
Author information
Authors and Affiliations
Contributions
Conceptualization: DD , VJ and AD; Model definition: DD; Model characterization, parametrization, simulations, and preparation of the figures: DD, FT and MD, writing of the original: DD, FT; All authors (DD, FT, MD, VJ, and AD) reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Action Editor: John Rinzel
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Depannemaecker, D., Tesler, F., Desroches, M. et al. Modeling impairment of ionic regulation with extended Adaptive Exponential integrate-and-fire models. J Comput Neurosci 53, 1–8 (2025). https://doi.org/10.1007/s10827-025-00893-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-025-00893-7