Abstract
Purpose
Both obstructive (OSA) and central sleep apnea (CSA) are associated with considerable cardiovascular morbidity which argues for treatment initiation with a positive airway pressure (PAP) device even in the absence of significant day-time sleepiness. While the long-term consequences of PAP treatment in patients with sleep disordered breathing have been investigated in several studies, less is known about the immediate hemodynamic effects. Therefore, the present study intended to investigate the immediate effect of PAP treatment on non-invasively measured hemodynamic parameters in 10 patients with either OSAS or CSA.
Methods
During diagnostic and therapeutic conditions, the routine polysomnographic assessment was extended with an impedance cardiography (ICG) system. Statistical analysis was performed to find differences between both groups and conditions. In addition, the relationship between the treatment associated effect on stroke volume (SV) with biometric, polysomnographic, and cardiovascular parameters was assessed.
Results
Comparing both subgroups, we found statistically significant differences for biometric, polysomnographic, and cardiovascular parameters. Patients with CSA were older (p = 0.0005) and had higher values for diagnostic (p = 0.015) and therapeutic (p = 0.029) pulse pressure and the pre-ejection period under diagnostic conditions (p = 0.031). In contrast to patients with CSA who exhibited a slight increase of SV and derived parameters under therapeutic conditions, a pronounced decrease was observed in patients with OSAS which was statistically significant for the cardiac index (p = 0.038).
Conclusion
Our results indicate that patients with OSAS and CSA who are characterized by unique clinical features may show a distinguishable hemodynamic response to PAP treatment that can be measured non-invasively with ICG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Background
Sleep apnea can be due to obstructive or central respiratory events which are classified as two distinct entities and are treated with different positive airway pressure (PAP) devices. Obstructive sleep apnea (OSA) is commonly explained by a collapse of the upper airway during inspiration which is due to decreased pharyngeal muscle tone and a comparably elevated extraluminal pressure during sleep [1]. Breathing excursions against a closed airway lead to negative intrapleural pressure swings, oxygen desaturations and sympathetic activation causing elevated blood pressure levels and a disturbed sleep quality [2,3,4]. Although there is currently a wide range of available treatment options including surgical approaches, oral appliances or tongue pacemakers [5, 6], PAP treatment with a continuous or adaptive pressure device remains the therapeutic mainstay in OSAS.
Central sleep apnea (CSA) can present in different clinical contexts including chronic heart failure, opioid intake, neurological disorders or as an adaptive state during low oxygen supply. Chronic heart failure is associated with pulmonary congestion and an increased sympathetic tone which predisposes to nocturnal hyperventilation [7]. Low cardiac output and circulatory delay result in a disturbed feedback circuit which leads to hypocapnia and a decreased apnoeic threshold, eventually causing a waxing and waning breathing pattern, called Cheynes-Stokes respiration. Besides treating the underlying condition, CSA is commonly addressed with adaptive servoventilation (ASV) which anticipates respiratory events by predicting the future tidal volume and proving ventilatory support if needed. The device can be set to auto-titration mode or work on two modifiable exspiratory and inspiratory pressure levels. Despite being highly efficient in preventing central respiratory events, ASV is contraindicated in patients with advanced heart failure being associated with an increased mortality among these patients [8].
Although OSA and CSA show a distinguishable pathogenesis, both are related to an increased risk for the development of cardiovascular pathologies like endothelial dysfunction, secondary hypertension, atrial fibrillation or stroke [9,10,11,12]. Comorbidities resulting from sleep disordered breathing are explained multifactorially involving intrathoracic pressure changes, apnea induced arousals and disturbances of the normal sleep architecture. Treatment with a positive airway pressure (PAP) device can reverse apnea related pathologies, but may have a potential for hemodynamic impairment which would remain undetected during routine sleep medicine practice. To investigate the immediate cardiovascular effects of PAP treatment in patients with CSA and OSAS, we extended our routine polysomnographic measurements with non-invasive hemodynamic monitoring using impedance cardiography (ICG). ICG strongly correlated with the reference method of thermodilution [13, 14] and had a high re-test reliability [15]. Unlike other techniques like echocardiography or thermodilution, ICG provides a continuous measurement without requiring catheterization. The present study intends to investigate the immediate hemodynamic effects of PAP treatment and tries to find differences in the therapeutic response between patients with CSA and OSAS.
Methods
Participants
To investigate the hemodynamic response of PAP treatment, we included 10 patients with CSA related to chronic heart failure (7 patients with preserved and 3 patients with mid-range reduced ejection fraction) and compared them to respiratory distress index (RDI) matched patients with OSAS. Inclusion criteria were a pathological home sleep study with either predominantly central or obstructive events and the indication for diagnostic polysomnography. Patients were excluded if they met any of the following criteria: age younger than 18 years, height below 150 cm or above 210 cm, weight less than 50 kg or greater than 150 kg. All included patients underwent both diagnostic and therapeutic measurements.
Protocol
Polysomnographic assessment was performed during two consecutive nights between 10 pm and 6 am. If diagnostic polysomnography revealed a pathologically elevated RDI, treatment was initiated with a PAP device. Patients with OSAS were treated with automated positive airway pressure (APAP) (AirSense 10 AutoSet™, ResMed, San Diego, CA, USA), while patients with CSA received treatment with ASV (AirCurve 10 CS Pacewave™, ResMed, San Diego, CA, USA). Both devices were set to auto-titration mode and data were gathered during both nights at a sampling rate of 1 Hz independent of the applied pressure level. Data sets of the impedance cardiograph were assessed and corrected for outliers that may have been caused by artifacts. Informed written consent was given by all participants. The study received ethical approval by the ethics committee of Marburg University and is listed in the German clinical trial register (DRKS).
Data collection
During two nights under both diagnostic and therapeutic conditions, the SOMNOscreen™ polysomnography system (SOMNOmedics GmbH, Randersacker, Germany) and the CardioScreen® 1000 impedance cardiograph (Medis, Illmenau, Germany) were applied. Airflow measurement was performed with a thermistor and a nasal pressure transducer during the diagnostic night and with the pneumotachograph of the PAP device under treatment conditions. A respiratory induction plethysmograph was used to monitor thoracoabdominal breathing excursions. Continuous monitoring of the systemic blood pressure was performed using the pulse transit technique [16]. Polysomnographic events were scored according to the American Association of Sleep Medicine guidelines [17]. An obstructive apnea is defined as a decrease of the thermal flow signal by ≥ 90% for at least 10 s accompanied by a continued or increased inspiratory effort. An obstructive hypopnea is scored if a decrease of the flow signal by ≥ 30% for at least 10 s which occurs with a drop of the oxygen saturation by at ≥ 4%. Central apneas are distinguished from obstructive events by an absence of thoracoabdominal breathing excursions.
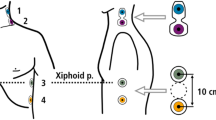
Hemodynamic monitoring was performed with the impedance cardiograph. Two electrodes connected to two dual sensors were placed on the patients´ lateral side of the neck and along the mid-axillary line of the left chest wall (Fig. 1). The outer sensors create a low amplitude alternating current, which is detected by the inner sensors to measure the change in thoracic impedance over time. To enhance the precision of the measurement of cardiac cycle intervals, an additional pulsoximetry sensor was attached to one ear lobe for recording of pulse volume curves by infrared light. Based on the blood flow dependent changes of the thoracic impedance and electrocardiographic time intervals (Fig. 2), the software “Cardiovascular Lab” calculates different hemodynamic parameters including SV, stroke volume index (SVI), cardiac output (CO), cardiac index (CI), pre-ejection period (PEP), left ventricular ejection time (LVET), ejection fraction (EF), and systolic time ratio (STR).
Time course of the electro- and impedance cardiogram with its characteristic turning points and the derived systolic time intervals. Abbreviations: B, opening of the aortic valve; C (dZ/dtmax), maximal systolic blood flow velocity; dZ/dt, maximal change in impedance; ECG, electrocardiogram; ICG, impedance cardiography; LVET, left-ventricular ejection time; O, closure of the mitral valve; PEP, pre-ejection period; X, closure of the aortic valve; Y, closure of the pulmonic valve, Z0, baseline impedance
Statistical analysis
The acquired data were gathered in EXCEL and analysed using the software XLSTAT© (Addinsoft Lumivero, New York, USA). To search for differences between diagnostic and therapeutic conditions, either a paired t-test or a Wilcoxon-rank test were conducted, after testing for normal distribution with the Shapiro-Wilk test. Mean values of both subgroups were compared with the unpaired t-test or the Mann-Whitney U test. The association between the treatment related change in SV and biometric, polysomnographic, and hemodynamic data was investigated by calculating the Pearson´s correlation coefficient or the Spearman´s rank correlation coefficient. Statistical significance was assumed for a p-value ≤ 0.05.
Results
Biometric data
On average participants in both groups were predominantly male and overweight (Table 1). In both groups a pathologically elevated body mass index (BMI) was observed with OSAS patients having a non-statistically significant higher BMI of 34.26 ± kg*m− 2 compared to patients with CSA (32.27 ± kg*m− 2, p = 0.547). The subgroups differed regarding their mean age with CSA patients (74.95 ± 7.56 years) being statistically significant older than patients with OSAS (56.89 ± 11.01 years, p = 0.0005) as shown in Fig. 3.
Polysomnographic parameters
Treatment with a PAP device proved to be efficient in reducing the respiratory distress index (RDI), the occurrence of obstructive apnea (OA) and hypopnea and in lowering the oxygen desaturation index (ODI) for both subgroups (Table 2). Central respiratory events were significantly reduced in patients with CSA (ΔCA -73.94 ± 62.74 h− 1, p ≤ 0.001). While a statistically significant reduction of the arousal-index was only observed in patients with CSA (ΔArousal-Index − 30.25 ± 99.28%, p ≤ 0.05), an effect on the number of counted sleep cycles under treatment conditions did only occur in patients with OSAS (ΔSC 125.00 ± 100.00%, p ≤ 0.001). Comparing both groups, statistically significant differences under diagnostic conditions were observed for the occurrence of central respiratory events (1.88 ± 2.07 h− 1 vs. 18.43 ± 18,79 h− 1, p = 0.013), hypopnea (27.35 ± 11.37 h− 1, 14.84 ± 14.31 h− 1, p = 0.044) and RERA (0.37 ± 0.39 h− 1 vs. 0.06 ± 0.19 h− 1, p = 0.010). Under therapeutic conditions, a statistically significant difference between both groups was only observed for OA (1.19 ± 1.39 vs. 0.15 ± 0.25, p = 0.010). Treatment with either APAP or ASV led to a statistically significant change in the occurrence of central respiratory events (224.39 ± 395.87% vs. -73.94 ± 62.74%, p = 0.0003).
Hemodynamic parameters
An overview of the hemodynamic data for both subgroups is given in Table 3. Overall, a trend towards a decrease of SV and derived parameters which was statistically significant for CI (ΔCI -6.59 ± 7.32%, p = 0.020) was observed in patients with OSAS. In contrast, no significant changes for SV and its related indices were seen in patients with CSA. For both groups, a trendwise significant increase of the PEP under therapeutic conditions and a statistically significant difference under diagnostic conditions was observed (105.67 ± 10.41 ms vs. 122.04 ± 19.50 ms, p = 0.031). Patients with CSA showed more elevated systolic blood pressure (SBP) levels with a statistically significant higher pulse pressure under both diagnostic (36.87 ± 9.70 mmHg vs. 59.69 ± 24.92 mmHg, p = 0.015) and treatment (38.99 ± 8.60 mmHg vs. 56.32 ± 21.40 mmHg, p = 0.029) conditions compared to patients with OSAS.
To assess the relationship between biometric, polysomnographic, and hemodynamic parameters with the relative change in SV, a correlational analysis was conducted for both subgroups. Regarding biometric data, a statistically significant association between patient age (r = 0.739, p = 0.015, Fig. 4a) and a negative relationship with patient weight (r=-0.653, p = 0.041, Fig. 4b) was observed for the subgroups with CSA. A positive correlation between the arousal-index under therapeutic conditions and the change in SV was seen for patients with CSA (r = 0.626, p = 0.071). Patients with OSAS exhibited a trendwise negative relationship between the change in counted sleep cycles (r=-0.577, p = 0.081) with the change in SV under treatment conditions. A trendwise negative correlation with the change in HR (r=-0.515, p = 0.096) and the STR (r=-0.607, p = 0.063) was shown. For patients with OSAS, a negative relationship with the EF (r=-0.715, p = 0.020, Fig. 4c) and a positive relationship between both the PEP (r = 0.624, p = 0.054) and the STR (r = 0.668, p = 0.035, Fig. 4d) were observed.
a. Correlation between age and relative change in SV in patients with CSA. Figure b. Correlation between body weight and relative change in SV in patients with CSA. Figure c. Correlation between diagnostic STR and relative change in SV in patients with OSAS. Figure d. Correlation between diagnostic EF and relative change in SV in patients with OSAS. Abbreviations. CSA, central sleep apnea; EF, ejection fraction; OSAS, obstructive sleep apnea; STR, systolic time ratio, SV, stroke volume
Discussion
In the present study, we could demonstrate the immediate hemodynamic effects of treatment initiation with a PAP device in patients with OSAS and CSA by applying ICG as a continuous hemodynamic monitoring technique. On polysomnography, the treatment efficacy of APAP and ASV were approved which both led to a decrease of obstructive and central respiratory events and related parameters like the ODI and the arousal-index.
Comparing both subgroups, patients with CSA were significantly older which confirms epidemiological studies reporting a mean age within the seventh and eighth decade of life [8]. Our study groups were also representative with regards to the predominance of male participants and an increased body weight. Relating to the pathophysiology of both entities, an advanced age in patients with CSA would partly be explained by the increasing incidence of chronic heart failure with concomitant CSA. Some authors conceptualize sleep disordered breathing on a pathophysiological spectrum beginning with OSAS as part of a metabolic syndrome which progresses to CSA as diastolic dysfunction and chronic heart failure become increasingly more prevalent [18].
Both OSAS and CSA are associated with increased sympathetic activation as a result of nocturnal arousals which lead to systemic hypertension as it was observed in both groups. Interestingly, SBP was more elevated in patients with CSA which was associated with a statistically significant higher PP compared to patients with OSAS. This could either be explained by a stronger sympathetic activation due to central respiratory events or by higher baseline SBP levels. Although sympathetic overactivation during periods of CSA is based on substantial evidence [19], the difference in PP between both subgroups is best explained by the pre-existing cardiovascular status. An increasing arterial stiffness with patient age would cause a higher PP in patients with CSA which would not be immediately reversible with PAP treatment [20, 21]. A treatment related decrease of the systemic blood pressure which was observed in several studies and most commonly related to reduced sympathetic activation [22, 23], could not be reproduced in the study presented. Instead, blood pressure values remained relatively stable during both conditions. However, the PEP which has been referred to as a valid surrogate parameter for cardiac sympathetic nervous system stimulation [24, 25], showed a trendwise statistically significant prolongation under treatment conditions. In addition, the duration of the PEP under diagnostic conditions was significantly shorter in patients with OSAS which could either be explained by a stronger sympathetic activation or by a reduced myocardial contractility in patients with CSA. With regards to biometric data, overweight is not only associated with an increasing likelihood in the occurrence of OSA [10], but would also explain a higher baseline sympathetic activation [26].
Besides these indirect effects of PAP treatment, intrathoracic pressure elevations can have an immediate hemodynamic impact which may depend on the underlying cardiovascular condition.
The presumed reduction of left ventricular pre- and afterload due to increased intrathoracic pressure levels would in sum cause a decreasing SV in the absence of heart failure. This is mainly explained by a decreasing transaortic (transmural) pressure gradient and a drop of the systemic venous return [27]. It should be mentioned that the cardiovascular response to changes in intrathoracic pressure levels may also depend on left ventricular morphology. While a treatment related reduction of the transmural pressure gradient may reduce left ventricular wall stress improving myocardial contractility in patients with eccentric hypertrophy [28], the drop of venous return may lead to hemodynamic impairment in patients with a stronger preload dependency, e.g. in concentric left-ventricular hypertrophy [29].
Most of these assumptions are based on experiments performed in the context of invasive positive pressure ventilation and may not be generalized to non-invasive PAP devices. The small number of studies investigating the immediate hemodynamic effects of PAP treatment is partly due to a lack of hemodynamic monitoring in routine sleep medicine practice. Bioimpedance appears to be most appropriate to monitor hemodynamic parameters during sleep studies since it enables a continuous assessment without the need for catheterization and is well tolerated by most patients.
Conclusion
In essence, by extending the routine polysomnography with a non-invasive hemodynamic monitoring device, we could demonstrate the effect of PAP treatment on hemodynamic parameters in patients with OSAS and CSA. Both subgroups differed with regards to biometric, polysomnographic and hemodynamic data. Our results approved treatment with ASV and APAP to be efficient in reducing the occurrence of central and obstructive events and indicate that PAP devices may have an immediate effect which can be measured non-invasively with ICG. Therefore, we conclude that non-invasive hemodynamic monitoring may be considered when PAP treatment is initiated in patients at risk of hemodynamic impairment.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APAP:
-
Automatic positive airway pressure
- ASV:
-
Adaptive servoventilation
- BMI:
-
Body mass index
- CI:
-
Cardiac index
- CO:
-
Cardiac output
- CSA:
-
Central sleep apnea
- DBP:
-
Diastolic blood presssure
- DRKS:
-
German clinical trials register
- ICG:
-
Impedance cardiography
- LVET:
-
Left-ventricular ejection time
- ODI:
-
Oxygen desaturation index
- OR:
-
Odds ratio
- OSA:
-
Obstructive sleep apnea
- OSAS:
-
Obstructive sleep apnea syndrome
- PAP:
-
Positive airway pressure
- PEP:
-
Pre-ejection period
- RDI:
-
Respiratory distress index
- REM:
-
Rapid eye movement
- SBP:
-
Systolic blood pressure
- STR:
-
Systolic time ratio
- SV:
-
Stroke volume
- SVI:
-
Stroke volume index
References
White DP (2005) Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 172:1363–1370. https://doi.org/10.1164/rccm.200412-1631SO
Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C (1995) Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79(2):581–588. https://doi.org/10.1152/jappl.1995.79.2.581
Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA (2002) Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med 165:61–66. https://doi.org/10.1164/rccm2009062
Tolle F, Judy W, Yu P, Markand O (1983) Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol Respiratory Environ Exerc Physiol 55(6):1718–1727. https://doi.org/10.1152/jappl.1983.55.6.1718
Bloch KE (2006) Alternatives to CPAP in the treatment of the obstructive sleep apnea syndrome. Swiss Med Wkly 136(1718):261–267
Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD (2010) Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep 33(19):1396–1407. https://doi.org/10.1093/sleep/33.10.1396
Yumino D, Bradley TD (2008) ‘Central Sleep Apnea and Cheyne-Stokes Respiration.’ Proceedings of the American Thoracic Society. 5(2), 226–236 https://doi.org/10.1513/pats.200708-129MG
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H (2015) Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 373(12):1095–1105. https://doi.org/10.1056/NEJMoa1506459
Feng J, Zhang D, Baoyuan C (2012) Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath 16:283–294. https://doi.org/10.1007/s11325-011-0519-8
Peppard P, Young TMP, Dempsey J, Skatrud J (2000) Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 284(23):3015–3021. https://doi.org/10.1001/jama.284.23.3015
Neilan TG, Farhad H, Dodson JA et al (2013) Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Association 2(6):1–10. https://doi.org/10.1161/JAHA.113.000421
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 19(186):2034–2041. https://doi.org/10.1056/NEJMoa043104
Albert NM, Hail MD, Li J, Young JB (2004) Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care 13:469–479. https://doi.org/10.4037/ajcc2004.13.6.469
Woltjer HH, Bogaard HJ, Scheffer GJ et al (1996) Standardization of non-invasive impedance cardiography for assessment of stroke volume: comparison with thermodiluation. Br J Anaesth 748–752. https://doi.org/10.1093/bja/77.6.748
Sherwood A, Mcfetridge J, Hutecheson J (1998) Ambulatory impedance cardiography: A feasibility study. Psychophysiology 27:1–23. https://doi.org/10.1111/j.14698986.1990.tb02171.x
Gesche H, Grosskurth D, Küchler G, Patzka A (2012) Continuous blood pressure measurement by using the pulse transit time: comparison to a cut-based method. Eur J Appl Physiol 112:309–315. https://doi.org/10.1007/s00421-011-1983-3
Iber C, Ancoli-Israel S, Chesson A, Quan S (2007) ‘The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification’. J Clin Sleep Med, 3(7)
Naughton MT, Kee K (2016) Sleep Apnoea in heart failure: to treat or not to treat. Respirology 22:217–229. https://doi.org/10.1111/resp.12964
Bradley TD, Floras JS (2003) Sleep apnea and heart failure. Circulation 107(13):1822–1826. https://doi.org/10.1161/01.cir.0000061757.12581.15
Dart AM, Kingwell BA (2001) Pulse Pressure - A review of mechanisms and clinical relevance. J Am Coll Cardiol 37(4):976–984
Nichols WW, Denardo SJIB, McEniery CM, O’Rourke MF (2008) Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens 10(4):295–303. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6217416
De Araujo MT, Bissoli NS, Gouvea SA (2013) ‘CPAP therapy prevents increase in blood pressure after upper airway surgery for obstructive sleep apnea’, Sleep, 1289–1299. https://doi.org/10.1007/s11325-013-0837-0
Pinto P, Barbara C, Montserrat JM (2013) Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC Pulm Med 13:1–13. http://www.biomedcentral.com/1471-2466/13/13
Johnson BF, Meeran MK, Frank A, Taylor SH (1981) Systolic time intervals in measurement of inotropic response to drugs. Br Heart J 46:513–521
Schächinger H, Weinbacher M, Kiss A, Ritz R, Lanngewitz W (2001) Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med 788–96. 0033-3174/01/6305-0788
Grassi G, Biffi A, Seravalle G, Trevano FQ, Dell´Oro R, Corrao G, Mancia G (2019) Sympathetic neural overdrive in the obese and overweight state. Hypertension 74:349–358. https://doi.org/10.1161/HYPERTENSIONAHA.119.12885
Singh I, Pinsky MR (2008) Heart-Lung interaction. In: Papdakos PJ, Lachmann B (eds) Mechanical ventilation: clinical applications and pathophysiology. Saunders Elsevier, Philadelphia, pp 173–184
Steiner S, Schannwell CM, Strauer BE (2008) Left ventricular response to continuous positive airway pressure: role of left ventricular geometry. Respiration 76(4):393–397. https://doi.org/10.1159/000150442
Grossmann W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64. https://doi.org/10.1172/JCI108079
Acknowledgements
We would like to thank the team of the sleep laboratory of Fachkrankenhaus Kloster Grafschaft GmbH and medis Medizinische Messtechnik GmbH for providing technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
All authors declare to have no financial interest or personal relationship that could have influenced the work presented in this manuscript. The authors did not receive any financial support that could have biased the results. No funding was received.
Author information
Authors and Affiliations
Contributions
All authors have seen and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was given by the Ethics Committee of Marburg University.
Informed consent
Informed written consent was received by all patients included in this study.
Consent for publication
Not applicable.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Müller, C., Kerl, J. & Dellweg, D. A comparative study on the hemodynamic effects of initiating positive airway pressure treatment in patients with obstructive and central sleep apnea. Sleep Breath 29, 154 (2025). https://doi.org/10.1007/s11325-025-03317-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-025-03317-z