Abstract
Urbanization can alter all types of ecological interactions, including parasitism. For urban-associated hosts, two hypotheses predict the response of parasites to urbanization: dense host populations can increase disease transmission, and/or healthy host populations can increase immunity against disease. Using a historical record of bird specimens, we examined the effect of historical urban environments on parasitic interactions by measuring the abundance of ectoparasites on two bird species: House Finch and Hairy Woodpecker. We counted adult chewing lice and their nits from museum study skins of these two species, which were collected in the western United States from 1900 to 1932. Our urbanness metric for each specimen’s collection site and year was extracted from the Built-Up Footprint Area dataset, which measures the area covered by human structures each decade. Despite finding many lice preserved on museum specimens, we found no significant relationship between urbanness and louse loads. This result could be because lice are not sensitive to their external environment, or because the impacts of urbanization on birds were still small in the temporal range documented by our specimens. We also found that House Finches had more nits but fewer adult Ischnocera than Hairy Woodpeckers, and that Ischnocera and nit loads exhibited slight seasonal variation. Our study emphasizes the value of museum collections for understanding the historical process of urbanization in an ecological context, including bird-louse dynamics, and provides an important initial evaluation of how chewing lice may or may not respond to urbanization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Over the past two centuries in the United States, rapid land conversion and human population growth have created novel urban landscapes (Gibson and Jung 2002; Leyk et al. 2020). Humans have changed urban ecosystems dramatically (Grimm et al. 2008), including alterations to physical habitat (Saunders et al. 1991; Arnold and Gibbons 1996; Fortuniak et al. 2006; Carey et al. 2013; Fletcher et al. 2013), trophic dynamics (Faeth et al. 2005; El-Sabaawi 2018; Sol et al. 1998; Shochat 2004), and community composition (Green and Baker 2003; Biamonte et al. 2011; Tomasevic and Marzluff 2017). Fischer et al. (2015) propose a classification system for how species respond to cities: urban dwellers are successful and form self-sustaining populations (McKinney 2006), urban utilizers can reproduce but do not form self-sustaining populations, and urban avoiders have low reproductive success (Lancaster and Rees 1979). A fundamental interaction which can synergistically affect and respond to host tolerance of the urban landscape is parasitism (Beldomenico and Begon 2010), potentially regulating host success and community structure.
There are two broad hypotheses for how parasitism changes in cities (Werner and Nunn 2020): (1) the ‘urban burden’, where transmission is exacerbated by dense populations (Bradley and Altizer 2007) and clumped resources (Wright and Gompper 2005; Giraudeau et al. 2014), or (2) the ‘urban refuge’, where abundant food and predation release create more immunocompetent populations which can expend more energy on defenses (Bradley and Altizer 2007). The two hypotheses may be linked to Fischer et al.’s (2015) classification of urban tolerance, such that urban avoiders are susceptible to parasites on the rare occasions they enter urban areas because they are less successful there, but urban dwellers are healthy in urban habitats and therefore resistant to parasites. A countervailing theory, the dilution effect, proposes that dispersed populations reduce transmission of diseases (Bradley and Altizer 2007; Civitello et al. 2015), which may apply to urban avoiders, whereas dense, homogeneous populations increase transmission, which may apply to urban dwellers. Which hypothesis dominates depends on a complex combination of host species, sex, age, and urban tolerance (Bentz et al. 2006; Delgado-V. & French 2012), as well as parasite life cycle complexity (Calegaro-Marques and Amato 2014; Bailly et al. 2016), transmission method (Evans et al. 2009; DeVore et al. 2020), endo- versus ectoparasitism (Gregoire et al. 2002; Arzua et al. 2003; Wright and Gompper 2005), and virulence (Bichet et al. 2020).
Birds have been widely studied for their urban responses, including higher boldness (Atwell et al. 2012; Minias 2015), a variety of stress responses (Bonier 2012), reduced body mass (Meillère et al. 2015), altered phenology (Tryjanowski et al. 2013; Hutton et al. 2021), and denser populations (Sorace 2002). In addition, nearly all bird species are associated with obligately ectoparasitic chewing lice in the clades Ischnocera and Amblycera (Psocodea: Phthiraptera) (Price et al. 2003; Clayton et al. 2008). In high numbers, chewing lice exact costs on their hosts by reducing feather mass (Booth et al. 1993; Clayton et al. 1999), increasing grooming time (Brown 1974; Redpath 1988; Price et al. 2003), making mates less competitive (Clayton 1990, 1991), and sometimes blood-feeding (Samuel et al. 1982; Price et al. 2003; Dik 2006). Chewing louse eggs, called nits, are cemented on feathers in hard-to-reach places (Eichler 1963; Foster 1969a, b; Price et al. 2003). Like many parasites, chewing lice follow an overdispersed count distribution, where a few hosts have high numbers of lice, and most hosts have few or none (Price et al. 2003). The two parvorders of avian chewing lice have important behavioral differences: Amblycera are more mobile both while a host is alive and in seeking a new host after death, and they often feed on blood as well as feathers, while Ischnocera are specialized to living and feeding on feathers, to the point of restricted mobility (Clayton et al. 2008; Kolenčík et al. 2024). For lice, low humidity is associated with lower prevalence and abundance, as well as lower diversity (Fabiyi 1996; Moyer et al. 2002; Bush et al. 2009, 2024), but Ischnocera mitigate this to some extent by absorbing atmospheric water vapor (Rudolph 1983; Bush et al. 2009). Although birds have often been studied in the context of urban environments or ectoparasitism, few studies have examined the intersection of these factors.
Urban development and ecosystems overall are temporally structured, but a lack of data on historical processes has hindered the study of past urban ecology (Ossola et al. 2021). Natural history collection specimens preserve chewing lice (Mey 2003), providing a unique opportunity to pinpoint when and how the development of urban spaces has affected parasitism (Winker 2004). This is especially true for the early 20th century, when high rates of urban growth presumably caused ecological transitions (Leyk et al. 2020; Ossola et al. 2021) and when American vertebrate collecting activity peaked (Joseph 2011). In the western United States, the House Finch, Haemorhous mexicanus (Statius Müller 1776), has been widely distributed across rural and urban habitats since at least the late 1800s (Bergtold 1913; Fig. 1a). The Hairy Woodpecker, Dryobates villosus (Linnaeus 1766), is more of an urban avoider (Ripper et al. 2007; Baz and Murphy 2023; Fig. 1b), but the two species overlap in distribution (Fink et al. 2023). The House Finch’s responses to urban environments include duller coloration (Giraudeau et al. 2018; Sykes et al. 2021), lower aggression (Hasegawa et al. 2014), lower sensitivity to artificial light (Hutton et al. 2024), and higher prevalence and severity of coccidiosis and poxvirus infections (Giraudeau et al. 2014; Sykes et al. 2021). The Hairy Woodpecker’s responses to cities have not been studied specifically, but as an urban avoider, it may be less healthy and less successful (Delgado-V. & French 2012). For these host species, chewing louse associations are documented (Table 1), but such interactions have received limited ecological attention, especially in urban spaces.
Therefore, to assess the major hypotheses of urban parasitism in a historical framework, we measured chewing louse abundance on House Finch and Hairy Woodpecker specimens collected from the western United States between 1905 and 1932 (Fig. 1c). As a predictor of louse abundance, we extracted values of historical urbanness from a time-series of land use from 1900 to 2010 (Leyk and Uhl 2018b). We also tested host species as a predictor, expecting different responses from urban dwellers and avoiders, and tested year, day of year, precipitation, host sex, and host age to control for plausible biological effects. We expected that urban environments in the western United States in the early 20th century would result in higher chewing louse loads, analogous to higher House Finch endoparasite loads in modern urban environments (Giraudeau et al. 2014). Alternatively, if urban habitat did not alter avian chewing louse abundance in this period, it would either mean that during this time urbanization was too limited to affect bird-lice interactions, or that, unlike endoparasites, chewing lice do not differ in abundance between rural and urban environments.
Annual mean relative abundance of (a) H. mexicanus and (b) D. villosus in 2022 in the western United States (Fink et al. 2023). Note that H. mexicanus is widely distributed, with dense populations around urban areas. In contrast, D. villosus is more patchily distributed, with a tendency to avoid cities. (c) Collection sites of the 104 H. mexicanus and 56 D. villosus UCLA Dickey Bird and Mammal Collection specimens used in this study, which were collected 1905–1932, and 2010 BUFA (Leyk and Uhl 2018b), a metric of how many m2 are covered by human structures per 250 × 250 m grid cell. Figure created in QGIS 3.36.1 (QGIS Development Team 2024), WGS 84 CRS
Methods
Bird specimens and louse abundance
To count lice, we searched 104 House Finch and 56 Hairy Woodpecker study skins (hosts) from the UCLA Dickey Bird and Mammal Collection. Specimens were collected between 1905 and 1932 across the states of Washington, Oregon, California, Idaho, Nevada, and Arizona (Fig. 1c). Specimens included information on collection date, geographic coordinates of collection, collector name, sex of individual specimen, and specimen age class (Juvenile, Immature, or Adult). Sex and age determinations were taken from specimen tags, while the rest of the information was derived from online catalog records. Geographic coordinates had precision of one arcsecond, giving a maximum uncertainty of 1861 m.
We applied standard search protocols to collect lice from each bird specimen (Clayton and Walther 1997; Mey 2003), with additional training and advice from Jessie Salter (pers. comm.). Under a magnifying circular LED light, we searched contour feathers around the ears, beak, neck, breast, and center of the dorsal side, but excluded the wings, lower belly, and thighs because neither host species is known to host wing lice and because it was difficult to search long, downy feathers consistently and thoroughly. We moved methodically across each section, lifting one row of feathers at a time, searching each dorsal and ventral surface, especially near the skin. Using forceps, we extracted (adult) lice and nits; in some cases, nits were counted but not removed because they were attached too strongly to remove without damaging the specimen. We anecdotally observed that most but not all nits were empty, though eggs may pop open as they dry, so empty nits were not necessarily hatched. While inspecting, we held each bird over glossy paper and examined fallen debris under a dissecting scope for additional lice. To ensure completeness, each specimen was searched separately by two individuals. All searchers (EXK, JMJ, AL, MCR, GAM) trained together for consistency. The median search time was 10 min, though times varied widely – search times were longer on birds with more lice because of the time required to remove ectoparasites.
We identified representative adult louse morphotypes by preserving them in ethanol, slide-mounting them in Hoyer’s medium, and imaging them with a Leica M205 FA Fluorescence Stereomicroscope and a compound light microscope. We identified lice with keys from taxonomic authorities (Table 1) and, in the case of Brueelia thorini, additional assistance from Dr. Daniel Gustafsson (pers. comm. 2023 Jul 30).
Historical urbanness index (BUFA)
Historical urbanness values were drawn from the Building Footprint Area (BUFA) raster dataset in the Historical Settlement Data Compilation for the United States (HISDAC-US) (Leyk and Uhl 2018b). Based on property records, BUFA calculates the land area covered by human structures in the contiguous United States at 250 m resolution, every decade 1900–2010 (Leyk and Uhl 2018a). Because we were interested in the environmental impacts of urbanization, the physical definition of urbanness measured by BUFA was preferable to a more sociological definition (e.g. Bretagnolle et al. 2015). Although 100 m2 covered by human structures may have quite different environmental effects in 1900 compared to 2010, this dataset is predictive of human population size at the county level (Leyk et al. 2020) and HISDAC-US provides by far the finest resolution metrics of historical urbanness currently available for the U.S. (Leyk and Uhl 2018a; Uhl et al. 2021).
With the terra package v.1.7–55, (Hijmans 2023) in R v.4.3.1 (R Core Team 2023), we extracted the average BUFA value in a 2000-m radius buffer around the collection site of each specimen to account for the 1861-m coordinate uncertainty, proportional to the exact fraction of each cell covered by the buffer. Values were extracted from the most recent layer before each collection date; for example, specimens collected in 1920 and 1929 both received BUFA values from the 1920 raster layer.
Precipitation
To account for effects of climatic variation, particularly humidity, we obtained bioclimatic variable BIO17, which is the total precipitation in the driest three months of the year in mm, in 2.5 arcminute resolution from WorldClim v.2.1, based on data from 1970 to 2000 (Fick and Hijmans 2017). We chose BIO17 because humidity is the main climatic variable thought to affect avian chewing lice (Bush et al. 2024). With the same terra package, we extracted precipitation values at the collection coordinates for each specimen. However, because of missing raster cell values, we extracted precipitation values at the exact coordinates using the package’s bilinear method, which interpolates the four nearest cells, without a buffer.
Statistical analysis
We conducted all statistical analyses in R v.4.3.1 (R Core Team 2023). We fit negative binomial generalized linear mixed models (GLMM) of louse abundance with the glmmTMB package (v.1.1.7, Brooks et al. 2017) using the ‘nbinom2’ family, which has a quadratic parameterization and a log link. Although our dataset potentially showed some zero-inflation, we found non-convergence of zero-inflation models during exploratory analyses, so we retained the simpler negative binomial GLMM for final analysis. Our model choice was supported by previous descriptions of louse abundance distributions (Price et al. 2003).
Because Amblycera and Ischnocera have important differences in their life histories, feeding patterns, and responses after host death, we separated them when modeling. However, we only encountered one amblyceran species, and it only occurred on a single Hairy Woodpecker. Therefore, there was an insufficient sample size for any amblyceran interaction, so we only modeled adult Ischnocera and total nits. The Ischnocera model included both host species, and its response variable was the summed abundances of Brueelia thorini, Philopterus sp., Brueelia straminea s. lat., Penenirmus auritus s. lat., and Picicola snodgrassi, with the first two occurring on House Finches and the latter three on Hairy Woodpeckers. We did not distinguish ischnoceran louse species because small sample sizes would have weakened our modelling power. We selected the best fitting model for each response variable based on the values of AICc calculated with the AICcmodavg package (v.2.3.3, Mazerolle 2023) and ΔAICc and Akaike weights calculated with the qpcR package (v.1.4-1, Spiess 2018; Anderson and Burnham 2002). As predictors, we tested BUFA, collection year, collection day of year (with linear and quadratic terms), precipitation, host sex, host age class, and host species. We z-transformed the continuous variables (BUFA, year, day of year, precipitation) before fitting. Because seasonal effects were unlikely to follow a linear pattern from January 1st to December 31st, day of year was always included as both a linear and a quadratic predictor, where the quadratic term was squared after scaling. We expected that the biological categories could alter the bird-louse interaction, and that there could be a seasonal effect. Collection year was mostly included as a nuisance variable, although it was possible that the interaction had changed linearly through time. Although collector name was available from the catalog, we excluded it because there were many collectors that only collected a few specimens, so there was a high risk of overfitting. We used BUFA as a predictor in every tested model because it was our variable of interest, and we used host species in every model except the BUFA-only model, because of the a priori assumption that two species of bird with two different louse communities would behave differently. We also tested the interactions of BUFA with collection year and BUFA with host species. These interactions were chosen because the same area covered by human structures could equate to very different impacts in 1900 versus 2010, and different bird species are known to respond differently to urban spaces, which might also affect their parasites. We would have tested host sex and age class with host species as the random intercept, but these models did not converge. Our full candidate model set can be found in SI Tables 1 and 2.
To evaluate the strength and effect of our variables, we used the ggeffects package (v.2.2.0, Lüdecke 2018) to predict counterfactual means, which reflect the distribution of the data by averaging the response of every observation as if they all belonged to each level or value of the predictor, for the two host species and across the ranges of BUFA and collection day of year. Estimates were back-transformed to the response scale (i.e., number of lice or nits).
Results
We documented two louse species parasitizing House Finches (host n = 104) and four parasitizing Hairy Woodpeckers (host n = 56), as well as abundant nits which were not identified to species (Figs. 2 and 3a). Of the 104 House Finches, 32 (31%) hosted Brueelia thorini (Fig. 2a) and 26 (25%) hosted Philopterus sp. (Fig. 2b), including 11 (11%) which hosted both species. Of the 56 Hairy Woodpeckers, just 1 (2%) hosted Menacanthus sp. (Fig. 2c), and this individual hosted 6 Menacanthus sp. and 27 total lice. Additionally, 7 (13%) woodpeckers hosted Brueelia straminea s. lat. (Fig. 2d), 4 (7%) hosted Picicola snodgrassi (Fig. 2e), and 11 hosted P. auritus s. lat. (Fig. 2f). Only 2 (4%) Hairy Woodpeckers hosted multiple louse species: one hosted both B. straminea s. lat. and P. auritus s. lat., while the other was an anomalously high-load individual which hosted all four species that we found on Hairy Woodpeckers.
The two louse species found in this study on House Finches were (a) Brueelia thorini and (b) Philopterus sp. The four louse species found in this study on Hairy Woodpeckers were (c) Menacanthus sp., (d) Brueelia straminea s. lat., (e) Picicola snodgrassi, and (f) Penenirmus auritus s. lat. Lice were slide-mounted in Hoyer’s medium and imaged with a Swiftcam SC1803R camera, Swift microscope SW380T, and Swift Imaging software v.3.0
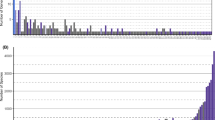
Ischnocera and nits were found on all host species (Fig. 3a), host sexes (Fig. 3b), and host age classes (Fig. 3c), with similar numbers between host sexes, though adult lice tended to be more abundant on immatures, and nits tended to be more abundant on House Finches. Specimens were collected between 1905 and 1932 (Fig. 4a), at sites with decadal BUFA values ranging from 0 to 2756 m2 built up area per 62,500 m2 grid cell (Fig. 4b). Specimens were collected across a gradient of 3–166 mm precipitation in the driest three months of the year (Fig. 4c) and throughout the year (Fig. 4d).
The top-ranked AICc model for nit abundance showed the same model structure as the second-highest ranked model for adult Ischnocera (SI Tables 1 and 2). Therefore, we selected this model structure for both response variables. Host species was a significant predictor in both models, though Hairy Woodpeckers were associated with higher Ischnocera and lower nit counts (Tables 2 and 3). Urbanness was not significant for either model (Tables 2 and 3), nor was precipitation (Tables 2 and 3), even though both variables were retained during model selection. Linear day of year had a significant negative effect only for nits, while day of year squared had a significant positive effect only for the adult Ischnocera model (Tables 2 and 3). These selected models did not include collection year, host sex, or host age as predictors.
Louse and nit abundance by species, host sex, and host age
On House Finches we found 0.86 ± 2.24 B. thorini and 0.38 ± 0.78 Philopterus sp. (mean ± s.d.) per specimen, with a total of 1.24 ± 2.35 lice per specimen (Fig. 3a). We also found 8.81 ± 19.69 nits per specimen (Fig. 3a), in two distinct types: larger, teardrop-shaped pairs on the underside of feathers and smaller, rounder ones in haphazard clumps, but we were unable to identify nits to species. On Hairy Woodpeckers, there were 0.11 ± 0.80 Menacanthus sp., 0.63 ± 2.35 B. straminea s. lat., 0.16 ± 0.68 P. mississippiensis, and 0.45 ± 1.16 P. auritus sp., with a total of 1.34 ± 3.87 lice per specimen, as well as 2.07 ± 5.90 nits (Fig. 3a). We again did not identify nits to species and did not notice obviously different types on Hairy Woodpeckers.
Host sex and age class were not predictors in our selected models. Excluding the six Menacanthus sp. on a single Hairy Woodpecker because they could not be modeled, on male hosts we found 1.22 ± 2.76 lice and 6.85 ± 19.00 nits, and on female hosts we found 1.27 ± 2.43 lice and 5.59 ± 9.60 nits (Fig. 3b). On juvenile hosts we found 0.36 ± 0.84 lice and 6.29 ± 13.70 nits, on immature hosts, we found 2.33 ± 3.75 lice and 4.60 ± 9.76 nits, and on adult hosts we found 1.21 ± 2.61 lice and 6.68 ± 17.50 nits (Fig. 3c).
Boxplots of Ischnocera and nit abundance across (a) host species, (b) host sex, and (c) host age class in the raw data. Note the logarithmic y-axes; louse and nit abundance values are plotted as (count + 1) to allow a log transformation for zero-counts. Amblycera are not included, because we only found six Menacanthus sp. on a single Hairy Woodpecker specimen
Scatterplots of Ischnocera and nit abundance on each specimen against (a) collection year, (b) the BUFA value at the collection site, (c) precipitation in the driest quarter of the year at the collection site, and (d) collection day of year in the raw data. Note the logarithmic y-axes; louse and nit abundance values are plotted as (count + 1) to allow the log transformation for zero-counts. Amblycera are not included, because we only found six Menacanthus sp. on a single Hairy Woodpecker specimen
GLMM selection and results
For predicting Ischnocera abundance, we found eleven models with ΔAICc < 2, of which all eleven included day of year, seven included year, four included precipitation, one included host sex, seven included host age, one included the interaction of BUFA with host species, and one included the interaction of BUFA with year (SI Table 1). For predicting nit abundance, there were eleven models with ΔAICc < 2, of which seven included day of year, two included year, six included precipitation, one included host sex, none included host age, four included the interaction of BUFA with host species, and none included the interaction of BUFA with year (SI Table 2). We selected the model parametrization that was highest weighted for nits (ΔAICc = 0), which was also second-highest weighted for Ischnocera (ΔAICc = 0.78). In contrast, the highest weighted parametrization for Ischnocera (ΔAICc = 0) was a poor model for nits (ΔAICc = 4.75). The selected model had BUFA, host species, day of year, and precipitation as its predictors, without interaction or random effects (Tables 2 and 3).
In the negative binomial GLMM of Ischnocera abundance (model I43 in SI Table 1), Hairy Woodpeckers had significantly higher louse loads than House Finches (Table 2; Fig. 5a). Urbanness was not a significant predictor (Table 2; Fig. 5b), nor were precipitation (Table 2; Fig. 5c) and linear day of year (Table 2; Fig. 5d), despite being retained in the model. However, day of year squared was a significant positive predictor (Table 2; Fig. 5d), indicating lower Ischnocera abundance in the summer than in the winter.
In the negative binomial GLMM of nit abundance (model N43 in SI Table 2), House Finches had significantly higher loads than Hairy Woodpeckers (Table 3; Fig. 5a), the inverse of the pattern for both adult louse models. Urbanness was not a significant predictor (Table 3; Fig. 5b), nor was precipitation (Table 3; Fig. 5c). However, linear day of year was a significant negative predictor (Table 3; Fig. 5d), whereas day of year squared was non-significant (Table 3; Fig. 5d), unlike the adult lice, indicating more abundant nits early in the year.
GLMM-predicted adult louse and nit abundances with 95% confidence intervals in response to (a) host species, (b) the urbanness metric BUFA, (c) precipitation in the driest quarter of the year, and (c) collection day of year. The prediction method was counterfactual. Ischnocera and nits are displayed together, but their abundances were not compared to each other, because they were modeled separately
Discussion
We found that urbanness did not have a significant effect on avian louse abundances, either demonstrating that unlike other parasites, chewing lice abundance is not affected by urban environments, or that ecological effects of urbanization in the western United States were much more limited a century ago compared with the present day. This held across both House Finches and Hairy Woodpeckers, even though we hypothesized that ectoparasitism on urban dwelling House Finches would be more strongly affected in urban environments, either suppressed by improved host health and increased preening or promoted by denser host populations. This null finding suggests that neither the urban tolerance/avoidance nor the dilution hypotheses can adequately explain avian chewing lice interactions, or that these effects only become large enough to be biologically meaningful in the western United States after the 1930s.
Our lack of evidence that urban environments change louse abundance must be understood in the context of different kinds of parasitic interaction and the source of our specimens. In contrast to our nonsignificant effect of urbanness, Giraudeau et al. (2014) documented higher severity and prevalence of coccidia and poxvirus infections for more urban House Finches. Potentially, lice are less responsive to urban environments than other infections because they are buffered from certain environmental factors, or because the changes that avian hosts undergo in urban environments have limited effects on anti-louse defenses. It should be noted that we only modeled Ischnoceran lice because we found very few Amblycera, and these parvorders may respond differently to the urban environment, as they do with humidity (Bush et al. 2009). Few other studies have examined the effect of urban environments on avian lice of any host species, except for some interactions of introduced species (Delgado-V. & French 2012), so future comparative research between bird species could elucidate if avian louse abundance is always unaffected by cities, or if abundance depends on the host species and the host’s tolerance for urban environments. Alternatively, because our specimens reflect urban and non-urban environments between 1905 and 1932, our findings may represent the state of urban ecosystems in the western United States a century ago and their differences from modern cities. More intensive sampling of specimens across a wider time series, and specifically comparison between historical and modern specimens, could elucidate whether the effects of cities on avian louse abundance have changed since 1905. If modern louse abundance is affected by cities, even though it was unaffected historically, this would be evidence that the intensification of urban environments through time has caused corresponding variation in parasitic interactions.
For House Finches and Hairy Woodpeckers collected in the western United States from 1905 to 1932, House Finches had fewer adult Ischnocera and more nits than Hairy Woodpeckers, precipitation did not have a significant effect, and linear day of year was a negative predictor for nits, while quadratic day of year was a positive predictor for both Amblycera and Ischnocera. It is not entirely clear why host species has opposite effects on adult lice and nits, but one potential factor is that they experience different infection dynamics. Adult lice are strongly controlled by host preening and other behaviors (Booth et al. 1993; Clayton et al. 1999; Clayton and Walther 2001), but nits are usually cemented in hard-to-reach places (Nelson and Murray 1971) and may escape preening pressure. Since one female louse can lay 12–20 nits every 4–10 days (Price et al. 2003), just one breeding pair of adult lice that escapes preening could cause high nit abundance. Therefore, adult louse abundance may represent the long-term infection success, modulated by energy the host allocates to anti-louse behaviors, whereas nit abundance may be a snapshot of a more variable metric that requires more frequent sampling to fully describe. Alternatively, the discrepancy for host species as a predictor may stem from differences in louse species life histories between hosts. For example, Brueelia thorini on House Finches may be more fecund than Brueelia straminea s. lat. on Hairy Woodpeckers. Such a pattern could potentially be due to evolutionary histories, host grooming pressure, or competition between louse species, but little concrete is known about variation among life histories of different louse species.
We additionally found a null ecological effect of precipitation, as a proxy for humidity, across the ranges of our louse species. The effect of humidity on the abundance of a single louse species which is adapted to an arid climate may be small (Carrillo et al. 2007), and the ecological scale of our study should be distinguished from the correlation of humidity with louse abundance and diversity at a larger biogeographic scale (Bush et al. 2024). The positive association of quadratic day of year with adult louse abundances indicates lower louse loads in the summer, but the nits instead showed high abundance early in the year. Foster (1969a) reported a peak in Ricinus nit loads corresponding with the host’s spring breeding season, and our data appear to show a similar pattern for nit abundance. There is wide variation in whether louse communities peak during breeding, during molt, or in response to unrelated seasonal patterns (Galloway and Lamb 2021), but since both host species that we studied have a single annual molt in late summer to fall (Pyle 1997), the decline in adult louse abundance in the middle of the year may be related to shedding and replacing feathers. It seems likely that the louse life stages were temporally decoupled in our study because adult lice and nits responded most strongly to different effects in their hosts’ life histories.
Notably, our study represents the first record of Philopterus sp. lice on House Finches (Table 1), with documented occurrences in our data originating from the U.S. states of Washington, California, and Nevada. We found Philopterus sp. so frequently on House Finches that they were almost certainly infesting those hosts in life, and Philopterus species have known associations with several other Fringillidae (Price et al. 2003). Brueelia thorini was already known from House Finches in Utah, California, and Mexico (Table 1). We did not find Myrsidea conspicua, Menacanthus alaudae, or Ricinus microcephalus on any specimens, despite previous reports from Hawaii for M. conspicua, Hawaii and Arizona for M. alaudae, and Hawaii and California for R. microcephalus (Table 1). The absence of these species may be partially because they are Amblycera, which are more mobile than Ischnocera (Price et al. 2003) and more likely to abandon a dead host (Mey 2003). For Hairy Woodpeckers, we found Menacanthus sp. on a single specimen from California, and this genus is widely reported, including on Hairy Woodpeckers in Manitoba (Table 1). Brueelia straminea s. lat., P. auritus s. lat., P. jungens, and Picicola snodgrassi have been reported across the U.S. (Table 1).
A complication for our findings is that we studied museum specimens collected in the early 1900s. We lack documentation on the exact preparation and handling methods used on our specimens over decades. However, chewing lice are often preserved with museum skins (Mey 2003), and the adult lice we found were still in identifiable condition. With the exception of Philopterus sp., we only found louse species that have documented associations with our host birds, so cross-contamination during specimen collection is of minimal concern. Mey (2003) notes that chewing lice will only leave a corpse if they encounter a new host, and Ischnocera are particularly reluctant to leave. Even assuming that a proportion of lice leave after death, high loads on specimens still must correlate with high loads in life. Moreover, since nits are not dislodged even after death, they are reliably correlated with nit abundance in life (Foster 1969b). Some modern collectors wash bird skins as they are prepared (Winker 2000; Szabo et al. 2013), which can remove ectoparasites (Szabo et al. 2014), but this is a modern practice that postdates most of our specimens (Mey 2003; e.g. Chapin 1923 does not mention washing), because it relies on modern dish detergents. We also note that nits are likely resistant to washing because they are cemented to the feathers, so they may be a reliable abundance metric even for washed specimens. These factors give us confidence that our ectoparasite counts are closely correlated with the true abundance of at time of collection. We urge future specimen preparators to keep records of preparation techniques and specifically to avoid washing skins when specimen condition and cleanliness permit.
Museum collections are a vital tool for understanding how urban ecology has changed through time. Because these museum specimens allowed us to study urban conditions from 1905 to 1932, we now have historical data that can track earlier phases of urbanization. The best interpretation of our findings is either that avian louse abundance on House Finches and Hairy Woodpeckers is not affected by urbanness, or that from 1905 to 1932 the degree of urbanization in the western United States was not yet severe enough to affect this louse abundance. By comparing louse abundance in present-day urban and rural habitats to our historical findings, future studies could provide insight into how urbanization and its effects on birds and their parasites have changed in the past century.
Data availability
Raw data used in this study are available on Dryad at https://doi.org/10.5061/dryad.bcc2fqzr0.
References
Anderson DR, Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. J Wildl Manag 66(3):912–918. https://doi.org/10.2307/3803155
Arnold CL Jr, Gibbons CJ (1996) Impervious surface coverage: the emergence of a key environmental indicator. J Am Plan Assoc 62(2):243–258. https://doi.org/10.1080/01944369608975688
Arzua M, Navarro da Silva MA, Famadas KM, Beati L, Barros-Battesti DM (2003) Amblyomma aureolatum and Ixodes auritulus (Acari: Ixodidae) on birds in Southern Brazil, with notes on their ecology. Exp Appl Acarol 31(3):283–296. https://doi.org/10.1023/B:APPA.0000010381.24903.1c
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23(5):960–969. https://doi.org/10.1093/beheco/ars059
Bailly J, Scheifler R, Belvalette M, Garnier S, Boissier E, Clément-Demange V-A, Gète M, Leblond M, Pasteur B, Piget Q, Sage M, Faivre B (2016) Negative impact of urban habitat on immunity in the great tit Parus major. Oecologia 182(4):1053–1062. https://doi.org/10.1007/s00442-016-3730-2
Baz A, Murphy MT (2023) Woodpeckers in the City: abundances are highest in large green spaces with complex understories. Ornithol Appl 125(3):duad013. https://doi.org/10.1093/ornithapp/duad013
Beldomenico PM, Begon M (2010) Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol Evol 25(1):21–27. https://doi.org/10.1016/j.tree.2009.06.015
Bentz S, Rigaud T, Barroca M, Martin-Laurent F, Bru D, Moreau J, Faivre B (2006) Sensitive measure of prevalence and parasitaemia of haemosporidia from European Blackbird (Turdus merula) populations: value of PCR-RFLP and quantitative PCR. Parasitol 133(6):685–692. https://doi.org/10.1017/S0031182006001090
Bergtold WH (1913) A study of the house Finch. Auk 30(1):40–73. https://doi.org/10.2307/4071894
Biamonte E, Sandoval L, Chacón E, Barrantes G (2011) Effect of urbanization on the avifauna in a tropical metropolitan area. Landsc Ecol 26(2):183–194. https://doi.org/10.1007/s10980-010-9564-0
Bichet C, Brischoux F, Ribout C, Parenteau C, Meillère A, Angelier F (2020) Physiological and morphological correlates of blood parasite infection in urban and non-urban house sparrow populations. PLoS ONE 15(8):e0237170. https://doi.org/10.1371/journal.pone.0237170
Bonier F (2012) Hormones in the City: endocrine ecology of urban birds. Horm Behav 61(5):763–772. https://doi.org/10.1016/j.yhbeh.2012.03.016
Booth DT, Clayton DH, Block BA (1993) Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc Royal Soc B 253(1337):125–129. https://doi.org/10.1098/rspb.1993.0091
Bradley CA, Altizer S (2007) Urbanization and the ecology of wildlife diseases. Trends Ecol Evol 22(2):95–102. https://doi.org/10.1016/j.tree.2006.11.001
Bretagnolle A, Delisle F, Mathian H, Vatin G (2015) Urbanization of the united States over two centuries: an approach based on a long-term database (1790–2010). Int J Geogr Inf Sci 29(5):850–867. https://doi.org/10.1080/13658816.2014.999681
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9(2):378–400. https://doi.org/10.32614/RJ-2017-066
Brown NS (1974) The effect of louse infestation, wet feathers, and relative humidity on the grooming behavior of the domestic chicken. Poult Sci 53(5):1717–1719. https://doi.org/10.3382/ps.0531717
Bush SE, Harbison CW, Slager DL, Peterson AT, Price RD, Clayton DH (2009) Geographic variation in the community structure of lice on Western Scrub-jays. J Parasitol 95(1):10–13
Bush SE, Waller MM, Davis KM, Clayton SF, Clayton DH (2024) Birds in arid regions have depauperate louse communities: climate change implications? Ecol Evol 14(9):e70280. https://doi.org/10.1002/ece3.70280
Calegaro-Marques C, Amato SB (2014) Urbanization breaks up host-parasite interactions: A case study on parasite community ecology of Rufous-bellied thrushes (Turdus rufiventris) along a rural-urban gradient. PLoS ONE 9(7):e103144. https://doi.org/10.1371/journal.pone.0103144
Carey RO, Hochmuth GJ, Martinez CJ, Boyer TH, Dukes MD, Toor GS, Cisar JL (2013) Evaluating nutrient impacts in urban watersheds: challenges and research opportunities. Environ Pollut 173:138–149. https://doi.org/10.1016/j.envpol.2012.10.004
Carrillo CM, Valera F, Barbosa A, Moreno E (2007) Thriving in an arid environment: High prevalence of avian lice in low humidity conditions. Ecoscience 14(2):241–249. https://doi.org/10.2980/1195-6860(2007)14[241:TIAAEH]2.0.CO;2
Chapin JP (1923) The Preparation of birds for study: instructions for the proper Preparation of bird skins and skeletons for study and future mounting. American Museum of Natural History, New York
Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, Ortega CN, Sauer EL, Sehgal T, Young S, Rohr JR (2015) Biodiversity inhibits parasites: broad evidence for the Dilution effect. PNAS 112(28):8667–8671. https://doi.org/10.1073/pnas.1506279112
Clay T (1965) Contributions towards a revision of Myrsidea Waterston (Mallophaga: Menoponidae). II. Proc Royal Entomol Soc B Taxon 34(9–10):117–122. https://doi.org/10.1111/j.1365-3113.1965.tb01704.x
Clayton DH (1990) Mate choice in experimentally parasitized rock doves: lousy males lose. Am Zool 30(2):251–262. https://doi.org/10.1093/icb/30.2.251
Clayton DH (1991) The influence of parasites on host sexual selection. Parasitol Today 7(12):329–334. https://doi.org/10.1016/0169-4758(91)90211-6
Clayton DH, Walther BA (1997) Appendix C: collection and quantification of arthropod parasites of birds. Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 419–440
Clayton DH, Walther BA (2001) Influence of host ecology and morphology on the diversity of Neotropical bird lice. Oikos 94(3):455–467. https://doi.org/10.1034/j.1600-0706.2001.940308.x
Clayton DH, Lee PLM, Tompkins DM, Brodie EDI (1999) Reciprocal natural selection on host-parasite phenotypes. Am Nat 154(3):261–270. https://doi.org/10.1086/303237
Clayton DH, Adams RJ, Bush SE (2008) Phthiraptera, the chewing lice. Parasitic diseases of wild birds. Wiley-Blackwell, pp 515–526
R Core Team (2023) R: A language and environment for statistical computing v.4.3.1. R Foundation for Statistical Computing. https://www.R-project.org/
Dalgleish RC (1969) The Picicola (Mallophaga: Ischnocera) of the Picidae (Aves: Piciformes). Proc Royal Entomol Soc B Taxon 38(7–8):101–113. https://doi.org/10.1111/j.1365-3113.1969.tb00239.x
Dalgleish RC (1971) The Brueelia (Mallophaga: Ischnocera) of the Picidae (Aves: Piciformes). J N Y Entomol Soc 79(3):139–146
Dalgleish RC (1972) The Penenirmus (Mallophaga: Ischnocera) of the Picidae (Aves: Piciformes). J N Y Entomol Soc 80(2):83–104
Denny H (1842) Monographia anoplurorum britanniae or an essay on the British species of parasitic insects belonging to the order of anoplura of Leach, with the modern divisions of the general according to the views of Leach, Nitzsch, and burmeister, with highly magnified figures of each species. H. G. Bohn, London
DeVore JL, Shine R, Ducatez S (2020) Urbanization and translocation disrupt the relationship between host density and parasite abundance. J Anim Ecol 89(4):1122–1133. https://doi.org/10.1111/1365-2656.13175
Dik B (2006) Erosive stomatitis in a white pelican (Pelecanus onocrotalus) caused by Piagetiella Titan (Mallophaga: Menoponidae). J Vet Med B 53(3):153–154. https://doi.org/10.1111/j.1439-0450.2006.00927.x
Eichler W (1963) Arthropoda. III. Insecta. 7b. Phthiraptera I. Mallophaga. Akademische Verlagsgesellschaft Geest & Portig K.G., Leipzig
El-Sabaawi R (2018) Trophic structure in a rapidly urbanizing planet. Funct Ecol 32(7):1718–1728. https://doi.org/10.1111/1365-2435.13114
Emerson KC (1972) Checklist of the mallophaga of North America (north of Mexico). Part IV bird host list. Deseret Test Center, Dugway
Emerson KC, Johnson JC (1961) The genus Penenirmus (Mallophaga) found on North American woodpeckers. J Kans Entomol Soc 34(1):34–43
Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ (2009) Effects of urbanisation on disease prevalence and age structure in Blackbird Turdus Merula populations. Oikos 118(5):774–782. https://doi.org/10.1111/j.1600-0706.2008.17226.x
Fabiyi JP (1996) Association between duration of humid season and geographical distribution patterns of different species of chewing lice (Mallophaga: Insecta) infesting domestic chickens in Nigeria. J Parasitol 82(6):1034–1036. https://doi.org/10.2307/3284220
Faeth SH, Warren PS, Shochat E, Marussich WA (2005) Trophic dynamics in urban communities. Bioscience 55(5):399–407. https://doi.org/10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km Spatial resolution climate surfaces for global land areas. Int J Climatol 37(12):4302–4315. https://doi.org/10.1002/joc.5086
Fink D, Auer A, Strimas-Mackey M, Ligocki S, Robinson O, Hochachka W, Jaromczyk L, Crowley C, Dunham K, Stillman A, Davies I, Rodewald A, Ruiz-Gutierrez V, Wood C (2023) eBird status and trends, data version: 2022; released: 2023. Cornell Lab Ornithol. https://doi.org/10.2173/ebirdst.2022
Fischer JD, Schneider SC, Ahlers AA, Miller JR (2015) Categorizing wildlife responses to urbanization and conservation implications of terminology. Conserv Biol 29(4):1246–1248. https://doi.org/10.1111/cobi.12451
Fletcher TD, Andrieu H, Hamel P (2013) Understanding, management and modelling of urban hydrology and its consequences for receiving waters: A state of the Art. Adv Water Resour 51:261–279. https://doi.org/10.1016/j.advwatres.2012.09.001
Fortuniak K, Kłysik K, Wibig J (2006) Urban–rural contrasts of meteorological parameters in Łódź. Theor Appl Climatol 84(1):91–101. https://doi.org/10.1007/s00704-005-0147-y
Foster MS (1969a) Synchronized life cycles in the Orange-crowned warbler and its mallophagan parasites. Ecology 50(2):315–323. https://doi.org/10.2307/1934858
Foster MS (1969b) The eggs of three species of mallophaga and their significance in ecological studies. J Parasitol 55(2):453–456. https://doi.org/10.2307/3277435
French D-VCA K (2012) Parasite–bird interactions in urban areas: current evidence and emerging questions. Landsc Urban Plan 105(1):5–14. https://doi.org/10.1016/j.landurbplan.2011.12.019
Galloway TD, Lamb RJ (2016) Chewing lice (Phthiraptera: amblycera and Ischnocera) infesting woodpeckers and sapsuckers (Aves: piciformes: Picidae) in Manitoba, Canada. Can Entomol 148(5):520–531. https://doi.org/10.4039/tce.2015.89
Galloway TD, Lamb RJ (2021) Population dynamics of chewing lice (Phthiraptera) infesting birds (Aves). Annu Rev Entomol 66(Volume 66, 2021):209–224. https://doi.org/10.1146/annurev-ento-041420-075608
Gibson C, Jung K (2002) Historical census statistics on population totals by race, 1790 to 1990, and by Hispanic origin. 1970 To 1990, for the united States, regions, divisions, and States. U.S. Bureau of the Census, Population Division, Washington, DC
Giraudeau M, Mousel M, Earl S, McGraw K (2014) Parasites in the City: degree of urbanization predicts poxvirus and Coccidian infections in house finches (Haemorhous mexicanus). PLoS ONE 9(2):e86747. https://doi.org/10.1371/journal.pone.0086747
Giraudeau M, Toomey MB, Hutton P, McGraw KJ (2018) Expression of and choice for condition-dependent carotenoid-based color in an urbanizing context. Behav Ecol 29(6):1307–1315. https://doi.org/10.1093/beheco/ary093
Green DM, Baker MG (2003) Urbanization impacts on habitat and bird communities in a Sonoran desert ecosystem. Landsc Urban Plan 63(4):225–239. https://doi.org/10.1016/S0169-2046(02)00195-0
Gregoire A, Faivre B, Heeb P, Cezilly F (2002) A comparison of infestation patterns by Ixodes ticks in urban and rural populations of the common Blackbird Turdus Merula. Ibis 144(4):640–645. https://doi.org/10.1046/j.1474-919X.2002.00102.x
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319(5864):756–760. https://doi.org/10.1126/science.1150195
Gustafsson DR, Bush SE (2019) Brueelia (Phthiraptera: ischnocera: Philopteridae) of North American nine-primaried Oscines (Aves: passeriformes: Passerida) with descriptions of nine new species. J Parasitol 105(6):858–873. https://doi.org/10.1645/19-82
Gustafsson DR, Adam C, Zou F (2022) One new genus and three new species of the Penenirmus-complex (Phthiraptera: Ischnocera) from China, with resurrection of Picophilopterus Ansari, 1947. Zootaxa 5087(3):401–426. https://doi.org/10.11646/zootaxa.5087.3.1
Hamstra TL, Badyaev AV (2009) Comprehensive investigation of ectoparasite community and abundance across life history stages of avian host. J Zool 278(2):91–99. https://doi.org/10.1111/j.1469-7998.2008.00547.x
Hasegawa M, Ligon RA, Giraudeau M, Watanabe M, McGraw KJ (2014) Urban and colorful male house finches are less aggressive. Behav Ecol 25(3):641–649. https://doi.org/10.1093/beheco/aru034
Hijmans RJ (2024) terra: Spatial data analysis. R package v.1.7–55. https://CRAN.R-project.org/package=terra
Hutton P, McKenna J, McGraw KJ (2021) Urban links to molt schedule, body condition and carotenoid-based coloration in the house Finch Haemorhous Mexicanus. J Avian Biol 52(5). https://doi.org/10.1111/jav.02761
Hutton P, Lendvai ÁZ, Németh J, McGraw KJ (2024) Urban house finches are more resistant to the effects of artificial light at night. Sci Total Environ 946:174525. https://doi.org/10.1016/j.scitotenv.2024.174525
Joseph L (2011) Museum collections in ornithology: today’s record of avian biodiversity for tomorrow’s world. Emu 111(3):i–xii. https://doi.org/10.1071/MUv111n3_ED
Kellogg VL (1896) New Mallophaga, II, from land birds, together with an account of the mallophagan mouth-parts. Leland Stanford Junior University, Palo Alto
Kellogg VL, Chapman BL (1902) Mallophaga from birds of the Hawaiian Islands. J N Y Entomol Soc 10(3):155–169
Kolenčík S, Loaiza-Muñoz MA, Londono GA, Allen JM, Plskova K, Sychra O (2024) Understanding the life history characteristics of bird lice: amblycera versus ischnocera. Ecol Entomol. https://doi.org/10.1111/een.13416
Lancaster RK, Rees WE (1979) Bird communities and the structure of urban habitats. Can J Zool 57(12):2358–2368. https://doi.org/10.1139/z79-307
Leyk S, Uhl JH (2018a) HISDAC-US, historical settlement data compilation for the conterminous united States over 200 years. Sci Data 5:180175. https://doi.org/10.1038/sdata.2018.175
Leyk S, Uhl JH (2018b) Historical built-up intensity layer series for the U.S. 1810–2015, V. 2. Harv Dataverse. https://doi.org/10.7910/DVN/1WB9E4
Leyk S, Uhl JH, Connor DS, Braswell AE, Mietkiewicz N, Balch JK, Gutmann M (2020) Two centuries of settlement and urban development in the united States. Sci Adv 6(23):eaba2937. https://doi.org/10.1126/sciadv.aba2937
Linnaeus C (1766) a Systema naturæ: Per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, 12th, reformata. edn. Laurentius Salvius, Stockholm
Lüdecke D (2018) Ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw 3(26):772. https://doi.org/10.21105/joss.00772
Malcomson RO (1960) Mallophaga from birds of North America. The Wilson Bulletin 72(2):182-197Mazerolle MJ (2023) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package v.2.3.3. https://cran.r-project.org/package=AICcmodavg
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127(3):247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Meillère A, Brischoux F, Parenteau C, Angelier F (2015) Influence of urbanization on body size, condition, and physiology in an urban exploiter: A multi-component approach. PLoS ONE 10(8):e0135685. https://doi.org/10.1371/journal.pone.0135685
Mey E (2003) Bird collections -- an essential resource for collecting ectoparasites, in particular chewing lice. Bonn Zool Beitr 51(2/3):131–135
Minias P (2015) Successful colonization of a novel urban environment is associated with an urban behavioural syndrome in a reed-nesting waterbird. Ethol 121(12):1178–1190. https://doi.org/10.1111/eth.12433
Moyer BR, Drown DM, Clayton DH (2002) Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos 97(2):223–228. https://doi.org/10.1034/j.1600-0706.2002.970208.x
Nelson BC, Murray MD (1971) The distribution of mallophaga on the domestic pigeon (Columba livia). Int J Parasitol 1(1):21–29. https://doi.org/10.1016/0020-7519(71)90042-7
Ossola A, Cadenasso ML, Meineke EK (2021) Valuing the role of time in urban ecology. Front Ecol Evol 9:620620. https://doi.org/10.3389/fevo.2021.620620
Price RD (1977) The Menacanthus (Mallophaga: Menoponidae) of the passeriformes (Aves). J Med Entomol 14(2):207–220. https://doi.org/10.1093/jmedent/14.2.207
Price RD, Emerson KC (1975) The Menacanthus (Mallophaga: Menoponidae) of the piciformes (Aves). Ann Entomol Soc Am 68(5):779–785. https://doi.org/10.1093/aesa/68.5.779
Price RD, Hellenthal RA, Palma RL, Johnson KP, Clayton DH (2003) The chewing lice: world checklist and biological overview. Illinois Natural History Survey, Champaign
Pyle P (1997) Identification guide to North American birds. Part I: Columbidae to Ploceidae. Slate Creek, Bolinas
QGIS Development Team (2024) QGIS geographic information system v.3.36.1. Open Source Geospatial Foundation. http://qgis.osgeo.org/
Redpath S (1988) Vigilance levels in preening Dunlin Calidris alpina. Ibis 130(6):555–557. https://doi.org/10.1111/j.1474-919X.1988.tb02723.x
Ripper D, Bednarz JC, Varland DE (2007) Landscape use by hairy woodpeckers in managed forests of Northwestern Washington. J Wildl Manag 71(8):2612–2623. https://doi.org/10.2193/2005-487
Rudolph D (1983) The water-vapour uptake system of the phthiraptera. J Insect Physiol 29(1):15–25. https://doi.org/10.1016/0022-1910(83)90101-4
Samuel WM, Williams ES, Rippin AB (1982) Infestations of Piagetiella Peralis (Mallophaga: Menoponidae) on juvenile white pelicans. Can J Zool 60(5):951–953. https://doi.org/10.1139/z82-130
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: A review. Conserv Biol 5(1):18–32. https://doi.org/10.1111/j.1523-1739.1991.tb00384.x
Scopoli IA (1763) Entomologia carniolica exhibens insecta carnioliae indigena et distributa in ordines, genera, species, varietates. Methodo Linnæana. Ioannes Thomas Trattner, Vienna
Shochat E (2004) Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106(3):622–626. https://doi.org/10.1111/j.0030-1299.2004.13159.x
Sol D, Santos DM, Garcia J, Cuadrado M (1998) Competition for food in urban pigeons: the cost of being juvenile. Condor 100(2):298–304. https://doi.org/10.2307/1370270
Sorace A (2002) High density of bird and pest species in urban habitats and the role of predator abundance. Ornis Fenn 79(2):60–71
Spiess A-N (2018) QpcR: modelling and analysis of real-time PCR data. R package v.1.4-1. https://CRAN.R-project.org/package=qpcR
Statius Müller PL (1776) Supplements- und Register-Band Über alle sechs theile Oder classen des Thierreichs. Mit einer ausführlichen erklärung ausgefertiget. Gabriel Nicolaus Raspe, Nuremberg
Sykes BE, Hutton P, McGraw KJ (2021) Sex-specific relationships between urbanization, parasitism, and plumage coloration in house finches. Curr Zool 67(3):237–244. https://doi.org/10.1093/cz/zoaa060
Szabo I, Bostwick K, Milensk CM, Proctor HC, Robbins MB, Scholes E III, Trail PW, Wieczorek J, Tan DK (2013) Bird specimens 7: How to wash and dry bird skins. UBC Beaty Biodiversity Museum YouTube Channel. https://youtu.be/5WwLVieJzr4?si=PBs1bbvSQrbEqKoP. Accessed 13 December 2024
Szabo I, Bostwick K, Milensk CM, Proctor HC, Robbins MB, Scholes E III, Trail PW, Wieczorek J, Tan DK (2014) Bird specimens 11: Washing birds for arthropod ectosymbionts UBC Beaty Biodiversity Museum YouTube Channel. https://youtu.be/iJZushKix2g?si=AcKQ78lmVq2ojPyU. Accessed 13 December 2024
Tomasevic JA, Marzluff JM (2017) Cavity nesting birds along an urban-wildland gradient: is human facilitation structuring the bird community? Urban Ecosyst 20(2):435–448. https://doi.org/10.1007/s11252-016-0605-6
Tryjanowski P, Sparks TH, Kuźniak S, Czechowski P, Jerzak L (2013) Bird migration advances more strongly in urban environments. PLoS ONE 8(5):e63482. https://doi.org/10.1371/journal.pone.0063482
Uhl JH, Leyk S, McShane CM, Braswell AE, Connor DS, Balk D (2021) Fine-grained, Spatiotemporal datasets measuring 200 years of land development in the united States. Earth Syst Sci Data 13(1):119–153. https://doi.org/10.5194/essd-13-119-2021
von Schrank F P (1776) Beiträge Zur naturgeschichte. Caspar Fritsch, Leipzig
Werner CS, Nunn CL (2020) Effect of urban habitat use on parasitism in mammals: A meta-analysis. Proc Royal Soc B 287(1927):20200397. https://doi.org/10.1098/rspb.2020.0397
Winker K (2000) Obtaining, preserving, and Preparing bird specimens. J Field Ornithol 71(2):250–297. https://doi.org/10.1648/0273-8570-71.2.250
Winker K (2004) Natural history museums in a postbiodiversity era. Bioscience 54(5):455–459. https://doi.org/10.1641/0006-3568(2004)054[0455:NHMIAP]2.0.CO;2
Wright AN, Gompper ME (2005) Altered parasite assemblages in raccoons in response to manipulated resource availability. Oecologia 144(1):148–156. https://doi.org/10.1007/s00442-005-0018-3
Acknowledgements
Jessie Salter provided invaluable support and expertise in developing our louse collection methods. Jonathan Marcot provided access to specimens, working space, and resources, which made this study possible. Daniel Gustafsson assisted with identification for Brueelia thorini, and Stanislav Kolenčík and Kevin Johnson assisted with identification for Philopterus sp.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no financial or proprietary interests in any material discussed in this article. This material uses data from the eBird Status and Trends Project at the Cornell Lab of Ornithology, eBird.org. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Cornell Lab of Ornithology.
Author information
Authors and Affiliations
Contributions
E.X.K., J.M.J, A.L., M.C.R, and G.A.M contributed to the initial study design and conception, and E.X.K, G.A.M., and M.W.T. refined the study questions. E.X.K., J.M.J, A.L., M.C.R, and G.A.M designed and performed louse data collection. E.X.K. performed urbanness methods. E.X.K. and M.W.T. performed the analysis. E.X.K. wrote the first draft of the manuscript. All authors contributed to and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kahn, E.X., Jacobsen, J.M., Leyel, A. et al. The response of avian chewing lice (Psocodea: Phthiraptera) loads to early-1900s urbanization in the Western United States. Urban Ecosyst 28, 113 (2025). https://doi.org/10.1007/s11252-025-01724-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-025-01724-4