Abstract
In a longitudinal cohort study with intervention (NCT05216796), we utilized multiorgan imaging to determine if metabolic dysfunction-associated steatotic liver disease (MASLD) is associated with elevated cerebral glutamate and myo-inositol and to determine their sensitivity to dietary intervention. Fifty-five adults with self-reported MASLD or high MASLD risk (3 + metabolic risk factors) received liver and brain magnetic resonance spectroscopy scans pre and post two-week low carbohydrate (≤30 g/d) or low-calorie (women ~ 1200 kcal/d; men ~ 1500 kcal/d) diet, both known for their ability to reduce liver fat. Forty-four adults completed the study (36 female, average age 54 years). Thirty out of 44 met clinical criterion for MASLD based on neuroimaging (≥ 5% hepatic triglycerides). Intervention was associated with significant decreases in liver fat fraction (mean difference = 3.101, 95% CI 2.104–4.099, p < 0.0001), glutamate (mean difference = 0.753, 95% CI 0.274–1.233, p = 0.0032) and myo-inositol (mean difference = 0.478, 95% CI 0.180–0.775, p = 0.0027) in patients with confirmed MASLD. Thus, MASLD may be a source of glutamate neurotoxicity and neuroinflammation and diet is an effective strategy for supporting brain as well as liver health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly non-alcoholic fatty liver disease (NAFLD), is the most common chronic liver disease globally, affecting an estimated 38% of adults, with projections predicting an increase to 55% by 2040 (Younossi et al. 2025). This rising burden of liver disease is closely tied to the global epidemic of obesity and is underpinned by systemic metabolic dysfunction—a defining feature of MASLD. The condition is widely recognized as the hepatic component of metabolic syndrome (MetS) and frequently coexists with obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension (Chan et al. 2024).
While MASLD is primarily characterized by hepatic fat accumulation, growing evidence points to a broader impact, including on deleterious effects on cognition and brain health. Individuals with MASLD often exhibit subtle cognitive symptoms, such as reduced attention, psychomotor slowing, and impaired executive function (George et al. 2022). However, the association between MASLD and cognitive impairment is far from settled, due to variability in diagnostic approaches to MASLD, often lacking granularity in stratification, inconsistent use of comprehensive neuropsychological batteries, and multiple potential pathways to neurodegeneration (Giuffrè et al. 2024). Here, we focus on one pathway linking metabolic liver disease to brain vulnerability - disruption in brain metabolism.
Altered levels of key neurometabolites are a hallmark of both metabolic health conditions (Zhao et al. 2018; Yates et al. 2012) and cognitive disorders (Maul et al. 2020). These neurometabolic alterations reflect impairments in energy metabolism and neurotransmission pathways crucial to cognitive function (Camandola and Mattson 2017) and may provide insight into early cognitive vulnerability. Consistent with this body of work, our group has reported elevated cerebral glutamate and myo-inositol levels in cognitively unimpaired adults with MetS (Haley et al. 2010), suggesting early neurometabolic changes associated with systemic metabolic stress. Thus, MASLD can also be expected to disrupt cerebral metabolism. The liver plays a central role in maintaining cerebral glutamate homeostasis through its exclusive ability to detoxify ammonia via the urea cycle. It is the only organ that converts ammonia into urea, a process essential for the safe removal of nitrogen waste from the body (Strong et al. 2021). Impaired hepatic function can lead to hyperammonemia, disrupting glutamatergic neurotransmission in later stages of disease. Excess cerebral glutamate has been reported in chronic liver disease via dysfunction of the glutamate–nitric oxide–cGMP pathway (Monfort and Felipo 2002). Given this connection, the role of cerebral glutamate in the liver–brain axis, especially earlier in the liver disease process, warrants further investigation.
The primary aim of this study was to determine whether diet-induced reductions in hepatic triglyceride content are accompanied by corresponding decreases in cerebral glutamate and myo-inositol concentrations in patients with MASLD. A secondary aim was to compare baseline cerebral glutamate and myo-inositol levels between individuals with MetS, with and without MASLD, in a cross-sectional analysis. We hypothesized that at baseline, individuals with MASLD would exhibit higher cerebral glutamate and myo-inositol levels than those without, and that dietary intervention would lead to a reduction in cerebral glutamate and myo-inositol levels in patients with MASLD.
Methods
Study design
This study was a single-center, longitudinal cohort study with intervention. We examined the effects of a 2-week low carbohydrate or low-calorie diet on hepatic triglyceride levels and cerebral glutamate and myo-inositol concentrations in participants with MASLD. This study was conducted in accordance with the Declaration of Helsinki and the Institutional Review Board at the University of Texas at Austin approved all study procedures (STUDY00002189). Written informed consent was obtained from all participants. This study was registered at ClinicalTrials.gov, NCT05216796, prior to onset of data collection.
Participants
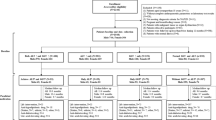
Participants were recruited from the Austin, Texas metropolitan area through flyers, newspaper advertisements, word-of-mouth, and social media outreach. To be eligible for this study, participants had to be at least 40 years of age, English speakers, live in the Austin area, report a prior diagnosis of MASLD or high MASLD risk (at least three MetS components - abdominal obesity, high blood pressure, impaired fasting glucose, high triglyceride levels, or low HDL cholesterol levels), be willing to engage in a 2-week diet intervention, and agree to partake in two Magnetic Resonance Imaging (MRI) sessions. Participants were excluded if they failed to meet standard MRI safety criteria, or if they reported traumatic brain injury with loss of consciousness > 5 min, alcohol use disorder, or significant neurological, cardiovascular or psychiatric disorder (e.g. stroke, epilepsy, bipolar disorder, schizophrenia). They were also excluded if they reported they have participated in a diet intervention within the past six months, are currently following a specialized diet plan (e.g., Atkins), or engage in exercise training program for weight loss. Data were collected between May 2022 and August 2024. Consort Diagram is Available as Supplemental Fig. 1. One hundred and eighty-two participants were screened, 70 fulfilled study inclusion criteria (reported a pre-existing diagnosis of MASLD or 3 + cardiometabolic risk factors). 55 participants were enrolled. 44 completed the study with good quality MRI data pre- and post-intervention (36 female, average age 54 years). Despite our best effort to recruit only patients with MASLD, approximately 30% of volunteers failed to meet clinical criteria for MASLD on neuroimaging (≥ 5% hepatic triglycerides). This was not surprising as prediction of clinically significant MASLD without neuroimaging is extraordinarily difficult (Eskridge et al. 2023). However, this situation allowed us to form and test our secondary aim to compare baseline concentrations of glutamate and myo-inositol in MetS patients with and without MASLD.
Diet intervention
Eligible participants were randomly assigned to a low carbohydrate or a low-calorie diet in a 1:1 ratio. The first five eligible participants were assigned to the low carbohydrate diet, and the next five assigned to the low-calorie diet. This assignment was alternated between the two diets in blocks of five participants per assignment for the remaining enrollment period. This was an open label study although group allocation was not directly disclosed to participants, neither was the intervention discussed during the imaging sessions. A commercial meal service (Snap Kitchen, https://www.snapkitchen.com) prepared all meals. Meals were delivered to, or picked up by, participants twice per week.
The two dietary interventions—calorie restriction and carbohydrate restriction—were selected based on current clinical guidelines and prior evidence. Weight loss is the primary therapeutic strategy for reducing liver triglyceride content in individuals with MASLD, and calorie restriction is the most widely adopted dietary approach to achieve this goal efficiently. Low-calorie meals were designed to include ~ 1200 kcal/d for women and ~ 1500 kcal/d for men. Additionally, previous studies (Browning et al. 2011) have shown that under conditions of calorie restriction, further limiting carbohydrate intake to very low levels (< 20 g/day) can lead to greater reductions in liver fat content. Our study aimed to replicate this approach with a modest modification: due to logistical constraints with the food supplier (Snap Kitchen), the carbohydrate-restricted meals contained approximately \(\:\le\:\)30 g/day of carbohydrate, which is slightly more liberal than in the referenced study.
Procedures
Informed consent
Informed consent was obtained from eligible participants in an online session through the Zoom online platform. Consenting participants were then invited to complete study questionnaires including demographics and medical history. Following questionnaire completion and 3 days on a weight-stable diet, participants were invited to the first study visit.
Health screening visit
This session involved measurements of height and weight, waist circumference, blood pressure and metabolic parameters relevant to MetS. Participants arrived fasted, having refrained from eating or drinking anything (except water) for 12 hours, nor caffeine or vigorous exercise 24 hours before the study visit. Arterial blood pressure was measured in duplicate after 10 minutes of rest in a supine position, using the Omron VP-1000 plus vascular screening device (Omron Healthcare Co., Kyoto Japan). Height and weight were measured using a balance beam scale. Waist circumference was measured in duplicate using a nonelastic tape measure and recorded to the nearest tenth of a centimeter. Blood concentrations of glucose, triglycerides, HDL-cholesterol, and LDL-cholesterol were determined using a standard enzymatic technique using Alere Cholestech LDX Analyzer (Abbott, Chicago, USA). MetS diagnosis was confirmed using Alberti et al. (2009) consensus criteria: fasting glucose ≥100 mg/dL or treatment for hyperglycemia, triglycerides ≥150 mg/dL, HDL-cholesterol ≤40 mg/dL in males and ≤50 mg/dL in females or treatment for dyslipidemia, systolic blood pressure ≥130 mmHg or diastolic ≥85 mmHg or antihypertensive medication, and waist circumference ≥102 cm for males and ≥88 cm for females. MetS diagnosis was indicated by 3 or more criteria (Alberti et al. 2009).
Pre-intervention liver and brain imaging
Participants completed an MRI safety screening form prior to the MRI scan. Brain and liver MRI data were acquired on a 3T Siemens Vida MRI scanner. Hepatic triglyceride levels were assessed using a high-speed T2-corrected multi-echo (HISTO) technique, employing a TR of 3000 ms, spectral width of 1200 Hz, a resolution of 1024 points, and five echo times: 12, 24, 36, 48, and 72-ms. A 27-cm3 voxel was positioned in the right lobe of the liver, carefully avoiding major vessels or bile ducts. Hepatic triglyceride levels were expressed as a percentage of the fat signalto the combined signal from both fat and water, proton density fat fraction (PDFF). Brain imaging included anatomical brain scans in the sagittal plane using a high-resolution ultrafast Gradient Echo 3D (MPRAGE) sequence, featuring a 256 × 256 matrix, flip angle of 7°, field of view measuring 24 × 24 cm2, 1 mm slice thickness with no gap. Brain spectra were acquired using a 30-ms TE point resolved spectroscopy (PRESS) sequence with a 4750-ms TR, 96 or 128 averages, and a spectral width of 1600 or 2500-Hz. Voxels were approximately 8 cm3 and localized in occipitoparietal gray matter including the posterior cingulate cortex (PCC). The PCC was selected because it is a highly metabolic and internally connected brain area, that plays a critical role in cognition, including memory and attention (Leech and Sharp 2014) and tends to be affected early in the disease processes of both metabolic (Kaur et al. 2015; Haley et al. 2010) and cognitive disorders (Ali et al. 2022).In addition, an eight-averaged unsuppressed water reference was acquired for phase correction and metabolite quantization, and a 24-point TE/TR water relaxation data set was acquired to separate the tissue and cerebral spinal fluid water compartments (Knight-Scott et al. 2005). The glutamate (Glu) and myo-inositol (mI) resonances were identified and quantified using LCModel (Version 6.3-R) (Provencher 1993) and their concentrations were reported in molality units relative to tissue water (Knight-Scott et al. 2003).
Post-intervention liver and brain imaging
Following a two-week period on their assigned diet, participants returned for a follow-up imaging visit, following the same procedures outlined in the pre-intervention liver and brain imaging visit.
Outcomes
The primary variables of interest in this study were liver triglycerides proton density fat fraction (PDFF), cerebral glutamate (Glu) and myo-inositol (mI) concentrations.
Statistical analysis
Sample size calculations were based on effect sizes for liver triglyceride reductions following short-term low carbohydrate and low calorie diet interventions published in Browning et al. in 2011 (Browning et al. 2011) using G*Power 3.1 (Faul et al. 2007). It was determined that a sample size of 40 was needed to detect a moderate effect size (0·3) with 95% power. All analyses were performed using R Statistical Software (v4.4.1; R Core Team 2024). Data were analyzed using descriptive statistics, repeated measures analysis of variance (ANOVA), paired t-tests, and Pearson correlations using the following packages: tidyverse, dplyr, ggplot2, ggpubr, rstatix, table1 (Wickham et al. 2019; Kassambara 2023; Rich 2023). All analyses were performed as intent-to-treat.
Results
Descriptive analyses
Detailed study procedure and sample descriptive statistics are available in Supplemental Fig. 1 and Table 1. On average, participants were 54 years of age (SD 9 years), with 16 years of education (SD 3 years), 22 participants (50%) identified as White, 14 (32%) as Hispanic, 36 (82%) were female. As described in health assessment section, participants were classified as meeting MetS criteria for abdominal obesity, elevated blood pressure, elevated blood glucose, high triglycerides or low HDL-cholesterol were based on consensus criteria (Alberti et al. 2009). Thirty-seven (84%) of participants had abdominal obesity, 44 (100%) of participants had elevated blood pressure, 44 (100%) had elevated blood glucose, 17 (39%) had elevated triglycerides, 29 (70%) had low HDL-Cholesterol. All but 1 participant met the clinical criterion for MetS diagnosis (3+ risk factors). Liver imaging revealed that 30/44 participants fulfilled clinical criterion for MASLD (≥5% hepatic triglycerides level as measured by their PDFF at study entry). Means and standard deviations for the main outcome variables including hepatic triglycerides are reported in Table2, for the full sample as well as by MASLD diagnosis.
Spectral quality check
Voxel position and example spectra are shown on Fig1. Spectral quality was assessed using Cramer-Rao lower bounds (CRLB). Spectral quality was excellent, even for the most challenging of metabolite (Glu) at 5.3% for both pre and post acquisitions.
Primary analyses: Diet effects on liver triglycerides and brain glutamate and myo-inositol in MASLD
Assumptions Testing
Assumptions of normality were tested using Shapiro-Wilk normality test on the residuals (or differences) for each participant’s scores across the two time points. Assumption of normality was met for all primary variables (hepatic triglycerides W = 0.936, p-value = 0.072; glutamate W = 0.982, p-value = 0.868, and myo-inositol W = 0.937, p-value = 0.075). Homogeneity of variance across diet groups was tested using Levene’s test. Assumption of homogeneity of variance was met for all primary variables (hepatic triglycerides F(1,28) = 1.096, p = 0.304; glutamate (F(1,28) = 0.145, p = 0.7065; and myo-inositol F(1,28)= 1.167, p = 0.6844). Therefore, parametric statistics were used and reported throughout.
Low carbohydrate vs low-calorie diet
Repeated measures ANOVA revealed no significant interactions between time (pre- and post- intervention) and diet (low carbohydrate vs low-calorie) for any of the primary outcome variables: hepatic triglycerides (F(1,28) = 0.856, p = 0.363), cerebral glutamate (F(1,28)= 0.180, p = 0.675) or myo-inositol (F(1,28) = 0.032, p=0.860). Therefore, data from the two diet interventions were pooled together.
Diet effects on liver triglycerides, brain glutamate and myo-inositol
Paired T-tests revealed that diet intervention was associated with significant decreases in liver fat fraction (mean difference = 3.101, 95% CI 2.104– 4.099, p < 0.0001, Fig. 2), glutamate (mean difference = 0.753, 95% CI 0.274– 1.233, p = 0.0032, Fig. 3) and myo-inositol (mean difference = 0.478, 95% CI 0.180– 0.775, p = 0.0027, Fig. 3) in patients with MASLD. Overall, 97% of MASLD participants experienced a decrease in liver fat fraction post intervention, 77% of participants experienced a decrease in glutamate, and 70% experienced a decrease in myo-inositol.
Secondary outcomes and exploratory correlational analyses
Baseline differences in cerebral glutamate and myo-inositol levels between individuals with MetS and MASLD (MASLD+) as compared to individuals with MetS without MASLD (MASLD-)
Baseline levels of myo-inositol were significantly higher in individuals with MetS and MASLD, compared to those with MetS and no MASLD (mean difference = 0.617, 95% CI -1.133– -0.1016, p = 0.0207, Supplemental Fig. 2). Baseline levels of glutamate were not significantly different between the two groups (mean difference = 0.424, 95% CI-1.458– 0.6094, p = 0.403, Supplemental Fig. 2).
Exploratory Correlational Analyses
Apart from significant decrease in liver triglycerides, diet was also significantly associated with weight loss in the full cohort (t(42) = 8.158, p < 0.0001). Weight loss and change in liver triglycerides were correlated (r = 0.44, CI95%= 0.17-0.66, npairs= 43, p = 0.0028, Supplemental Fig. 3). However, while changes in liver triglycerides were significantly associated with changes in cerebral glutamate (r = 0.33, CI95%= 0.04-0.57, npairs = 44, p = 0.03, Supplemental Fig. 4), the correlation between cerebral glutamate and weight loss was weaker and did not reach statistical significance (r = 0.27, CI95%= -0.03-0.53, npairs = 43, p = 0.08). The change in glutamate was significantly related to change in myo-inositol (r = 0.69, CI95%= 0.50-0.82, npairs = 44, p < 0.0001, Supplemental Fig. 5). Change in myo-inositol alone was not significantly related to change in liver triglycerides (r = 0.21, CI95%= -0.10-0.47, npairs= 44, p = 0.18) or weight loss (r = 0.23, CI95%= -0.07-0.50, npairs= 43, p = 013).
Discussion
The results of the current study showed that both low calorie and low carbohydrate diets can effectively reduce free cerebral glutamate and myo-inositol concentrations in adults with MASLD, in parallel with decreases in hepatic triglycerides. Since adults at high metabolic risk generally exhibit higher glutamate levels than healthy controls (Haley et al. 2010), and excessive release of glutamate can have neurotoxic effects and lead to neurodegeneration (Hynd 2004), these results suggest that altering diet can support both liver and brain health in patients vulnerable to disruptions in glutamatergic neurotransmission.
To our knowledge, this is one of the first studies to show glutamate changes in the human brain in response to a restrictive diet intervention at clinical field strengths. While glutamate changes have been reported at high field strengths (7T), in response to acute dietary supplementation (Hone-Blanchet et al. 2023), only one other study has reported related neurochemical changes in response to a restrictive diet intervention (intermittent fasting) at 3T (Kapogiannis et al. 2024). The authors reported a significant reduction in brain glucose and ascorbate, a modulator of glutamatergic neurotransmission, in cognitively intact older adults with insulin resistance following an 8-week diet. While links between liver and brain have been well documented in hepatic encephalopathy (Monfort and Felipo 2002), the liver-brain axis has never been shown to be sensitive to intervention at such an early stage of liver disease development. Diet interventions are known to quickly and efficiently improve liver health by reducing hepatic triglycerides (Schwimmer et al. 2019), but to date, concomitant improvements in neurometabolic health have only been speculated in this patient population. Here, we demonstrated that a reduction in hepatic triglyceride is accompanied by a reduction in cerebral glutamate and myo-inositol, both related to neurotoxicity at high levels. We speculate that the results indicate a beneficial reduction of neurotoxicity related to improved liver function, though long-term follow-up with cognitive testing would be needed to clarify if the changes in glutamate and myo-inositol levels are directly related to changes in cognition.
Glutamate is the major excitatory neurotransmitter in the central nervous system, which is also responsible for regulating neuronal growth and differentiation, synaptic plasticity, learning and memory (Mattson 2008). However, excessive release of glutamate can have neurotoxic effects and lead to neurodegeneration (Hynd 2004). Chronic liver disease and subsequent hyperammonemia are known to disrupt the glutamate-nitric oxide-cGMP pathway in the brain (Monfort and Felipo 2002), leading to extracellular glutamate accumulation and a range of neuropsychiatric symptoms including cognitive impairment (Hynd 2004). Ammonia induced astrocyte swelling and cerebral edema can lead to dangerous increases in intracranial pressure, brain herniation and death (Felipo and Butterworth 2002). However, cytotoxic edema is only detected in a small proportion of chronic liver failure patients (4-8%); therefore, it cannot account for the wide range of neurological symptoms experienced by patients with mild hepatic encephalopathy (Hadjihambi et al. 2018). Cognitive and motor dysfunction is also reported in animal models of mild hepatic encephalopathy in the absence of edema (Cauli et al. 2009). These findings are consistent with data from our lab showing increased cerebral glutamate and poorer cognitive performance in adults with MetS suggestive of some type of preclinical mild hepatic encephalopathy (Haley et al. 2010; Foret et al. 2021). Therefore, our target population of adults with MASLD, all of whom also fulfilled criteria for MetS, was an ideal candidate for early intervention aimed at supporting brain function by targeting hepatic triglyceride.
In comparison to elevated glutamate levels, which are consistently identified as a risk factor, elevated myo-inositol levels can be a marker of risk as well as an early protective factor. Myo-inositol is an organic osmolyte and precursor of secondary messengers such as inositol-triphosphate (IP3) and phosphatidyl-myo-inositol (Ross 1991). Since increases in myo-inositol are detected in many neurodegenerative conditions, it is often considered a marker of glial proliferation. In end-stage liver disease, severe depletions of myo-inositol are attributed to osmotic stress due to increased ammonia in the blood (Tran et al. 2020). In early-stage liver disease, however, detected increases in myo-inositol are likely related to the detoxifying function of astrocytes. We had previously hypothesized that mild early increases in neurotoxic load may be compensated by elevations of myo-inositol break down product glucoronate, a detoxifying substrate in the liver (Haley et al. 2010). If myo-inositol serves the same function in the brain, its acute depletion in hepatic encephalopathy may also be due to the high exposure of the brain to circulating toxins (Ross 1991). The fact that myo-inositol and glutamate “travel together” under conditions of mild somatic stress gives support to the hypothesis that brain vulnerability in MASLD may be reversible by early intervention. This is particularly important in light of evidence supporting the significance of peripheral organ health in neuropsychiatric disorders (Tian et al. 2023). A study by Tian et al. (2023) revealed that in dementia, which is often associated with progressive gray matter loss, the most significant deviations from normative ranges were observed in the metabolic and hepatic organ systems. This suggests that the interplay between insulin resistance and hepatic dysfunction may hinder the brain's clearance of amyloid and other toxic metabolites, leading to brain inflammation and neuropathology.
It is interesting to speculate on the specificity of glutamate and myo-inositol in their identification of brain vulnerability in MASLD. Dietary-induced changes in cerebral glutamate levels appear on average across the entire MetS sample in this study regardless of MASLD status, despite some attenuation of the effect with the inclusion of the entire sample. In comparison, changes in myo-inositol levels appear to be highly specific to the MASLD group. In our original identification of brain vulnerability in MetS (Haley et 2010), we did not test for the presence of MASLD, therefore we do not how much MASLD might have contributed to the elevation of glutamate and myo-inositol levels in MetS, compared to healthy controls. In the current pilot study, we did not include healthy controls and therefore do not know their neurometabolic sensitivity to dietary changes, neither did we have the power to explore the effects of MASLD versus the effects of the metabolic conditions with which it often co-exists (e.g., hypertension, dyslipidemia and hyperglycemia). However, given the clear influence of MASLD on cerebral glutamate and myo-inositol levels in adults, all brain 1H magnetic resonance spectroscopy studies should at the very least test for MALSD in suspect cases, to control for its effects.
There are many limitations of this study that must be acknowledged. This was a proof-of-concept study, which included a small number of individuals at a single time point in life. Thus, we are unable to speculate about the long-term cognitive and behavioral consequences of increased glutamate or myo-inositol levels in MASLD or about the behavioral consequences of diet induced reductions in these neurometabolites. The lack of a healthy control group also receiving the intervention is a major hindrance to establishing both association and causality. Additionally, the study sample was majority female, and we are unable to explore sex as a biological variable. Finally, glutamate and myo-inositol measurements can be improved with the use of very short TE sequences (Kim et al. 2005; Wijtenburg and Knight‐Scott 2011). Future work should also include both younger and older individuals, as well as healthy controls to map the lifespan trajectories of glutamate and myo-inositol and their cognitive correlates.
In conclusion, we found that low calorie/low carbohydrate diets effectively reduce free cerebral glutamate and myo-inositol concentrations in MASLD, in parallel with reductions in hepatic triglycerides. Since increases in free cerebral glutamate can be neurotoxic, this work might provide a quantitative way to track brain health in adults with early-stage liver disease. Our findings enhance our understanding of the neurometabolic impact of MASLD in adults with MetS and support MRS as a useful research tool in examining liver-brain relationships.
Data availability
Data used to generate tables and figures for this paper will be made available through Figshare, after manuscript publication.
References
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Karen A, Donato J-C, Fruchart W, Philip T, James CM, Loria, and Sidney C. Smith (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood Institute. Circulation 120(16):1640–1645. American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesityhttps://doi.org/10.1161/CIRCULATIONAHA.109.192644
Ali DG, Ahmed A, Bahrani JM, Barber, Riham H, El Khouli, Brian T, Gold JP, Harp Y, Jiang DM, Wilcock, Jicha GA (2022) Amyloid-PET levels in the precuneus and posterior cingulate cortices are associated with executive function scores in preclinical Alzheimer’s disease prior to overt global amyloid positivity. J Alzheimer’s Disease 88(3):1127–1135. https://doi.org/10.3233/JAD-220294
Browning JD, Jonathan A, Baker T, Rogers J, Davis S, Satapati, Shawn CB (2011) Short-Term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 93(5):1048–1052. https://doi.org/10.3945/ajcn.110.007674
Camandola S, Mark PM (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J 36(11):1474–1492. https://doi.org/10.15252/embj.201695810
Cauli O, Rodrigo R, Llansola M, Montoliu C, Monfort P, Piedrafita B, Mlili NE, Boix J, Agustí A, and Vicente Felipo (2009) Glutamatergic and Gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 24(1):69–80. https://doi.org/10.1007/s11011-008-9115-4
Chan KE, Ong EYH, Chung CH et al (2024) Longitudinal outcomes associated with metabolic dysfunction-associated steatotic liver disease: a meta-analysis of 129 studies. Clin Gastroenterol Hepatol 22(3):488-498.e44. https://doi.org/10.1016/j.cgh.2023.09.018
Eskridge W, Cryer DR, Schattenberg JörnM, Gastaldelli A, Malhi H, Allen AM, Noureddin M, Arun JS (2023) Metabolic Dysfunction-Associated steatotic liver disease and metabolic Dysfunction-Associated steatohepatitis: the patient and physician perspective. J Clin Med 12(19):6216. https://doi.org/10.3390/jcm12196216
Faul F, Erdfelder E, Lang A-G, and Axel Buchner (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/BF03193146
Felipo V, Roger F, Butterworth (2002) Neurobiology of Ammonia. Prog Neurobiol 67(4):259–279. https://doi.org/10.1016/S0301-0082(02)00019-9
Foret JT, Stephanie Oleson B, Hickson S, Valek H, Tanaka, Andreana PH (2021) Metabolic syndrome and cognitive function in midlife. Arch Clin Neuropsychol 36(6):897–907. https://doi.org/10.1093/arclin/acaa112
George ES, Sood S, Daly RM, Sze-Yen T (2022) Is there an association between Non-Alcoholic fatty liver disease and cognitive function?? A systematic review. BMC Geriatr 22(1):47. https://doi.org/10.1186/s12877-021-02721-w
Giuffrè M, Merli N, Pugliatti M, and Rita Moretti (2024) The metabolic impact of nonalcoholic fatty liver disease on cognitive dysfunction: A comprehensive clinical and pathophysiological review. Int J Mol Sci 25(6):3337. https://doi.org/10.3390/ijms25063337
Hadjihambi A, Arias N, Sheikh M, and Rajiv Jalan (2018) Hepatic encephalopathy: A critical current review. Hep Intl 12(S1):135–147. https://doi.org/10.1007/s12072-017-9812-3
Haley AP, Mitzi M, Gonzales T, Tarumi SC, Miles KG, and Hirofumi Tanaka (2010) Elevated cerebral glutamate and Myo-Inositol levels in cognitively normal Middle-Aged adults with metabolic syndrome. Metab Brain Dis 25(4):397–405. https://doi.org/10.1007/s11011-010-9221-y
Hone-Blanchet A, Antal B, McMahon L, Lithen A, Smith NA, Stufflebeam S, Yen Y-F et al (2023) Acute administration of ketone Beta-Hydroxybutyrate downregulates 7T proton magnetic resonance Spectroscopy-Derived levels of anterior and posterior cingulate GABA and glutamate in healthy adults. Neuropsychopharmacology 48(5):797–805. https://doi.org/10.1038/s41386-022-01364-8
Hynd M (2004) Glutamate-Mediated excitotoxicity and neurodegeneration in Alzheimer?S disease. Neurochem Int 45(5):583–595. https://doi.org/10.1016/j.neuint.2004.03.007
Kapogiannis D, Manolopoulos A, Mullins R, Avgerinos K, Delgado-Peraza F, Mustapic M, Nogueras-Ortiz C et al (2024) Brain responses to intermittent fasting and the healthy living diet in older adults. Cell Metabol 36(8):1668–1678e5. https://doi.org/10.1016/j.cmet.2024.05.017
Kassambara A (2023) _rstatix: Pipe-Friendly framework for basic statistical tests_. https://CRAN.R-project.org/package=rstatix
Kaur S, Gonzales MM, Strasser B, Pasha E, McNeely J, Tanaka H, Haley AP (2015) Central adiposity and cortical thickness in midlife. Psychosom Med 77(6):671–678. https://doi.org/10.1097/PSY.0000000000000202
Kim H, Thompson RB, Hanstock CC, and Peter S. Allen (2005) Variability of metabolite yield using STEAM or PRESS sequences in vivo at 3.0 T, illustrated with Myo-inositol. Magn Reson Med 53(4):760–769. https://doi.org/10.1002/mrm.20434
Knight-Scott J, Haley AP, Rossmiller SR, Farace E, Mai VM, Christopher JM, Manning CA, Virginia I, Simnad, and Helmy M. Siragy (2003) Molality as a unit of measure for expressing 1H MRS brain metabolite concentrations in vivo. Magn Reson Imaging 21(7):787–797. https://doi.org/10.1016/S0730-725X(03)00179-6
Knight-Scott, Jack SA, Dunham, Shanbhag DD (2005) Increasing the speed of Relaxometry-Based compartmental analysis experiments in STEAM spectroscopy. J Magn Reson 173(1):169–174. https://doi.org/10.1016/j.jmr.2004.12.001
Leech R, and David J. Sharp (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137(1):12–32. https://doi.org/10.1093/brain/awt162
Mattson MP (2008) Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci 1144(1):97–112. https://doi.org/10.1196/annals.1418.005
Maul S, Giegling I, Rujescu D (2020) Proton magnetic resonance spectroscopy in common dementias—current status and perspectives. Front Psychiatry 11. https://doi.org/10.3389/fpsyt.2020.00769
Monfort P, and Vicente Felipo (2002) Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab Brain Dis 17(4):237–250
Provencher S (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30(6):672–679
Rich B (2023) _table1: tables of descriptive statistics in HTML_. https://CRAN.R-project.org/package=table1
Ross BD (1991) Biochemical considerations in 1 H spectroscopy. Glutamate and glutamine; Myo -inositol and related metabolites. NMR Biomed 4(2):59–63. https://doi.org/10.1002/nbm.1940040205
Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, Alazraki A et al (2019) Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: A randomized clinical trial. JAMA 321(3):256. https://doi.org/10.1001/jama.2018.20579
Strong A, Gold J, Gold NB, and Marc Yudkoff (2021) Hepatic manifestations of Urea cycle disorders. Clin Liver Disease 18(4):198–203. https://doi.org/10.1002/cld.1115
Tian Y, Ella MA, Di Biase PE, Mosley, Michelle K, Lupton Y, Xia J, Fripp M, Breakspear V, Cropley, and Andrew Zalesky (2023) Evaluation of Brain-Body health in individuals with common neuropsychiatric disorders. JAMA Psychiatry 80(6):567. https://doi.org/10.1001/jamapsychiatry.2023.0791
Tran TT, Wei K, Cole S, Mena E, Csete M, King KS (2020) Brain MR spectroscopy markers of encephalopathy due to nonalcoholic steatohepatitis. J Neuroimaging 30(5):697–703. https://doi.org/10.1111/jon.12728
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G et al (2019) Welcome to the tidyverse. J Open Source Softw 4(43):1686. https://doi.org/10.21105/joss.01686
Wijtenburg S, Andrea, and Jack Knight-Scott (2011) Very short echo time improves the precision of glutamate detection at 3T in1 H magnetic resonance spectroscopy. J Magn Reson Imaging 34(3):645–652. https://doi.org/10.1002/jmri.22638
Yates KF, Sweat V, Yau PL, Turchiano MM, and Antonio Convit (2012) Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arterioscler Thromb Vasc Biol 32(9):2060–2067. https://doi.org/10.1161/ATVBAHA.112.252759
Younossi ZM, Kalligeros M, and Linda Henry (2025) Epidemiology of metabolic Dysfunction-Associated steatotic liver disease. Clin Mol Hepatol 31(Suppl):S32–50. https://doi.org/10.3350/cmh.2024.0431
Zhao X, Han Q, Gang X, Wang G (2018) Altered brain metabolites in patients with diabetes mellitus and related Complications– Evidence from 1H MRS study. Biosci Rep 38(5):BSR20180660. https://doi.org/10.1042/BSR20180660
Acknowledgements
We sincerely thank all participants for their time, Snap Kitchen for cooking and delivering the meals, and the University of Texas at Austin for providing funding for this study.
Funding
The University of Texas at Austin.
Author information
Authors and Affiliations
Contributions
APH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Visualization, Writing – original draft; JKS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing; MC: Data curation, Investigation, Project administration, Supervision, Writing – review & editing; IG: Data curation, Investigation, Project administration, Supervision, Writing – review & editing; JP: Data curation, Investigation, Project administration, Supervision, Writing – review & editing; YL: Investigation, Writing – review& editing; TW: Investigation, Writing – review& editing; HT: Conceptualization, Methodology, Writing – review & editing; JB: Conceptualization, Methodology, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare no competing interests. Snap Kitchen was paid for the meals they provided and had no input into study design or data collection and interpretation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haley, A.P., Knight-Scott, J., Caillaud, M. et al. Low carbohydrate and low-calorie diets reduce liver fat and lower brain glutamate and myo-inositol levels in patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Metab Brain Dis 40, 199 (2025). https://doi.org/10.1007/s11011-025-01624-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11011-025-01624-8