Abstract
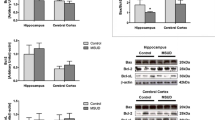
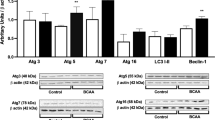
Maple Syrup Urine Disease (MSUD) is an autosomal recessive inherited disorder that affects the activity of the branched-chainα-keto acid dehydrogenase complex (BCDK). This deficiency on BCDK complex results in the accumulation of branched-chain amino acids (BCAA) leucine, isoleucine, valine, and their corresponding α-keto acids. Epigenetic changes can negatively affect the metabolism of BCAA. These changes are catalyzed by the epigenetic regulatory enzymes, e.g., DNA methyltransferase (DNMT), histone deacetylases (HDAC), and histone acetyltransferases (HAT). However, the impacts of BCAA administration on the activity of epigenetic regulatory enzymes in the brain of MSUD patients are still unknown. In this study, we aimed to demonstrate the impact of BCAA administration on the activity of DNMT, HDAC, and HAT in the brain structures of infant rats, an animal model of MSUD. For that, we administered a BCAA pool to infant rats for 21 days. We demonstrated that BCAA administration significantly increased the DNMT and HDAC activities in the hippocampus and striatum, but not in the cerebral cortex of MSUD infant rats. A positive correlation was observed between HDAC and DNMT activities in the hippocampus and striatum of animals exposed to BCAA injections. Our results showed that the BCAA administration could modulate epigenetic regulatory enzymes, mainly DNMT and HDAC, in the brains of infant rats. Therefore, we suggest that the increase in the activity of DNMT and HDAC in the hippocampus and striatum could partially explain the neurological impairments presented in animal models of MSUD.




Similar content being viewed by others
References
Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42(6):947–959. https://doi.org/10.1016/j.neuron.2004.05.021
Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM et al (2011) Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology 36(8):1545–1556. https://doi.org/10.1038/npp.2011.61
Bird AP (1986) CpG-rich islands and the function of DNA methylation. Nature 321(6067):209–213. https://doi.org/10.1038/321209a0
Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, Klee EW, Atwal PS (2017) Maple syrup urine disease: mechanisms and management. Appl Clin Genet 10:57–66. https://doi.org/10.2147/TACG.S125962
Bridi R, Fontella FU, Pulrolnik V, Braun CA, Zorzi GK, Coelho D, Wajner M, Vargas CR, Dutra-Filho CS (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230. https://doi.org/10.1016/j.jneumeth.2006.01.005
Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC et al (2011) Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet 20(4):631–640. https://doi.org/10.1093/hmg/ddq507
Cañas CA, Cañas F, Bonilla-Abadia F, Ospina FE, Tobón GJ (2016) Epigenetics changes associated to environmental triggers in autoimmunity. Autoimmunity 49(1):1–11. DOI:https://doi.org/10.3109/08916934.2015.1086996
Chuang DT, Shih VE (2001) Maple syrup urine disease (branchedchain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Day JJ, Sweatt JD (2010) DNA methylation and memory formation. Nat Neurosci 13:1319–1323. https://doi.org/10.1038/nn.2666
Day JJ, Sweatt JD (2011) Epigenetic mechanisms in cognition. Neuron 70(5):813–829. https://doi.org/10.1016/j.neuron.2011.05.019
Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E, Tahir A, Sweatt JD (2013) DNA methylation regulates associative reward learning. Nat Neurosci 16:1445–1452. https://doi.org/10.1038/nn.3504
Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M et al (2001) DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci 21:788–797. https://doi.org/10.1523/JNEUROSCI.21-03-00788.2001
Fernstrom JD (2005) Branched-chain amino acids and brain function. J Nutr 135(6 Suppl):1539S–1539S46S. https://doi.org/10.1093/jn/135.6.1539S
Fisher CR, Fisher CW, Chuang DT, Cox RP (1991) Occurrence of a Tyr393Asn (Y393N) mutation in the E1 alpha gene of the branched-chain alpha-keto acid dehydrogenase complex in maple syrup urine disease patients from a Mennonite population. Am J Hum Genet 49:429–434
Fraziera DM, Allgeierb C, Homerc C, Marriageb BJ, Ogatad B, Rohre F, Splettf PL, Stembridgeh A, Singhh RH (2014) Nutrition management guideline for maple syrup urine disease: An evidence- and consensus-based approach. Mol Genet Metab 112:210–217. https://doi.org/10.1016/j.ymgme.2014.05.006
Ganai SA, Banday S, Farooq Z, Altaf M (2016) Modulating epigenetic HAT activity for reinstating acetylation homeostasis: A promising therapeutic strategy for neurological disorders. Pharmacol Ther 166:106–122. https://doi.org/10.1016/j.pharmthera.2016.07.001
Goto K, Numata M, Komura J-I, Ono T, Bestor TH, Kondo H (1994) Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation 56:39–44. https://doi.org/10.1046/j.1432-0436.1994.56120039.x
Gregory RI, Randall TE, Johnson CA, Sanz LA, Feil R, Hata K, Arnaud P (2001) DNA methylation is linked to deacetylation of histone H3, but not H4, on the imprinted genes Snrpn and U2af1-rs1. Mol Cell Biol 21(16):5426–5436. https://doi.org/10.1128/MCB.21.16.5426-5436.2001
Grunstein M (1997) Histone acetylation in chromatin structure and transcription. Nature 389(6649):349–352. https://doi.org/10.1038/38664
Gu Z, Liu Y, Cai F, Patrick M, Zmajkovic J, Cao H et al (2019) Loss of EZH2 reprograms BCAA metabolism to drive leukemic transformation. Cancer Discov 9(9):1228–1247. https://doi.org/10.1158/2159-8290.CD-19-0152
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J et al (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459(7243):55–60. https://doi.org/10.1038/nature07925
Holliday R, Pugh JE (1975) DNA modification mechanisms and gene activity during development. Science 187:226–232
Iannitti T, Palmieri B (2011) Clinical and experimental applications of sodium phenylbutyrate. Drugs R D 11(3):227–249. https://doi.org/10.2165/11591280-000000000-00000
Imtiaz F, Al-Mostafa A, Allam R, Ramzan K, Al-Tassan N, Tahir AI et al (2017) Twenty novel mutations in BCKDHA, BCKDHB and DBT genes in a cohort of 52 Saudi Arabian patients with maple syrup urine disease. Mol Genet Metab Rep 11:17–23. https://doi.org/10.1016/j.ymgmr.2017.03.006
Javaid N, Choi S (2017) Acetylation- and methylation-related epigenetic proteins in the context of their targets. Genes (Basel) 8(8):196. https://doi.org/10.3390/genes8080196 (Published 2017 Aug 7)
Kumar R, Jain V, Kushwah N, Dheer A, Mishra KP, Prasad D, Singh SB (2018) Role of DNA methylation in hypobaric hypoxia-induced neurodegeneration and spatial memory impairment. Ann Neurosci 25(4):191–200. https://doi.org/10.1159/000490368
Landgrave-Gómez J, Mercado-Gómez O, Guevara-Guzmán R (2015) Epigenetic mechanisms in neurological and neurodegenerative diseases. Front Cell Neurosci 9:58. https://doi.org/10.3389/fncel.2015.00058
Lei MZ, Li XX, Zhang Y, Li JT, Zhang F, Wang YP et al (2020) Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Signal Transduct Target Ther 5(1):70. https://doi.org/10.1038/s41392-020-0168-0
Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD (2006) Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281:15763–15773. https://doi.org/10.1074/jbc.M511767200
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mai A, Rotili D, Valente S, Kazantsev AG (2009) Histone deacetylase inhibitors and neurodegenerative disorders: holding the promise. Curr Pharm Des 15(34):3940–3957. https://doi.org/10.2174/138161209789649349
Mariño-Ramírez L, Kann MG, Shoemaker BA, Landsman D (2005) Histone structure and nucleosome stability. Expert Rev Proteomics 2(5):719–729. https://doi.org/10.1586/14789450.2.5.719
Mescka CP, Rosa AP, Schirmbeck G, da Rosa TH, Catarino F, de Souza LO et al (2016) L-carnitine prevents oxidative stress in the brains of rats subjected to a chemically induced chronic model of MSUD. Mol Neurobiol 53:6007–6017. https://doi.org/10.1007/s12035-015-9500-z
Miller JL, Grant PA (2013) The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell Biochem 61:289–317. https://doi.org/10.1007/978-94-007-4525-4_13
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38(1):23–38. https://doi.org/10.1038/npp.2012.112
Moosavi A, Motevalizadeh Ardekani A (2016) Role of epigenetics in biology and human diseases. Iran Biomed J 20(5):246–258. https://doi.org/10.22045/ibj.2016.01
Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, Strauss KA (2013) Biochemical correlates of neuropsychiatric illness in maple syrup urine disease. J Clin Invest 123:1809–1820. https://doi.org/10.1172/JCI67217
Nellis MM, Danner DJ (2001) Gene preference in maple syrup urine disease. Am J Hum Genet 68:232–237. https://doi.org/10.1086/316950
Putiri EL, Robertson KD (2011) Epigenetic mechanisms and genome stability. Clin Epigenetics 2(2):299 314. https://doi.org/10.1007/s13148-010-0017-z
Qian H, Xu X (2014) Reduction in DNA methyltransferases and alteration of DNA methylation pattern associate with mouse skin ageing. Exp Dermatol 23:357–359. https://doi.org/10.1111/exd.12375
Quental S, Macedo-Ribeiro S, Matos R et al (2008) Molecular and structural analyses of maple syrup urine disease and identification of a founder mutation in a Portuguese Gypsy community. Mol Genet Metab 94(2):148–156. https://doi.org/10.1016/j.ymgme.2008.02.008
Rosa L, Scaini G, Furlanetto CB, Galant LS, Vuolo F, Dall’Igna DM et al (2016) Administration of branched-chain amino acids alters the balance between pro-inflammatory and anti-inflammatory cytokines. Int J Dev Neurosci 48:24–30. https://doi.org/10.1016/j.ijdevneu.2015.11.002
Rudenko A, Tsai LH (2014) Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology 80:70–82. https://doi.org/10.1016/j.neuropharm.2014.01.043
Scaini G, Jeremias IC, Morais MO, Borges GD, Munhoz BP, Leffa DD et al (2012a) DNA damage in an animal model of maple syrup urine disease. Mol Genet Metab 106(2):169–174. https://doi.org/10.1016/j.ymgme.2012.04.009
Scaini G, Teodorak BP, Jeremias IC, Morais MO, Mina F, Dominguini D et al (2012b) Antioxidant administration prevents memory impairment in an animal model of maple syrup urine disease. Behav Brain Res 231(1):92–96. https://doi.org/10.1016/j.bbr.2012.03.004
Scaini G, de Rochi N, Jeremias IC, Deroza PF, Zugno AI, Pereira TCB et al (2012c) Evaluation of acetylcholinesterase in an animal model of maple syrup urine disease. Mol Neurobiol 45(2):279–286. https://doi.org/10.1007/s12035-012-8243-3
Scaini G, Mello-Santos LM, Furlanetto CB, Jeremias IC, Mina F, Schuck PF et al (2013) Acute and chronic administration of the branched-chain amino acids decreases nerve growth factor in rat hippocampus. Mol Neurobiol 48(3):581–589. https://doi.org/10.1007/s12035-013-8447-1
Scaini G, Jeremias GC, Furlanetto CB, Dominguini D, Comim CM, Quevedo J et al (2014a) Behavioral responses in rats submitted to chronic administration of branched-chain amino acids. JIMD Rep 13:159–167. https://doi.org/10.1007/8904_2013_274
Scaini G, Morais MOS, Galant LS, Vuolo F, Dall’Igna DM, Pasquali MAB et al (2014b) Coadministration of branched-chain amino acids and lipopolysaccharide causes matrix metalloproteinase activation and blood–brain barrier breakdown. Mol Neurobiol 50:358–367. https://doi.org/10.1007/s12035-013-8618-0
Schonberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82(1):69–75. https://doi.org/10.1016/j.ymgme.2004.01.016
Shukla S, Tekwani BL (2020) Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol 11:537. https://doi.org/10.3389/fphar.2020.00537
Strand JM, Skinnes R, Scheffler K, Rootvelt T, Woldseth B, Bjoras M, Eide L (2014) Genome instability in Maple Syrup Urine Disease correlates with impaired mitochondrial biogenesis. Metabolism 63(8):1063–1070. https://doi.org/10.1016/j.metabol.2014.05.003
Urdinguio RG, Sanchez-Mut JV, Esteller M (2009) Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol 8(11):1056–1072. https://doi.org/10.1016/S1474-4422(09)70262-5
Walterfang M, Bonnot O, Mocellin R, Velakoulis D (2013) The neuropsychiatry of inborn errors of metabolism. J Inherit Metab Dis 36:687–702. https://doi.org/10.1007/s10545-013-9618-y
Ward-Caviness CK, Agha G, Chen BH, Pfeiffer L, Wilson R et al (2018) Analysis of repeated leukocyte DNA methylation assessments reveals persistent epigenetic alterations after an incident myocardial infarction. Clin Epigenetics 10(1):161. https://doi.org/10.1186/s13148-018-0588-7
Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D (2007) Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39:457–466. https://doi.org/10.1038/ng1990
Weinhold B (2006) Epigenetics: the science of change. Environ Health Perspect 114(3):A160–A167. https://doi.org/10.1289/ehp.114-a160
Wessler LB, de Miranda Ramos V, Pasquali MAB, Moreira JCF, de Oliveira J, Scaini G, Streck EL (2019a) Administration of branched-chain amino acids increases the susceptibility to lipopolysaccharide-induced inflammation in young Wistar rats. Int J Dev Neurosci 78:210–214. https://doi.org/10.1016/j.ijdevneu.2019.07.007
Wessler LB, Farias HR, Ronsani JF, Candiotto G, Dos Santos PCL, de Oliveira J, Rico EP, Streck EL (2019) Acute exposure to leucine modifies behavioral parameters and cholinergic activity in zebrafish. Int J Dev Neurosci 78:222–226. https://doi.org/10.1016/j.ijdevneu.2019.10.001
Wessler LB, Ise K, Lemos IC, Rezende VL, Duarte MB, Damiani AP et al (2020) Melatonin ameliorates oxidative stress and DNA damage of rats subjected to a chemically induced chronic model of Maple Syrup Urine Disease. Metab Brain Dis. https://doi.org/10.1007/s11011-020-00572-9
Xu X (2015) DNA methylation and cognitive aging. Oncotarget 6:13922–13932. https://doi.org/10.18632/oncotarget.4215
Yen CY, Huang HW, Shu CW, Hou MF, Yuan SS, Wang HR, Chang YT, Farooqi AA, Tang JY, Chang HW (2016) DNA methylation, histone acetylation and methylation of epigenetic modifications as a therapeutic approach for cancers. Cancer Lett 373:185–192. https://doi.org/10.1016/j.canlet.2016.01.036
Yu NK, Baek SH, Kaang BK (2011) DNA methylation-mediated control of learning and memory. Mol Brain 4:5. https://doi.org/10.1186/1756-6606-4-5
Zeng P, Shi Y, Wang XM, Lin L, Du YJ, Tang N et al (2019) Emodin Rescued Hyperhomocysteinemia-Induced Dementia and Alzheimer’s Disease-Like Features in Rats. Int J Neuropsychopharmacol 22(1):57–70. https://doi.org/10.1093/ijnp/pyy090
Zhang ZY, Schluesener HJ (2013) Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J Neuropathol Exp Neurol 72(3):178–185. https://doi.org/10.1097/NEN.0b013e318283114a
Zovkic IB, Guzman-Karlsson MC, Sweatt JD (2013) Epigenetic regulation of memory formation and maintenance. Learn Mem 20(2):61–74. https://doi.org/10.1101/lm.026575.112
Acknowledgements
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), Instituto Cérebro e Mente and UNESC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have reviewed and approved the contents of the Manuscript and validated the accuracy of the data. Finally, they have no financial or personal conflicts of interest related to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Streck, E.L., Bussular, F.P., Wessler, L.B. et al. Administration of branched-chain amino acids alters epigenetic regulatory enzymes in an animal model of Maple Syrup Urine Disease. Metab Brain Dis 36, 247–254 (2021). https://doi.org/10.1007/s11011-020-00631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00631-1