Abstract
Purpose of Review
The use of statins has transformed approaches to the prevention of cardiovascular disease. However, many patients remain at a major risk of experiencing cardiovascular events, due to a range of factors including suboptimal control of low-density lipoprotein cholesterol (LDL-C). Accordingly, there is an ongoing need to develop additional strategies, beyond the use of statins, to achieve more effective reductions in cardiovascular risk.
Recent Findings
Genomic studies have implicated the causal role of LDL in atherosclerosis and identified that polymorphisms influencing factors involved in lipid metabolism influence both the level of LDL-C and cardiovascular risk. These findings have highlighted the potential for cardiovascular benefit from development of therapies targeting these factors and incremental benefit when used in combination with statins. Clinical trials have demonstrated that these new agents have favourable effects on both atherosclerotic plaque and cardiovascular events. Additional work has sought to improve intensification of statin therapy and adherence with lipid lowering therapy, to achieve more effective cardiovascular prevention via lipid lowering.
Summary
Emerging therapies, beyond statins, have the potential to optimise lipid levels and play an effective role in the prevention of cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
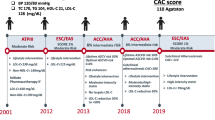
Low-density lipoproteins play a pivotal role in the pathogenesis of atherosclerotic cardiovascular disease. This has been well established by preclinical investigations that demonstrate an association between increasing levels of LDL cholesterol (LDL-C) and atherogenesis, genetic studies that implicate polymorphisms and mutations associated with higher LDL-C levels and cardiovascular risk and by cohort studies that demonstrate a curvilinear relationship between LDL-C and the prospective risk of cardiovascular events [1, 2]. The finding that lowering levels of LDL-C results in a reduction in cardiovascular risk, further supports the importance of LDL-C lowering as a cornerstone for the prevention of cardiovascular disease [3,4,5]. 2024 represents the thirty-year anniversary of the report of the first large cardiovascular outcomes trial demonstrating clinical benefit with statins in patients with a prior myocardial infarction [6]. While efforts have concentrated on increasing use of statins in clinical practice, more recent advances in lipid therapeutics have focused on the development of additional agents with the potential to both lower LDL-C and cardiovascular risk.
Statins and Cardiovascular Risk Reduction
Statins are pharmacological inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase and are widely used for lowering levels of LDL-C. Mendelian randomization studies have established that the presence of genetic polymorphisms resulting in less HMGCoA reductase associate with lower levels of LDL-C and cardiovascular events [7, 8]. Use of statins have produced cardiovascular benefit across the clinical spectrum from high-risk primary prevention through to those who have had a recent acute coronary syndrome [3,4,5]. The use of more intensive statin doses, achieving lower LDL-C levels, have produced benefit compared with more moderate lipid lowering [3,4,5]. In parallel, a range of forms of plaque imaging have permitted evaluation of the impact of statin therapy on atherosclerotic disease. Use of carotid intima-medial thickness [9,10,11], coronary angiography [12, 13], intravascular ultrasound [14,15,16,17,18] and computed tomography coronary angiography [19,20,21] have demonstrated that statins can both slow progression of disease or promote plaque regression. More advanced techniques including virtual histology [22,23,24,25], magnetic resonance [26], high resolution computed tomography [19,20,21] and optical coherence tomography [27,28,29,30,31,32,33] have extended these findings to demonstrate plaque composition changes consistent with stabilization. The findings of these studies and the direct association between LDL-C lowering and clinical benefit have prompted successive updates to clinical guidelines that link the degree of clinical risk with LDL-C target. Additional analyses have demonstrated an independent association between lowering levels of high sensitivity C-reactive protein (hsCRP) and both slowing of plaque progression [34] and reducing cardiovascular event rates [35] observed with statins. This would support the concept that statins may possess additional properties, beyond their ability to lower LDL-C that may influence cardiovascular risk.
However, several challenges remain regarding the use of statins in clinical practice. Clinical registries demonstrate that patients at high cardiovascular risk are undertreated, both from the perspective of failure to either intensify statin doses or use combination therapy [36,37,38]. Many patients stop taking their statin within the first year of initiation [39,40,41], some of which is due to the experience of muscle symptoms or concerns regarding the reported increased risk of developing diabetes [42]. The combination of intensity and adherence to lipid lowering therapy is critical in determining achieved LDL-C levels and potential reductions in cardiovascular risk [38]. When statins are taken in clinical trials, there remains a residual risk of plaque progression [43] and clinical events [44], suggesting that additional approaches are required, in order to achieve more effective prevention of cardiovascular disease.
Ezetimibe
Ezetimibe inhibits intestinal cholesterol absorption, via its ability to inhibit Niemann-Pick C1-Like 1 (NPC1L1), resulting in the ability to lower LDL-C by approximately 20% [45]. While ezetimibe monotherapy does not lower hsCRP levels, when used in combination with statins the degree of hsCRP lowering is greater than that observed with statin monotherapy [45]. Mendelian randomization studies demonstrate not only less cardiovascular risk in the setting of less NPC1L1, but greater protection in the combination with less HMGCoA reductase, suggesting the potential for incremental clinical benefit using the combination of statins and ezetimibe [7].
Clinical trials have demonstrated the impact of therapeutic regimens including ezetimibe on cardiovascular outcomes. The Plaque Regression with Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound (PRECISE-IVUS) trial demonstrated that addition of ezetimibe to atorvastatin produced greater regression of coronary atherosclerosis on serial intravascular ultrasound imaging, compared with atorvastatin monotherapy [46]. The Study of Heart and Renal Protection (SHARP) demonstrated that the combination of simvastatin and ezetimibe reduced the risk of the combination of myocardial infarction, coronary death, non-hemorrhagic stroke or arterial revascularization by 17% compared with placebo in patients with chronic kidney disease, who have yet to experience a cardiovascular event [47]. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) demonstrated that the combination of simvastatin and ezetimibe produced a modest (6.4%), but significant, reduction in the combination of cardiovascular death, myocardial infarction, unstable angina requiring hospitalization, coronary revascularization or stroke, compared with simvastatin monotherapy in patients with an acute coronary syndrome [48]. The clinical benefit of these studies directly associated with the extent of LDL-C lowering and confirmed the use of ezetimibe as an important approach to achieving more effective lipid control in high cardiovascular risk patients.
PCSK9 Inhibitors
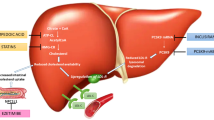
Proprotein convertase subtilisin kexin type 9 (PCSK9) plays a role in LDL metabolism. Synthesized within the liver, limits removal of LDL particles from the circulation, via binding to the LDL receptor and preventing its recirculation to the hepatocyte surface [49]. Early genetic studies identified gain of function PCSK9 mutations as a monogenic cause of familial hypercholesterolemia, while loss of function mutations was demonstrated in healthy individuals with very low LDL-C levels [49]. Mendelian randomization studies similarly revealed cardiovascular protection with low PCSK9 levels, which was greater in the presence of less HMGCoA reductase [50]. Early clinical studies of PCSK9 inhibitory monoclonal antibodies demonstrated dose-dependent lowering of LDL-C by up to 60%, when used as monotherapy or in combination with statins [51, 52]. Tolerability was good, with mild injection site reactions experienced by some patients. This presented the opportunity to both enable more patients to achieve their LDL-C goals and for high-risk patients to achieve very low LDL-C levels. These benefits extended to the setting of familial hypercholesterolemia [53, 54], particularly the most severe forms in which the requirement for LDL apheresis was decreased [53, 55].
Serial plaque imaging studies made several seminal discoveries regarding the impact of adding a PCSK9 inhibitor to statin therapy [56]. The Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) trial demonstrated that addition of the PCSK9 inhibitor, evolocumab, to existing statin therapy for 18 months produced plaque regression compared with continuing statin monotherapy [57]. Greater degrees of plaque regression were observed in patients achieving the lowest LDL-C levels [57]. The High-Resolution Assessment of Coronary Plaques in a Global Evolocumab Randomized Study (HUYGENS) studied the impact of adding evolocumab to high intensity statin therapy for 12 months on coronary atheroma using multimodality imaging, in patients following an acute coronary syndrome [58]. Serial intravascular ultrasound imaging also demonstrated greater plaque regression with the combination of evolocumab and statin, compared with statin monotherapy [58]. Greater regression was observed in HUYGENS, compared with GLAGOV, despite a shorter treatment period, suggesting potential greater modifiability of atherosclerosis in patients with an acute coronary syndrome. Optical coherence tomography imaging demonstrated that the combination of statin and PCSK9 inhibitor produced greater thickening of the fibrous cap and reduction in plaque lipid and macrophages, consistent with plaque stabilization. Similar findings were observed in the Effects of the PCSK9 Antibody Alirocumab on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction (PACMAN-AMI) study, in which addition of alirocumab to a statin produced plaque regression on intravascular ultrasound, reductions in plaque macrophages on optical coherence tomography and lipid cores on near infrared spectroscopy [59]. While these trials involved at least 12 months of treatment, observational studies have suggested that plaque stabilization features begin to appear as early as 4 weeks after commencement of combination therapy with a PCSK9 inhibitor and a statin (https://doi.org/10.1016/j.jjcc.2019.08.002). The Effect of Evolocumab on Coronary Plaque Characteristics in Stable Coronary Artery Disease: a Multimodality Imaging Study (YELLOW 3) extended the findings of plaque stabilization with statin and evolocumab to patients with stable disease [60].
The impact of PCSK9 inhibitory monoclonal antibodies on cardiovascular outcomes have been investigated in three clinical trials. The Further Cardiovascular Outcomes Research with PCSK9 Inhibition Subjects with Elevated Risk (FOURIER) study demonstrated that addition of evolocumab to statin therapy produced a 15% reduction in the risk of the composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina or coronary revascularization in patients with stable atherosclerotic cardiovascular disease [61]. A direct association was observed between achieved LDL-C levels and the rate of cardiovascular events [61]. No increase in adverse events was observed in those patients achieving the lowest LDL-C levels [62]. In addition, a cognitive function substudy demonstrated no adverse impact of treatment with evolocumab [63]. Post hoc analyses demonstrated a greater absolute risk reduction with evolocumab in patients with multivessel disease, polyvascular disease, recurrent events and multiple risk factors [8, 64, 65]. The long-term extension study demonstrated increasing benefit, with evidence of a significant reduction in cardiovascular death [66]. An ongoing clinical trial is evaluating the impact of evolocumab on cardiovascular risk in patients deemed to be at high risk, yet have not experienced a clinical event (https://doi.org/10.1016/j.ahj.2023.12.004).
The Evaluation of Cardiovascular Outcomes After an Acute Coronary Synrdome During Treatment with Alirocumab (ODYSSEY Outcomes) study demonstrated that addition of alirocumab to statin therapy produced a 15% reduction in the risk of the composite of coronary heart disease death, myocardial infarction, ischemic stroke or unstable angina requiring hospitalization in patients with an acute coronary syndrome within the preceding 4 to 52 weeks [67]. A nominal reduction in mortality was observed [67]. The study design required that patients achieving very low LDL-C levels (< 0.39 mmol/L or 15 mg/dL) underwent blinded back titration of therapy or swapping to placebo. These patients demonstrated lower rates of cardiovascular events, suggesting that even short periods of achieving very low LDL-C levels could produce a legacy effect on cardiovascular risk [68]. Subsequent analyses of the ODYSSEY study demonstrated that lowering levels of Lp(a) with alirocumab, independently associated with its clinical benefit [69].
Monoclonal antibodies bind circulating PCSK9 that has been synthesized in the liver. An alternative approach involves inhibition of hepatic PCSK9 synthesis, leading to the development of short interfering RNA interference (siRNA) therapeutics that are selectively targeted to the hepatocyte [70,71,72]. Inclisiran is the first siRNA targeting PCSK9 to advance in clinical development. Early studies have demonstrated that inclisiran reduces LDL-C levels by more than 45%, but also with good durability of effect. LDL-C lowering by more than 38% has been observed up to 6 months following a single injection of inclisiran, providing the opportunity to develop twice yearly administration [70,71,72,73]. This has important implications for the treatment of patients, where adherence may be a challenge. Longer term administration has demonstrated both sustained efficacy and good tolerability by patients [74]. The impact of inclisiran on cardiovascular events is currently being evaluated in three ongoing clinical trials, one in the primary prevention setting and two in patients with clinically manifest atherosclerotic cardiovascular disease.
Most jurisdictions have limited access to these agents, due to their cost, which has influenced cost effectiveness analyses. This typically involves delaying administration to the outpatient setting, downstream of a clinical event. Clinical trials have investigated the impact of early initiation of either evolocumab [75] or inclisiran [76] in the setting of an acute coronary syndrome. These studies have demonstrated good tolerability and early lipid lowering, which then subsequently translates to favorable effects on coronary atheroma with more sustained use. The impact of early administration of evolocumab in the acute coronary syndrome patient on cardiovascular outcomes is being evaluated in an ongoing clinical trial.
Ongoing work is aiming to develop alternative PCSK9 inhibitor formulations for administration. Lerodalcibep is a recombinant fusion protein of adnectin, a PCSK9 gene binding domain, and human serum albumin. Administered in small volumes monthly, lerodalcibep reduced LDL-C levels by up to 65% in patients with heterozygous familial hypercholesterolemia [77]. PCSK9 inhibitors have also been developed in an oral form, with a macrocyclic peptide lowering LDL-C in a dose-dependent fashion by up to 61% [78]. These agents will need to be evaluated in larger and longer trials.
Bempedoic Acid
Bempedoic acid is an oral lipid lowering agent that inhibits ATP citrate lyase, a factor involved in hepatic cholesterol synthesis [79]. Mendelian randomization demonstrated that polymorphisms associated with less ATP citrate lyase resulted in lower cardiovascular risk, which was additive to lower levels of HMGCoA reductase [80]. Like statins, bempedoic acid lowers LDL-C levels by promoting upregulation of hepatic LDL receptors and removal of LDL from the circulation. Bempedoic acid is ingested in an inactive form, with the isozyme responsible for activation present in the liver, but not skeletal muscle [79, 81, 82]. In theory, bempedoic acid should produce LDL-C lowering, without the myalgia symptoms experienced by up to 20% of patients treated with a statin. Early clinical studies demonstrated that bempedoic acid lowered LDL-C by 15–25% and hsCRP by 20–30%, in studies conducted in both statin intolerant patients and those receiving maximally tolerated, with lesser effects observed in those taking a statin [83,84,85,86,87,88,89,90]. Further studies had demonstrated LDL-C lowering by 38–45% with the combination of bempedoic acid and ezetimibe [91] and by 64% with the combination of low dose statin, bempedoic acid and ezetimibe [92]. The CLEAR Outcomes study was conducted in patients at high risk of a cardiovascular event, with elevated LDL-C levels and documented statin intolerance. Treatment with bempedoic acid produced a 13% reduction in the risk of cardiovascular death, myocardial infarction, stroke or coronary revascularization [93,94,95,96]. A greater benefit was observed in patients who had yet to experience a clinical event [97]. Bempedoic acid treated patients demonstrated a greater likelihood of gout and liver enzyme abnormalities [93, 94].
CETP Inhibitors
Cholesteryl ester transfer protein (CETP) plays an important role in lipid metabolism, facilitating the exchange of esterified cholesterol from high-density lipoprotein (HDL) to LDL and very low-density lipoprotein (VLDL) particles [98,99,100]. Early development of CETP inhibitors was stimulated on the basis of their ability to raise HDL cholesterol (HDL-C), yet several clinical trials proved disappointing with either toxicity [101] or no impact on cardiovascular outcomes [102, 103]. While one CETP inhibitor, anacetrapib, did reduce cardiovascular risk, the benefit was modest and not related to HDL-C raising, rather due to lowering of non-HDL-C [104, 105]. This complemented studies from Mendelian randomization, which demonstrated that genetic polymorphisms associated with less CETP also resulted in less cardiovascular events, directly related to lower levels of LDL-C [106,107,108,109]. Additional analyses demonstrated additive effects of having less CETP and HMGCoA reductase. This led to a rethink on development of CETP inhibitors, focusing on their ability to lower LDL-C levels, rather than raising HDL-C. Studies of the potent CETP inhibitor obicetrapib have demonstrated LDL-C lowering by 40–50% as monotherapy and in combination with high-intensity statin therapy, in addition to lowering triglyceride by up to 11% and Lp(a) by up to 56% and raising HDL-C levels by up to 165% [110,111,112]. The impact of obicetrapib on cardiovascular outcomes is currently being evaluated in a large clinical trial of high cardiovascular risk patients with elevated LDL-C levels [113].
Additional Approaches to Lowering LDL-C
A number of additional therapeutic approaches are used in clinical practice for lowering LDL-C in patients with severe forms of familial hypercholesterolemia. Mipomersen is an antisense oligonucleotide targeting apolipoprotein B-100, with LDL-C lowering by up to 36% [114, 115]. Lomitapide inhibits microsomal transfer protein, essential for assembly of VLDLs in the liver and chylomicrons in the intestine, with LDL-C lowering by 50% [116]. Both of these agents result in increases in hepatic fat content and liver enzymes [114,115,116]. Angiopoietin-like 3 (ANGPTL3) influences lipid metabolism in a number of ways. As an inhibitor of lipoprotein lipase, early studies of ANGPTL3 inhibition primarily focused on their potential to influence triglyceride levels [117]. However, ANGPTL3 inhibition also has the potential to lower LDL-C levels, via a mechanism independent of LDL receptor expression [117]. Genetic studies have demonstrated that loss of function ANGPTL3 variants associate with lower levels of triglycerides, LDL-C and cardiovascular risk [118]. Administration of the ANGPTL3 inhibitory monoclonal antibody, evanicumab, was well tolerated and produced LDL-C lowering by 49% [119]. Novel ANGPTL3 inhibitors aimed at targeting RNA are in clinical development, primarily on the basis of their ability to lower triglyceride levels [120, 121]. They may provide an additional approach to treating LDL-C in the resistant patient. Apheresis remains a therapeutic option for treatment of severe familial hypercholesterolemia, which is refractory to treatment with conventional therapies. While effective, it is a costly and invasive therapeutic option, stimulating the search to develop alternative approaches for patients [122].
Models of Care
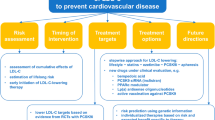
Prescribing more intensive lipid lowering regimens can only be effective in cardiovascular prevention if patients remain adherent with therapy. The combination of greater adherence to more intensive lipid lowering therapy results in more effective lowering of both LDL-C and cardiovascular risk [38]. Accordingly, approaches to facilitate shared decision making by both patient and clinician, with a view to improving long term therapeutic adherence is critical to more effectively prevent the risk of cardiovascular events [123]. Educational tools that assist in demonstrating the level of risk for patients can assist in decision making, whether they distil risk scores or use additional information from imaging or genetic scores. Additional digital tools, such as text messages or use of chatbots, can assist in promoting long term adherence to therapies. Efforts are underway to reinstate LDL-C measurements as a quality metric for patients with acute coronary syndromes, as an approach to ensuring more effective lipid management [124]. An alternative approach to achieving more effective long term lipid control involves the use of combination therapy, with lower statin doses, to minimise drug discontinuation and maintenance of cardiovascular risk lowering [125]. More work is required in each of these areas to develop effective models of care that can be integrated into local management plans and used in a fit for purpose strategy with patients.
Gene Editing
Advances in gene editing now permit selective targeting of gene editing of factors involved in lipid metabolism within the hepatocyte. Early human studies of gene editing have targeted both PCSK9 and ANGPTL3, with evidence of durable and robust LDL-C lowering in non-human primates [126]. While longer studies are required to characterize both efficacy and safety of these interventions, they do present the potential for a once in a lifetime approach to management of dyslipidemia.
Conclusion
While statins have played a major role in lowering levels of LDL-C and cardiovascular events, residual risk and challenges with achieving treatment targets highlight the need to develop additional lipid lowering agents. Genetic studies have identified a number of pharmacological targets that have led to new therapies, which favorably modulate lipid levels in the blood, plaque burden and composition within the artery wall and ultimately cardiovascular risk. The use of these therapies, both in combination with statins and as monotherapy in patients unable to tolerate statins, have the potential to achieve more effective lipid lowering and prevention of cardiovascular disease.
Key References
-
1.
Raal F, Fourie N, Scott R, et al.: Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: the LIBerate-HeFH trial. Eur Heart J 2023, 44(40):4272–4280. New injectable form of PCSK9 inhibitor in development.
-
2.
Ballantyne CM, Banka P, Mendez G, et al.: Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616. J Am Coll Cardiol 2023, 81(16):1553–1564. First human experience of an oral PCSK9 inhibitor.
-
3.
Nissen SE, Lincoff AM, Brennan D, et al.: Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med 2023, 388(15):1353–1364. Impact of bempedoic acid on cardiovascular outcomes in patients with statin intolerance.
-
4.
Nicholls SJ, Ditmarsch M, Kastelein JJ, et al.: Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: a randomized phase 2 trial. Nat Med 2022, 28(8):1672–1678. Effects of a potent CETP inhibitor on atherogenic lipid levels in patients treated with high-intensity statins.
Data Availability
No datasets were generated or analysed during the current study.
References
Boren J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72.
Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–8.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504.
Randomised trial of cholesterol lowering. In 4444 patients with coronary heart disease: the scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383–9.
Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–61.
Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of Cardiovascular Disease and Diabetes. N Engl J Med. 2016;375(22):2144–53.
Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35(12):2902–9.
Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357(9256):577–81.
Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: arterial Biology for the investigation of the Treatment effects of reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106(16):2055–60.
Ballantyne CM. Clinical trial endpoints: angiograms, events, and plaque instability. Am J Cardiol. 1998;82(6A):M5–11.
Ballantyne CM, Raichlen JS, Nicholls SJ, et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117(19):2458–66.
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291(9):1071–80.
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65.
Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87.
Okazaki S, Yokoyama T, Miyauchi K, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. 2004;110(9):1061–8.
Hiro T, Kimura T, Morimoto T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54(4):293–302.
Auscher S, Heinsen L, Nieman K, et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: Assessment with serial coronary CT angiography. Atherosclerosis. 2015;241(2):579–87.
Shin S, Park HB, Chang HJ, et al. Impact of intensive LDL cholesterol lowering on coronary artery atherosclerosis progression: a serial CT Angiography Study. JACC Cardiovasc Imaging. 2017;10(4):437–46.
Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198–204.
Puri R, Libby P, Nissen SE, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging. 2014;15(4):380–8.
Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, et al. Integrated Biomarker and Imaging Study 3 (IBIS-3) to assess the ability of rosuvastatin to decrease necrotic core in coronary arteries. EuroIntervention. 2016;12(6):734–9.
Raber L, Taniwaki M, Zaugg S, et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur Heart J. 2015;36(8):490–500.
Banach M, Serban C, Sahebkar A, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med. 2015;13:229.
Underhill HR, Yuan C, Zhao XQ et al., Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J 2008, 155(3):584 e581-588.
Kataoka Y, Puri R, Hammadah M, et al. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol. 2014;114(4):549–54.
Dai J, Hou J, Xing L, et al. Is age an important factor for vascular response to statin therapy? A serial optical coherence tomography and intravascular ultrasound study. Coron Artery Dis. 2017;28(3):209–17.
Hattori K, Ozaki Y, Ismail TF, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging. 2012;5(2):169–77.
Hou J, Xing L, Jia H, et al. Comparison of Intensive Versus Moderate lipid-lowering therapy on fibrous Cap and Atheroma volume of coronary lipid-rich plaque using serial Optical Coherence Tomography and Intravascular Ultrasound Imaging. Am J Cardiol. 2016;117(5):800–6.
Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol. 2014;64(21):2207–17.
Nishio R, Shinke T, Otake H, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234(1):114–9.
Takarada S, Imanishi T, Kubo T, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009;202(2):491–7.
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352(1):29–38.
Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8.
Ray KK, Haq I, Bilitou A, et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023;29:100624.
Ray KK, Molemans B, Schoonen WM, et al. EU-Wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279–89.
Khunti K, Danese MD, Kutikova L, et al. Association of a combined measure of Adherence and Treatment Intensity with Cardiovascular outcomes in patients with atherosclerosis or other Cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open. 2018;1(8):e185554.
De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78(4):684–98.
Lin I, Sung J, Sanchez RJ, et al. Patterns of statin use in a Real-World Population of patients at High Cardiovascular Risk. J Manag Care Spec Pharm. 2016;22(6):685–98.
Banach M, Rizzo M, Toth PP, et al. Statin intolerance - an attempt at a unified definition. Position paper from an international lipid Expert Panel. Archives Med Science: AMS. 2015;11(1):1–23.
Cholesterol Treatment Trialists’ Collaboration. Electronic address cnoau, cholesterol treatment trialists C: effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis. Lancet Diabetes Endocrinol. 2024;12(5):306–19.
Bayturan O, Kapadia S, Nicholls SJ, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010;55(24):2736–42.
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8.
Savarese G, De Ferrari GM, Rosano GM, Perrone-Filardi P. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015;201:247–52.
Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering Strategy with Ezetimibe and Atorvastatin on Coronary Plaque regression in patients with percutaneous coronary intervention: the Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol. 2015;66(5):495–507.
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after Acute Coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Scherer DJ, Nelson AJ, Psaltis PJ, Nicholls SJ. Targeting low-density lipoprotein cholesterol with PCSK9 inhibitors. Intern Med J. 2017;47(8):856–65.
Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–53.
Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–82.
Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–19.
Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–50.
Hovingh GK, Raal FJ, Dent R, et al. Long-term safety, tolerability, and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia. J Clin Lipidol. 2017;11(6):1448–57.
Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016;37(48):3588–95.
Barbieri L, Tumminello G, Fichtner I, et al. PCSK9 and coronary artery plaque-new opportunity or Red Herring? Curr Atheroscler Rep. 2024;26(10):589–602.
Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in statin-treated patients: the GLAGOV Randomized Clinical Trial. JAMA. 2016;316(22):2373–84.
Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of Evolocumab on Coronary Plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging; 2022.
Raber L, Ueki Y, Otsuka T et al., Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022.
https://.health.mountsinai.org/blog/yellow-iii-trial-finds-that-lipid-lowering-with-a-pcsk9-inhibitor-could-benefit-patients-on-statin-therapy/[ https://health.mountsinai.org/blog/yellow-iii-trial-finds-that-lipid-lowering-with-a-pcsk9-inhibitor-could-benefit-patients-on-statin-therapy/]
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical outcomes in patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–22.
Giugliano RP, Keech A, Murphy SA, et al. Clinical efficacy and safety of Evolocumab in High-Risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a Randomized Clinical Trial. JAMA Cardiol. 2017;2(12):1385–91.
Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a Randomized Trial of Evolocumab. N Engl J Med. 2017;377(7):633–43.
Sabatine MS, De Ferrari GM, Giugliano RP et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: analysis from FOURIER. Circulation 2018, 138(8):756–66.
Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–50.
O’Donoghue ML, Giugliano RP, Wiviott SD, et al. Long-term evolocumab in patients with established atherosclerotic Cardiovascular Disease. Circulation. 2022;146(15):1109–19.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and Cardiovascular outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379(22):2097–107.
Schwartz GG, Szarek M, Bhatt DL, et al. Transiently achieved very low LDL-cholesterol levels by statin and alirocumab after acute coronary syndrome are associated with cardiovascular risk reduction: the ODYSSEY OUTCOMES trial. Eur Heart J. 2023;44(16):1408–17.
Bittner VA, Szarek M, Aylward PE, et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk after Acute Coronary Syndrome. J Am Coll Cardiol. 2020;75(2):133–44.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
Ray KK, Landmesser U, Leiter LA et al. Inclisiran in patients at High Cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of Inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Ray KK, Kallend D, Leiter LA, et al. Effect of inclisiran on lipids in primary prevention: the ORION-11 trial. Eur Heart J. 2022;43(48):5047–57.
Ray KK, Troquay RP, Visseren FL, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL-C (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lanc Diab Endo; 2022.
Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL Cholesterol Levels in patients with Acute Coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452–62.
Koren MJ, Rodriguez F, East C, et al. An Inclisiran First Strategy vs Usual Care in patients with atherosclerotic Cardiovascular Disease. J Am Coll Cardiol. 2024;83(20):1939–52.
Raal F, Fourie N, Scott R, et al. Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: the LIBerate-HeFH trial. Eur Heart J. 2023;44(40):4272–80.
Ballantyne CM, Banka P, Mendez G, et al. Phase 2b Randomized Trial of the oral PCSK9 inhibitor MK-0616. J Am Coll Cardiol. 2023;81(16):1553–64.
Bilen O, Ballantyne CM. Bempedoic Acid (ETC-1002): an investigational inhibitor of ATP citrate lyase. Curr Atheroscler Rep. 2016;18(10):61.
Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and Cardiovascular Disease. N Engl J Med. 2019;380(11):1033–42.
Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134–51.
Cramer CT, Goetz B, Hopson KL, et al. Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J Lipid Res. 2004;45(7):1289–301.
Ballantyne CM, Davidson MH, Macdougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62(13):1154–62.
Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34(3):676–83.
Thompson PD, Rubino J, Janik MJ, et al. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015;9(3):295–304.
Ballantyne CM, McKenney JM, MacDougall DE, et al. Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol. 2016;117(12):1928–33.
Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203.
Ray KK, Bays HE, Catapano AL, et al. Safety and Efficacy of Bempedoic Acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–32.
Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of Bempedoic Acid vs Placebo added to maximally tolerated statins on Low-Density Lipoprotein Cholesterol in patients at High Risk for Cardiovascular Disease: the CLEAR Wisdom Randomized Clinical Trial. JAMA. 2019;322(18):1780–8.
Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of Bempedoic Acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603.
Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis. 2021;320:122–8.
Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic Acid and Cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64.
Nicholls S, Lincoff AM, Bays HE, et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–12.
Cho L, Plutzky J, Brennan D, et al. Impact of Bempedoic Acid on Cardiovascular outcomes by Sex. Circulation. 2024;149(22):1775–7.
Lincoff AM, Ray KK, Sasiela WJ, et al. Comparative Cardiovascular benefits of Bempedoic Acid and Statin drugs. J Am Coll Cardiol. 2024;84(2):152–62.
Nissen SE, Menon V, Nicholls SJ, et al. Bempedoic Acid for Primary Prevention of Cardiovascular events in statin-intolerant patients. JAMA. 2023;330(2):131–40.
Reeskamp LF, Meessen ECE, Groen AK. Transintestinal cholesterol excretion in humans. Curr Opin Lipidol. 2018;29(1):10–7.
Li J, Pijut SS, Wang Y, et al. Simultaneous determination of biliary and intestinal cholesterol secretion reveals that CETP (Cholesteryl Ester Transfer Protein) alters Elimination Route in mice. Arterioscler Thromb Vasc Biol. 2019;39(10):1986–95.
Nurmohamed NS, Ditmarsch M, Kastelein JJP. Cholesteryl Ester transfer protein inhibitors: from high-density lipoprotein cholesterol to low-density lipoprotein cholesterol lowering agents? Cardiovasc Res. 2022;118(14):2919–31.
Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22.
Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Nicholls SJ, Lincoff AM, Barter PJ, et al. Assessment of the clinical effects of cholesteryl ester transfer protein inhibition with evacetrapib in patients at high-risk for vascular outcomes: Rationale and design of the ACCELERATE trial. Am Heart J. 2015;170(6):1061–9.
Hps Timi Reveal Collaborative Group, Bowman L, Hopewell JC, et al. Effects of Anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–27.
Group HTRC, Writing C, Sammons E, et al. Long-term safety and efficacy of anacetrapib in patients with atherosclerotic vascular disease. Eur Heart J. 2022;43(14):1416–24.
Inazu A, Jiang XC, Haraki T, et al. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J Clin Invest. 1994;94(5):1872–82.
Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of Genetic Variants Related to CETP inhibitors and statins with lipoprotein levels and Cardiovascular Risk. JAMA. 2017;318(10):947–56.
Holmes MV, Smith GD. Dyslipidaemia: revealing the effect of CETP inhibition in cardiovascular disease. Nat Rev Cardiol. 2017;14(11):635–6.
Nelson AJ, Sniderman AD, Ditmarsch M et al. Cholesteryl Ester Transfer Protein Inhibition reduces major adverse Cardiovascular events by lowering apolipoprotein B levels. Int J Mol Sci 2022, 23(16).
Hovingh GK, Kastelein JJ, van Deventer SJ, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386(9992):452–60.
Nicholls SJ, Ditmarsch M, Kastelein JJ, et al. Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: a randomized phase 2 trial. Nat Med. 2022;28(8):1672–8.
Ballantyne CM, Ditmarsch M, Kastelein JJ, et al. Obicetrapib plus Ezetimibe as an adjunct to high-intensity statin therapy: a randomized phase 2 trial. J Clin Lipidol. 2023;17(4):491–503.
Nicholls SJ, Nelson AJ, Ditmarsch M, et al. Obicetrapib on top of maximally tolerated lipid-modifying therapies in participants with or at high risk for atherosclerotic cardiovascular disease: rationale and designs of BROADWAY and BROOKLYN. Am Heart J. 2024;274:32–45.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006.
Fogacci F, Ferri N, Toth PP, Ruscica M, Corsini A, Cicero AFG. Efficacy and safety of Mipomersen: a systematic review and Meta-analysis of Randomized clinical trials. Drugs. 2019;79(7):751–66.
Underberg JA, Cannon CP, Larrey D, Makris L, Blom D, Phillips H. Long-term safety and efficacy of lomitapide in patients with homozygous familial hypercholesterolemia: five-year data from the Lomitapide Observational Worldwide evaluation Registry (LOWER). J Clin Lipidol. 2020;14(6):807–17.
Lim GB. ANGPTL3 inhibition for hypercholesterolaemia. Nat Reviews Cardiol. 2021;18(2):72.
Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med. 2017;377(3):211–21.
Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20.
Carugo S, Sirtori CR, Gelpi G, Corsini A, Tokgozoglu L, Ruscica M. Updates in small interfering RNA for the treatment of Dyslipidemias. Curr Atheroscler Rep. 2023;25(11):805–17.
Rosenson RS, Gaudet D, Hegele RA, et al. Zodasiran, an RNAi therapeutic targeting ANGPTL3, for mixed hyperlipidemia. N Engl J Med. 2024;391(10):913–25.
Kayikcioglu M. LDL apheresis and lp (a) apheresis: a clinician’s perspective. Curr Atheroscler Rep. 2021;23(4):15.
Nelson AJ, Pagidipati NJ, Bosworth HB. Improving medication adherence in cardiovascular disease. Nat Reviews Cardiol. 2024;21(6):417–29.
Ballantyne CM, Agarwala A. Reinstating LDL-C measurement as a Quality Metric: this is the way. JACC Adv. 2024;3(1):100749.
Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380–90.
Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–34.
Acknowledgements
NA.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
This was received no funding.
Author information
Authors and Affiliations
Contributions
Both authors wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
No animal or human subjects were used in this study.
Competing Interests
SJN has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx and Sanofi-Regeneron and is a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Daiichi Sankyo, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, Novo Nordisk, CSL Sequiris and Vaxxinity. AJN has received research support from AstraZeneca, Amgen, Eli Lilly, Novartis and is a consultant for Amgen, AstraZeneca, Boehringer Ingelheim, CSL Sequiris, Eli Lilly, GSK, Sanofi Pasteur and Novo Nordisk.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicholls, S.J., Nelson, A.J. Achieving More Optimal Lipid Control with Non-Statin Lipid Lowering Therapy. Curr Atheroscler Rep 27, 32 (2025). https://doi.org/10.1007/s11883-025-01280-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-025-01280-4