Abstract
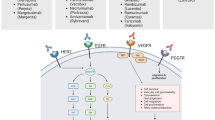
Monoclonal antibodies (MAbs) are a relatively new innovation in cancer treatment. At present, some monoclonal antibodies have increased the efficacy of the treatment of certain tumors with acceptable safety profiles. When monoclonal antibodies enter the body and attach to cancer cells, they function in several different ways: first, they can trigger the immune system to attack and kill that cancer cell; second, they can block the growth signals; third, they can prevent the formation of new blood vessels. Some naked MAbs such as rituximab can be directed to attach to the surface of cancer cells and make them easier for the immune system to find and destroy. The ability to produce antibodies with limited immunogenicity has led to the production and testing of a host of agents, several of which have demonstrated clinically important antitumor activity and have received U.S. Food & Drug Administration (FDA) approval as cancer treatments. To reduce the immunogenicity of murine antibodies, murine molecules are engineered to remove the immunogenic content and to increase their immunologic efficiency. Radiolabeled antibodies composed of antibodies conjugated to radionuclides show efficacy in non-Hodgkin’s lymphoma. Antivascular endothelial growth factor (VEGF) antibodies such as bevacizumab intercept the VEGF signal of tumors, thereby stopping them from connecting with their targets and blocking tumor growth. Trifunctional antibodies have revealed a new perspective in cancer therapy extending beyond primary destruction of tumor cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Rosa DD, Ismael G, Lago LD, et al. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev 2008; 34: 61–80.
Myron A, Philip A, Al T, et al. The Therapeutic value of monoclonal antibodies directed against immunogenic tumor glycoproteins. J Cancer 2010; 1: 209–222.
Katharina S, Tom ND, Matthew EF et al. Generation of tumour-rejecting anti-carbohydrate monoclonal antibodies using melanoma modified with Fas ligand. Int Immunol 2008; 20: 525–534.
Margaret VM, Gregory P, et al. Monoclonal antibody therapy for cancer. Annu Rev Med 2003; 54: 343–369.
Ignacio M, Sandra H, Martin G. Immunostimulatory monoclonal antibodies for cancer therapy. Nature 2007; 7: 95–106.
Mitchell R. Rituximab (monoclonal anti-CD20 antibody)—mechanisms of action and resistance. Oncogene 2003; 22: 7359–7368.
Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood 2001; 98: 1326–1331.
Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood 2002; 99: 3554–3561.
Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and nonsmall-cell lung cancer: current knowledge and future directions. J Clin Oncol 2004; 23: 2556–2568.
Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist 2007; 12: 1084–1095.
Tracy H. Monoclonal Antibody Therapies Shine in Breast Cancer Clinical Trials. JAMA 2005; 293: 2985–2989.
Andreas A, Haralabos P. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med, Publication 2010; 15: 183–191.
Dalle S, Thieblemont C, Thomas L, et al. Monoclonal antibodies in clinical oncology. Anticancer Agents Med Chem 2008; 8:523–532.
Yan L, Hsu K, Beckman RA, Antibody-based therapy for solid tumors. Cancer J 2008; 14:178–183.
Bagshawe KD. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev Anticancer Ther 2006; 6: 1421–1431.
Maruyama K. PEG-immunoliposome. Biosci Rep 2002; 22: 251–266.
Azinovic I, DeNardo GL, Lamborn KR, et al. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother 2006; 55: 1451–1458.
Surender K, Vaswani MD and Robert G, et al. Humanized antibodies as potentialtherapeutic drugs. Ann Allergy Asthma Immunol 1998; 81: 105–119.
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992; 89: 4285–4289.
Presta LG, Lahr SJ, Shields RL, et al. Humanization of an antibody directed against IgE. J Immunol 1993; 151: 2623–2632.
Beerli RR, Bauer M, Buser RB, et al. Isolation of human monoclonal antibodies by mammalian cell display. Proc Natl Acad Sci U S A. 2008; 105: 14336–14341
Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol 2005; 5: 543–549.
Raymond M, Monoclonal antibody and peptide-targeted radiotherapy of cancer. A John Wiley & Sons, Inc., Publication 201; 28–57.
Napoleone F, Kenneth J, William N. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005; 333: 328–335.
Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002; 2: 795–803.
Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol 2008; 181: 131–150.
Gregory P, Louis MW. Monoclonal antibody therapy of cancer. Nature Biotechnology 2005; 23: 1147–1157.
Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–3712.
Diane S. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab®). J Cancer 2011; 2: 309–316.
Ruf P, Gires O, Jäger M, et al. Characterisation of the new Ep-CAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br J Cancer 2007; 97: 315–321.
Zeidler R, Reisbach G, Wollenberg B, et al. Simultaneous activation of T-cells and accessory cells by a new class of intact bispecific antibody results in efficient tumour cell killing. J Immunol 1999; 163: 1246–1252.
Zeidler R, Mysliwietz J, Csanady M, et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer 2000; 83: 261–266.
Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 2010; 127: 2209–2221.
Frank G, Hensin T, Helen W, et al. Genetic Alterations in Signaling Pathways in Melanoma. Clin Cancer Res 2006; 12: 2301–2307.
Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 2008; 26: 1774–1777.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Guo, YT., Hou, QY. & Wang, N. Monoclonal antibodies in cancer therapy. Clin. Oncol. Cancer Res. 8, 215–219 (2011). https://doi.org/10.1007/s11805-011-0583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11805-011-0583-7