Abstract
Purpose
To investigate the molecular mechanisms underlying resistance to dopamine agonists (DA).
Methods
LC–MS/MS analysis was performed on rat pituitary neuroendocrine tumors (PitNET) cell line GH3 to identify differentially expressed proteins induced by cabergoline (CAB) treatment. A total of 180 human PITNET samples were subjected to transcriptome analysis. Immunohistochemistry (IHC) was conducted on 29 tumor samples to validate NDFIP1 alteration. A xenograft mouse model was established by subcutaneously injecting GH3 cells, with or without NDFIP1 overexpression, into nude mice to investigate tumor growth. PitNET cell lines were treated with CAB. Cell proliferation was assessed using the CCK-8, and protein expression levels were examined through Western blot analysis.
Results
CAB treatment upregulated FDFT1 and NDFIP1 protein expression in GH3 cells, with NDFIP1 showing a significant positive correlation with tumor size, as confirmed by IHC results. MMQ and GH3 cells overexpressing NDFIP1 exhibited enhanced viability and reduced sensitivity to CAB. In vivo experiments demonstrated that subcutaneous injection of NDFIP1-overexpressing GH3 cells led to enhanced tumor growth compared to parental GH3 cells. Although the total levels of PTEN remained unaltered, NDFIP1 overexpression induced PTEN nuclear translocation, potentially activating the mTOR pathway. This was supported by increased phosphorylation of key mTOR pathway components, including p-AKT and p-4EBP1, in cells overexpressing NDFIP1.
Conclusion
CAB treatment induces the upregulation of NDFIP1 in PitNET cells, which correlates with tumor size and contributes to reduced CAB sensitivity, potentially through activation of the mTOR pathway. NDFIP1 as a potential therapeutic target for overcoming DA resistance in PitNET patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Pituitary neuroendocrine tumors (PitNET) are benign tumors that arise from the anterior lobe of the pituitary gland, representing approximately 17% of all intracranial neoplasms [1]. Prolactin-secreting PitNET, i.e., prolactinomas, are the most common PitNET, accounting for 40–50% of cases, followed by non-functioning PitNET, somatotroph adenomas, and corticotroph adenomas [2]. PitNET can cause various clinical syndromes, such as hyperprolactinemia, acromegaly, and Cushing’s disease [3]. Morbidity associated with PitNET can be substantial due to hormonal imbalances and their potential to compress adjacent brain structures [4]. Dopamine agonists (DAs) like cabergoline (CAB) and bromocriptine are the first-line treatments for prolactinomas, with around 70–90% of patients responding positively [5]. However, 10–30% of prolactinomas can become resistant to DA treatment, posing a therapeutic challenge [6]. DA resistance in prolactinomas is not fully understood, but potential factors include alterations in dopamine receptor expression and changes in downstream signaling pathways [7]. Therefore, further research is needed to uncover the mechanisms behind DA resistance in prolactinomas and develop novel therapeutic strategies.
The mammalian target of rapamycin (mTOR) is a crucial serine/threonine protein kinase that regulates cell growth, proliferation, and survival [8]. Additionally, Phosphatase and Tensin Homolog (PTEN) is a tumor suppressor gene, acting as an antagonist of the PI3K/Akt signaling pathway, which is one of the upstream regulators of mTOR. Mutations or downregulation of PTEN can lead to hyperactivation of the mTOR pathway, promoting uncontrolled cell growth and contributing to tumorigenesis [9]. In PitNET, the upregulation and/or overactivation of the PI3K/Akt/mTOR pathway have been observed, highlighting its potential as a therapeutic target [10]. Clinical studies have demonstrated that mTOR inhibitors, such as everolimus, effectively inhibit the growth of PitNET cells [11]. Interestingly, DEP domain-containing mTOR-interacting protein (DEPTOR), an endogenous mTOR inhibitor, is found to be downregulated in PitNET. Overexpressing DEPTOR not only suppresses the proliferation of PitNET cells but also enhances their responsiveness to CAB, suggesting that mTOR pathway inhibition could be a key mechanism to counteract CAB resistance in PitNET [12]. Additionally, we previously observed that CAB induces autophagy in rat PitNET cell lines MMQ and GH3 by inhibiting the mTOR pathway [13]. Based on these findings, we hypothesize that mTOR pathway activation may represent a primary resistance mechanism to DA, and identifying the mechanism underlying may help overcome this resistance.
In this study, we aimed to uncover the molecular mechanisms underlying DA resistance in prolactinoma patients. We conducted a comprehensive analysis, starting with LC–MS/MS analysis on GH3 cells to identify proteins linked to CAB treatment and tumor size. We confirmed the involvement of Nedd4 family-interacting protein 1 (NDFIP1) through transcriptome analysis of 180 human PitNET samples. We also assessed the impact of NDFIP1 overexpression on cell viability and CAB responsiveness in vitro, as well as tumor growth in vivo, while exploring its role in PTEN translocation and mTOR pathway activation. Our findings highlight NDFIP1 as a key player in DA resistance, indicating its potential as a therapeutic target for enhancing treatment outcomes in prolactinoma patients.
Materials and methods
Human sample collection
The study was conducted in accordance with ethical guidelines and received approval from the Ethical Review Board at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). Between 2016 and 2020, a total of 180 consecutive patients diagnosed with various subtypes of PitNET who were undergoing transsphenoidal surgery at Ruijin Hospital were enrolled in this study. We collected 180 tumor samples to conduct transcriptome analysis. This cohort included all the 2017 WHO-defined PitNET subtypes, comprising 65 (36%) gonadotroph, 40 (22%) lactotroph, 35 (19%) corticotroph (TPIT lineage), 26 (14%) somatotroph, 2 (1%) mixed growth hormone/prolactin (GH-PRL), 6 (3%) null cell, 2 (1%) thyrotroph, and 4 (2%) plurihormonal PIT-1 PitNET. The sample database used in this study has been reported in our previous research [14] and summarized in Table S1. Data on normal pituitary tissue samples were obtained from public database (https://ngdc.cncb.ac.cn/databasecommons/database/id/6329).
Xenograft mouse model
Four-week-old female athymic nude mice (BALB/c-nu) were purchased from Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, China) and were maintained under specific pathogen-free conditions. Subcutaneous injections of 1 × 107 parental GH3 cells or NDFIP1-overexpressing GH3 cells were administered in a 100 μL volume at the flank region. Tumor dimensions were measured every 3 days post-injection using a caliper ruler. Tumor volumes were calculated using the formula: V (mm3) = (ab2)/2, where “a” represented the length, and “b” the width of the tumor [15]. When the largest tumor dimension reached approximately 2 cm, all mice were euthanized through cervical dislocation, and the tumors were photographed, weighed, and used for Western blot, immunohistochemistry and immunofluorescence analysis.
1 × 107 GH3 cells were mixed with PBS and injected subcutaneously into the flanks of 10 mice. The animals were randomly assigned to 2 groups with 5 animals in each group: control group and CAB group. The animals were injected intraperitoneally with 100 mL vehicle control (0.1% dimethyl sulfoxide in 0.9% saline) or CAB (0.5 mg/kg, intragastric administration once every 2 days,) for 12 days. The tumors were used for immunofluorescence analysis.
The study was approved by the Ethical Review Board at Ruijin Hospital of Shanghai Jiao Tong University School of Medicine, with the ethics number: RJ2023009.
Cell culture
Rat PitNET cell lines MMQ (CRL-10609™) and GH3 (CCL-82.1™) were purchased from the American Type Culture Collection (Manassas, VA, USA). These cells were cultured in Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY, USA) and F12 medium (Gibco), respectively, supplemented with 15% horse serum (Gibco) and 2.5% fetal bovine serum (Gibco). Both cell lines were maintained in a humidified atmosphere with 5% CO2 at 37 °C.
LC–MS/MS analysis
LC–MS/MS analysis was performed to identify differentially expressed proteins in MMQ cells in response to CAB treatment. Cells were treated with DMSO or CAB (25 μM and 50 μM) for 24 h. The cells were collected, lysed in SDS lysis buffer (4% SDS, 100 mM Tris–HCl, 100 mM dithiothreitol at pH 7.6), denatured at 95 °C for 10 min, and centrifuged at 12,000 g for 15 minuntes to obtain the supernatants. The protein concentration was determined using a tryptophan fluorescence emission assay and confirmed with Coomassie staining. Proteins were digested following the Filter Assisted Sample Preparation method. Briefly, 50 μg of proteins were loaded in 10 kDa centrifugal filter tubes, washed thrice with 200 μL urea buffer (8 M urea, 100 mM Tris–HCl, pH 8.5), alkylated with 50 mM iodoacetamide in urea buffer in the dark for 30 min, and then washed thrice with urea buffer, followed by three washes with 50 mM ammonium bicarbonate. Protein digestion was carried out overnight with trypsin at a ratio of 1:50 (protein: trypsin, w/w) at 37 °C. The concentration of digested peptides was measured using a BCA protein quantification kit (Beyotime, Shanghai, China). Peptides were desalted using C18 solid-phase extraction and resuspended in 0.1% formic acid for LC–MS/MS analysis.
The LC–MS/MS analysis was performed on an Orbitrap Q Exactive mass spectrometry platform (Thermo Fisher Scientific, USA) coupled to an online nano-flow EASY-nLC1000 HPLC system (Thermo Fisher Scientific). Peptides were loaded onto a self-packed column (75 μm × 150 mm; 3 μm ReproSil-Pur C18 beads, 120 Å, Dr. Maisch GmbH) and separated over a 180-min gradient at a flow rate of 300 nL/min. The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). A data-dependent acquisition MS method was employed, beginning with an MS1 full scan (300 to 1600 m/z) at a resolution of 70,000 at 200 m/z, with an AGC target of 3e6 ions. Subsequently, 15 data-dependent MS/MS scans with higher-energy collisional dissociation were performed at a resolution of 17,500 at 200 m/z. The normalized collision energy was set at 27%, and the isolation window was 2.0 m/z. Raw MS spectra were analyzed using MaxQuant (version 1.6.5.0) against the UniProt Rat database (downloaded in September 2020). For both peptide and protein identification, the false discovery rate was set at 0.01. Carbamidomethyl cysteine was considered a fixed modification, while oxidized methionine and protein N-terminal acetylation were treated as variable modifications. Enzyme specificity was defined as trypsin/P. Label-free quantification intensity values were used for subsequent data analysis.
Lentiviral vector transfection
A lentiviral vector, pCDH-puro, was purchased from BioSune Biotechnologies (Shanghai, China). Recombinant plasmids (pCDH-puro-NDFIP1) were constructed and sequenced by Boshang Biotechnologies (Shanghai, China). After 48 h of transduction with lentiviral vectors, MMQ and GH3 cells stably expressing NDFIP1 or empty vectors were selected with 2 μg/mL puromycin for 1–2 weeks.
Immunohistochemistry (IHC)
IHC validation of NDFIP1 expression was conducted on 29 patients with PitNET, including various subtypes and a range of tumor sizes. The tumor subtypes were categorized according to The 2017 World Health Organization classification of tumors of the pituitary gland [16], consisting of gonadotroph (n = 10), lactotroph (n = 4), corticotroph (n = 1), somatotroph (n = 5), null (n = 6), mixed growth hormone/prolactin (GH-PRL; n = 1), and plurihormonal PIT-1 PitNET (n = 2). Tumor sizes were distributed as < 1 cm (n = 1), ≥ 1 cm and < 2 cm (n = 13), ≥ 2 cm and < 3 cm (n = 11), and ≥ 3 cm (n = 4). The clinical characteristics of these samples are summarized in Table S2. Tissue samples were fixed in 4% formalin and embedded in paraffin. Tissue slices were cut into a thickness of 4 μm. IHC was performed using the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA). Rabbit primary antibodies for NDFIP1 (15602-1-AP, dilution: 1:1000; Proteintech, Wuhan, China) were used. Slices were developed with DAB and counterstained with hematoxylin. Slices were scanned using the Pannoramic 1000 slide digitalization system (3DHISTECH, Budapest, Hungary). Images were captured at × 20 magnification using CaseViewer 2.3 (3DHISTECH). IHC scores were assessed by two pathologists in a blinded manner. The staining was scored as negative (0+), low positive (1+), positive (2+), and high positive (3+), as previously described [17].
Cell counting kit-8 (CCK-8) assay
Cell proliferation was measured using a CCK-8 kit (Bimake, Houston, TX, USA) following the manufacturer’s instructions. MMQ and GH3 cells were plated in 96-well plates at 1 × 104 cells per well and treated with CAB ranging from 0 to 200 µM. The absorbance was measured at a wavelength of 450 nm using a microplate reader (Bio-Tek, USA).
Western blot analysis
Total proteins of tumor samples were extracted using the Total Protein Extraction Kit (Millipore Corporation, Billerica, MA, USA). Cultured cells were lysed with RIPA lysis Buffer (Beyotime). The proteins were separated in SDS-PAGE and transferred onto PVDF membranes (Millipore). The membranes were blocked with 5% skim milk in TBST buffer for 1 h and incubated overnight at 4 °C with primary antibodies against S6K1 (9202S, Cell Signaling Technology, 1:1000), p-S6K1 (Thr389,9205S, Cell Signaling Technology,1:1000), 4EBP1 (9644S, Cell Signaling Technology, 1:1000), p-4EBP1 (Thr37/46, 2855S, Cell Signaling Technology, 1:1000), Tubulin (ab7291, Abcam, 1:5000), mTOR (2983, Cell Signaling Technology, 1:1000), p-mTOR (5536, Cell Signaling Technology, 1:1000), AKT (4691, Cell Signaling Technology, 1:1000), p-AKT (4060, Cell Signaling Technology, 1:2000), PTEN (ab267787, Abcam, 1:1000), NDFIP1(15602-1-AP, Proteintech, 1:500), Peroxidase-conjugated AffiniPure Goat Anti-Mouse lgG (H+L) (115-035-003, Jackson ImmunoResearch), Peroxidase-conjugated AffiniPureGoat Anti-Rabbit lgG (H+L) (111-035-003, Jackson ImmunoResearch). Signals were detected using ECL detection reagent (Millipore) according to the manufacturer’s instructions.
Immunofluorescence
Tumor sections were deparaffinized and rehydrated, followed by antigen retrieval via high-pressure treatment in EDTA buffer to restore epitope accessibility. Subsequently, endogenous peroxidase activity was quenched using 3% hydrogen peroxide, and non-specific binding sites were blocked with 10% goat serum for 30 min at room temperature. The anti-PTEN antibody (ab267787, Abcam) and anti-NDFIP1 antibody (15602-1-AP, Proteintech) were applied and allowed to incubate overnight at 4 °C. After thorough washing, a secondary antibody (Goat Anti-Rabbit IgG HRP) was incubated, followed by labeling with CY5 for 10 min at room temperature and further washing steps. A second round of antigen retrieval was performed to enhance staining. Subsequently, donkey serum was used for blocking for 30 min at room temperature, and the sections were incubated with the secondary antibody (Alexa Fluor®488 donkey anti-rabbit IgG H+L) for 45 min at 37 °C and counterstained with DAPI to visualize cell nuclei. Finally, the sections were sealed with fluorescence mounting medium and analyzed under a fluorescence microscope.
Isolation and analysis of cytoplasm and nucleus proteins
Plasma membrane, cytoplasm, and nucleus proteins were isolated from GH3 and MMQ cells using the Minute™ Plasma Membrane Protein Isolation Kit (SM-005; Invent Biotechnologies). These proteins were separated by SDS-PAGE and immunoblotted using the specific antibodies.
Statistical analysis
Data were expressed as the mean ± standard deviation. All data were analyzed using GraphPad Prism (version 5; San Diego, CA, USA) and R (version 4.0.0) software. Differences among categorical variables were assessed using one-way ANOVA followed by the Student–Newman–Keuls (SNK) post-hoc test, independent-sample Student’s t-test, or two-sided Wilcoxon test, as appropriate. A P value less than 0.05 was considered statistically significant.
Results
NDFIP1 is upregulated in CAB-treated PitNET cells and positively correlates with tumor size in PitNET patients
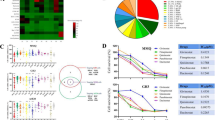
To investigate the underlying mechanism of DA resistance in PITNET patients, we employed LC–MS/MS analysis to identify differentially expressed proteins in MMQ cells induced by CAB treatment. At a concentration of 50 µM CAB, FDFT1 and NDFIP1 were the top two genes that exhibited increased expression levels (Fig. 1A). When analyzing human PitNET samples, we observed a significant elevation in NDFIP1 gene expression across various PitNETtypes (Fig. 1B). Pearson’s correlation analysis revealed a substantial positive correlation between NDFIP1 expression and tumor size (R2 = 0.26, P = 0.00033; Fig. 1C). In contrast, the correlation between FDFT1 and tumor size was weaker and not statistically significant (R = 0.035, P = 0.64; Fig. 1D).
NDFIP1 expression and correlation with tumor size. A MMQ cells were treated with DMSO or cabergoline (CAB; 25 µM or 50 µM). A heatmap of protein expression is shown. B A boxplot shows NDFIP1 mRNA expression across various human pituitary neuroendocrine tumor (PitNET) types according to WHO 2017 classification. C Correlation between NDFIP1 expression and tumor size. D Correlation between FDFDT1 expression and tumor size
Next, we sought to validate NDFIP1 upregulation and its positive correlation with tumor size using IHC. The results demonstrated that among the 29 PitNET patients with varying tumor types and tumor sizes, tumors with a diameter greater than 2 cm tended to have higher IHC scores than smaller tumors (P = 0.0092; Fig. 2A). Representative IHC staining from three cases with varying IHC scores is shown. Case 1, with a tumor diameter of 1.4 cm, exhibited a negative IHC score (0+); Case 2, with a tumor diameter of 1.8 cm, showed a low positive IHC score (1+); and Case 3, with a tumor diameter of 3.1 cm, had a positive IHC score (2+) (Fig. 2B). These findings suggest that NDFIP1 upregulation and its positive correlation with tumor size in PitNET potentially play a role in tumor progression and the tumor response to CAB therapy.
Correlation between NDFIP1 immunohistochemistry (IHC) score and tumor diameter in selected PitNET cases. A A scatter plot comparing the IHC score of NDFIP1 staining between tumors with diameters < 2 cm and those > 2 cm. **P = 0.0092 (t-test). B Representative IHC staining from three cases with varying IHC scores is presented. Case 1, with a tumor diameter of 1.4 cm, exhibited a negative IHC score (0+). Case 2, with a tumor diameter of 1.8 cm, showed a low positive IHC score (1+). Case 3, with a tumor diameter of 3.1 cm, had a positive IHC score (2+). Data are expressed as the mean ± standard deviation (SD). **P < 0.01
NDFIP1 overexpression enhances tumor growth while reducing CAB sensitivity
To investigate the effects of NDFIP1 overexpression on tumor growth, we established stable MMQ and GH3 cell lines overexpressing NDFIP1 or an empty vector as a control. For both cell lines, NDFIP1 overexpression significantly increased cell viability at 72 h compared to the control (Fig. 3A). CAB treatment reduced the cell viability of both cell lines with or without NDFIP1 overexpression in a dose-dependent manner. However, both cell lines with NDFIP1 overexpression exhibited higher cell viability compared to the control (Fig. 3B). In addition, NDFIP1-overexpressing cells formed larger tumors, and mice bearing these larger tumors exhibited a significant reduction in body weight compared to the control (Fig. 3C). These findings suggest that NDFIP1 overexpression in PitNET cell lines enhances cell viability, potentially contributing to increased tumor growth and resistance to CAB treatment,
The impact of NDFIP1 overexpression on cell viability and tumor growth. A MMQ and GH3 cells stably overexpressing NDFIP1 or an empty vector were treated with CAB (50 µM). Cell viability was determined at 0, 24, 48, or 72 h using CCK-8 assay. B Both cell lines were treated with CAB ranging from 0 to 100 µM. Cell viability was assessed at 72 h after treatment. C Four-week-old female nude mice were subcutaneously injected with either 1 × 107 parental GH3 cells or NDFIP1-overexpressing GH3 cells, and tumor growth was monitored until the largest tumor dimension reached approximately 2 cm. The tumors were subjected to photography, weighing, and volume measurement. EV, empty vector; OV, overexpression; CAB, cabergoline. Data are expressed as the mean ± SD. **P < 0.01, ***P < 0.001
NDFIP1 overexpression induces subcellular redistribution of PTEN
To elucidate the downstream effects of NDFIP1 overexpression in the mTOR signaling pathway, we focused on PTEN and p-4EBP1 based on the negative regulatory role of PTEN in mTOR signaling [18] and the fact that p-4EBP1 is positioned as a downstream target of mTOR [19]. IHC analysis revealed a pronounced increase in p-4EBP1 intensity in tumors formed from NDFIP1-overexpressing cells, while PTEN levels remained relatively unchanged (Fig. 4). Given PTEN in the cytoplasm acts as an inhibitor of the PI3K/AKT pathway and subsequently suppresses mTOR activation, we explored its subcellular distribution in MMQ and GH3 cells after NDFIP1 overexpression. We noted a rise in its nuclear localization, coupled with a reduction in the cytoplasm (Fig. 4B and C). This shift was further validated through immunofluorescence staining (Fig. 4D).
Impact of NDFIP1 overexpression on PTEN subcellular localization. A IHC analysis was performed to detect PTEN and p-4EBP1 intensity in mouse tumor tissues. (B, C) Western blot analysis was conducted to determine PTEN’s nuclear and cytoplasmic distribution in MMQ and GH3 cells upon NDFIP1 overexpression. Data are expressed as the mean ± SD. ***P < 0.001, ****P < 0.0001. D Immunofluorescence staining was carried out to visualize the enhanced nuclear localization of PTEN upon NDFIP1 overexpression tumor sections and CAB-treated tumor sections. Scale bar: 5 µm
These findings suggest that overexpression of NDFIP1 may promote the translocation of PTEN, potentially leading to enhanced mTOR activation due to reduced inhibition of the PI3K/AKT pathway in the cytoplasm.
NDFIP1 overexpression activates AKT/mTOR signaling in PitNET cells with potential implications in CAB treatment resistance
Next, we tested the effect of NDFIP1 overexpression on the AKT/mTOR pathway in PitNET cells. Our results revealed significant activation of the AKT/mTOR signaling pathway in MMQ and GH3 cells upon NDFIP1 overexpression as evidenced by the remarkable increase in the phosphorylation of AKT and mTOR, as well as the increased phosphorylation of S6K1 and 4EBP1, which are crucial downstream effectors of the mTOR pathway. Notably, the total PTEN levels remained relatively constant between control and NDFIP1-overexpressing cells (Fig. 5A).
Effects of NDFIP1 overexpression and CAB treatment on the AKT/mTOR pathway and apoptosis in PitNET cells. A Western blot analysis was performed to determine the effects of NDFIP1 overexpression on total and phosphorylated levels of AKT, mTOR, S6K1, and 4EBP1, as well as PTEN levels in MMQ and GH3 cells. B Time-course analysis of protein responses to CAB treatment (0, 24, 48, and 72 h)
Considering the involvement of mTOR in apoptosis induced by CAB [20], we further explored the protein responses to CAB treatment at different time points (0, 24, 48, and 72 h). Western blot analysis revealed a progressive increase in caspase-3 levels over time, indicating enhanced apoptosis. Intriguingly, the expression levels of NDFIP1 also exhibited an increase in a time-dependent manner, accompanied by a simultaneous decrease in the levels of p-AKT and p-mTOR in both PitNET cell lines (Fig. 5B). Additionally, CAB treatment substantially augmented the levels of caspase-3 and NDFIP1 proteins, which could be partially reversed by Z-VAD-FMK, a pan-caspase inhibitor (Fig. 5C). These data suggest that NDFIP1 overexpression activates the AKT/mTOR pathway in PitNET cells, potentially promoting cell survival. However, CAB treatment appears to induce simultaneous apoptosis and an increase in NDFIP1 expression. This dual effect may play a role in the development of resistance to CAB treatment in PitNET patients.
Discussion
The results of this study provide valuable insights into the molecular mechanisms associated with CAB resistance in PitNET. The upregulation of NDFIP1 in both CAB-treated GH3 cells and PitNET patient samples, along with its positive correlation with tumor size, suggests its potential role as a biomarker for tumor progression and treatment response. The observed increase in cell viability and tumor size in cells overexpressing NDFIP1 further supports the idea that NDFIP1 promotes tumor growth and resistance to CAB. NDFIP1 plays a pivotal role in the ubiquitin–proteasome system, mediating the ubiquitination and degradation of diverse substrate proteins to modulate various cellular processes like immune response, cell signaling, and cell cycle [21,22,23]. NDFIP1 has been implicated in the development of cancers, autoinflammatory diseases, and neurodegenerative disorders [24, 25]. However, its role in PitNET has not been reported yet. This is the first study to elucidate the potential role of NDFIP1 in the pathogenesis and treatment resistance of PitNET, opening avenues for further research and potential therapeutic interventions.
The role of PTEN as a negative regulator of the PI3K/AKT pathway is well-established, and its altered localization could contribute to enhanced mTOR activation, often associated with cell survival and proliferation [26,27,28]. Our results showed that in MMQ and GH3 cells, NDFIP1 overexpression promoted the translocation of PTEN from the cytoplasm to the nucleus, potentially leading to enhanced mTOR activation due to reduced inhibition of the PI3K/AKT pathway in the cytoplasm. Consistent with our findings, NDFIP1 has been shown to facilitate PTEN translocation by promoting its monoubiquitination. This monoubiquitination of PTEN, orchestrated by NEDD4 family E3 ubiquitin ligases recruited by NDFIP1, allows PTEN to be shuttled into the cell nucleus and exosomes, affecting its subcellular localization and, consequently, its activity in regulating PI3K/AKT signaling [29,30,31]. In our study, despite NDFIP1 overexpression leading to pronounced increases in downstream signaling, including p-4EBP1 and activation of the AKT/mTOR pathway, the levels of total PTEN remained relatively constant. Similar to our data, previous studies have indicated that Ndfip1 loss in the developing brain does not significantly alter total PTEN levels [32, 33]. However, studies of PTEN redistribution to the nucleus following cerebral ischemia have demonstrated that overexpression of NDFIP1 increased PTEN import into the nucleus [30]. This shift in PTEN localization may provide a mechanistic link between NDFIP1 overexpression and AKT/mTOR pathway activation.
Our findings highlight the significance of the activated AKT/mTOR signaling pathway in PitNET cells overexpressing NDFIP1. This activation may promote cell proliferation and potentially contribute to resistance against DA treatment, ultimately leading to the increase in tumor size. These results align with previous research that has shown the therapeutic potential of targeting the activated mTOR pathway in refractory prolactinomas. Specifically, everolimus, an mTOR inhibitor, can have antiproliferative effects when used in combination with CAB, resulting in reduced prolactin levels and tumor regression [11, 34]. Additionally, inhibiting the mTOR pathway has been shown to inhibit the growth of aggressive PitNET in vitro [35]. Furthermore, research by Gorvin et al. has highlighted the role of specific prolactin receptor variants in promoting cell proliferation through the mTOR pathway, which can be counteracted by everolimus [36]. Additionally, Zhu et al. have demonstrated that the compound ACT001 can reverse CAB resistance in GH3 cells by inhibiting the mTOR signaling pathway and inducing cell death through autophagy [37]. These findings collectively underscore the potential therapeutic relevance of targeting the mTOR pathway in prolactinoma treatment, particularly in cases of DA resistance.
The dual effect of CAB treatment, inducing both apoptosis and NDFIP1 upregulation, raises intriguing questions about the complex interplay between NDFIP1 and the therapeutic response. It suggests that NDFIP1 may have a role in cellular survival mechanisms that counteract the apoptotic effects of CAB. Supporting our findings, Goh et al. have shown that NDFIP1 facilitates the nuclear trafficking of PTEN in response to brain trauma, promoting neuron survival by preventing apoptosis [38]. In spinal cord injury, NDFIP1 overexpression abolished the inhibition effect of miR-155-5p mimic on PTEN nuclear translocation and reversed astrocyte activation induced by miR-155-5p mimic, ultimately contributing to the inhibition of apoptosis and cell survival [39]. While these previous findings mainly originate from the central nervous system, our novel observation of CAB increasing caspase-3 levels while simultaneously upregulating NDFIP1 suggests the need for extensive exploration into the precise underlying mechanisms of CAB resistance in prolactinoma.
The primary limitation of this study is that our results are largely based on in vitro cell culture models and murine xenografts, which may not fully recapitulate the complexity and heterogeneity of human PA. Additionally, the mechanism by which CAB therapy leads to an increase in NDFIP1 expression while inducing apoptosis was not completely elucidated.
Conclusions
In conclusion, this study demonstrates that NDFIP1 activates the AKT/mTOR pathway, potentially through altering PTEN localization, leading to increased tumor cell viability and reduced sensitivity to CAB treatment. While further research is needed to elucidate the precise mechanisms and validate these findings in clinical settings, this study provides new avenues for overcoming DA resistance in PitNET patients.
Data availability
All data generated or analysed during this study are included in this published article.
References
Ezzat S, Asa SL, Couldwell WT et al (2004) The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619
Varlamov EV, McCartney S, Fleseriu M (2019) Functioning pituitary adenomas–current treatment options and emerging medical therapies. Eur Endocrinol 15:30
Asa SL, Mete O, Perry A et al (2022) Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol 33:6–26
Asa SL, Mete O, Cusimano MD et al (2021) Pituitary neuroendocrine tumors: a model for neuroendocrine tumor classification. Mod Pathol 34:1634–1650
Lin S, Zhang A, Zhang X et al (2020) Treatment of pituitary and other tumours with cabergoline: new mechanisms and potential broader applications. Neuroendocrinology 110:477–488
Maiter D (2019) Management of dopamine agonist-resistant prolactinoma. Neuroendocrinology 109:42–50
Souteiro P, Karavitaki N (2020) Dopamine agonist resistant prolactinomas: any alternative medical treatment? Pituitary 23:27–37
Popova NV, Jücker M (2021) The role of mTOR signaling as a therapeutic target in cancer. Int J Mol Sci 22:1743
Wang Q, Wang J, Xiang H et al (2021) The biochemical and clinical implications of phosphatase and tensin homolog deleted on chromosome ten in different cancers. Am J Cancer Res 11:5833
Derwich A, Sykutera M, Bromińska B et al (2023) The role of activation of PI3K/AKT/mTOR and RAF/MEK/ERK pathways in aggressive pituitary adenomas—new potential therapeutic approach—a systematic review. Int J Mol Sci 24:10952
Zhang D, Way JS, Zhang X et al (2019) Effect of everolimus in treatment of aggressive prolactin-secreting pituitary adenomas. J Clin Endocrinol Metab 104:1929–1936
Yao H, Tang H, Zhang Y et al (2019) DEPTOR inhibits cell proliferation and confers sensitivity to dopamine agonist in pituitary adenoma. Cancer Lett 459:135–144
Lin SJ, Leng ZG, Guo YH et al (2015) Suppression of mTOR pathway and induction of autophagy-dependent cell death by cabergoline. Oncotarget 6:39329
Yao H, Xie W, Dai Y et al (2022) TRIM65 determines the fate of a novel subtype of pituitary neuroendocrine tumors via ubiquitination and degradation of TPIT. Neuro Oncol 24:1286–1297
Fan K, Ding X, Zang Z et al (2022) Drp1-mediated mitochondrial metabolic dysfunction inhibits the tumor growth of pituitary adenomas. Oxid Med Cell Longev 2022:1
Lopes MBS (2017) The 2017 world health organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol 134:521–535
Varghese F, Bukhari AB, Malhotra R et al (2014) IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 9:e96801
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13:283–296
Murugan AK (2019) mTOR: Role in cancer, metastasis and drug resistance. Seminars in cancer biology, Elsevier, Amsterdem, pp 92–111
Lin SJ, Wu ZR, Cao L et al (2017) Pituitary tumor suppression by combination of cabergoline and chloroquine. J Clin Endocrinol Metab 102:3692–3703
Tang Y-L, Zhu L, Yang H-F et al (2023) Research progress on the role of Ndfip1 (Nedd4 family interacting protein 1) in immune cells. Allergol Immunopathol 51:77–83
Altin JA, Daley SR, Howitt J et al (2014) Ndfip1 mediates peripheral tolerance to self and exogenous antigen by inducing cell cycle exit in responding CD4+ T cells. Proc Natl Acad Sci 111:2067–2074
O’Leary CE, Riling CR, Spruce LA et al (2016) Ndfip-mediated degradation of Jak1 tunes cytokine signalling to limit expansion of CD4+ effector T cells. Nat Commun 7:11226
Layman AAK, Deng G, O’Leary CE et al (2017) Ndfip1 restricts mTORC1 signalling and glycolysis in regulatory T cells to prevent autoinflammatory disease. Nat Commun 8:15677
Zhang Y, Zhang C, Zhao Q et al (2019) The miR-873/NDFIP1 axis promotes hepatocellular carcinoma growth and metastasis through the AKT/mTOR-mediated Warburg effect. Am J Cancer Res 9:927
Haddadi N, Lin Y, Travis G et al (2018) PTEN/PTENP1:’Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol Cancer 17:1–14
Chen J-H, Zhang P, Chen W-D et al (2015) ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 11:239–252
Martelli A, Evangelisti C, Chappell W et al (2011) Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia 25:1064–1079
Putz U, Howitt J, Doan A et al (2012) The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Science Signaling 5:ra70–ra70
Howitt J, Lackovic J, Low L-H et al (2012) Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J Cell Biol 196:29–36
Howitt J, Low L-H, Putz U et al (2015) Ndfip1 represses cell proliferation by controlling Pten localization and signaling specificity. J Mol Cell Biol 7:119–131
Goh C-P, Low L-H, Putz U et al (2013) Ndfip1 expression in developing neurons indicates a role for protein ubiquitination by Nedd4 E3 ligases during cortical development. Neurosci Lett 555:225–230
Hammond VE, Gunnersen JM, Goh C-P et al (2014) Ndfip1 is required for the development of pyramidal neuron dendrites and spines in the neocortex. Cereb Cortex 24:3289–3300
Lin AL, Geer EB, Lala N et al (2023) The treatment of aggressive prolactinomas with everolimus. Pituitary 26:474–481
Monsalves E, Juraschka K, Tateno T et al (2014) The PI3K/AKT/mTOR pathway in the pathophysiology and treatment of pituitary adenomas. Endocr Relat Cancer 21:R331–R344
Gorvin CM, Newey PJ, Rogers A et al (2019) Association of prolactin receptor (PRLR) variants with prolactinomas. Hum Mol Genet 28:1023–1037
Zhu J, Tang C, Cong Z et al (2022) ACT001 reverses resistance of prolactinomas via AMPK-mediated EGR1 and mTOR pathways. Endocr Relat Cancer 29:33–46
Goh C-P, Putz U, Howitt J et al (2014) Nuclear trafficking of Pten after brain injury leads to neuron survival not death. Exp Neurol 252:37–46
He L, Chang Q, Zhang Y et al (2023) MiR-155-5p aggravated astrocyte activation and glial scarring in a spinal cord injury model by inhibiting Ndfip1 expression and PTEN nuclear translocation. Neurochem Res 48:1912–1924
Acknowledgements
Not applicable
Funding
This work was supported by the National Natural Science Foundation of China under Grant Number 82141114, 81972339 (Z.B.W.), National Research Center for Translational Medicine under grant number NRCTM (SH)- 2019-05 (Z.B.W.), Shanghai Municipal Science and Technology Commission under Grant Number 18XD1403400 (Z.B.W.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Zhebao Wu conceived and supervised the study; Weiting Gu designed and performed experiments; Weifeng Zhang analysed data; Weiting Gu wrote the manuscript; Yu Cai made manuscript revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Human sample: The study was conducted in accordance with ethical guidelines and received approval from the Ethical Review Board at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). Mouse model: The study was approved by the Ethical Review Board at Ruijin Hospital of Shanghai Jiao Tong University School of Medicine, with the ethics number: RJ2023009.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, W., Zhang, W., Wu, Z. et al. Cabergoline-induced NDFIP1 upregulation in pituitary neuroendocrine tumor cells activates mTOR signaling and contributes to cabergoline resistance. J Neurooncol 172, 587–597 (2025). https://doi.org/10.1007/s11060-025-04949-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-025-04949-7