Abstract
Purpose of Review
Despite significant efforts to understand pathophysiological processes underlying cardiac diseases, the molecular causes for the most part remain unresolved. Rapid advancements in -omics technologies, and their application in cardiac research, offer new insight into cardiac remodeling in disease states. This review aims to provide an accessible overview of recent advances in omics approaches for studying cardiac remodeling, catering to readers without extensive prior expertise.
Recent Findings
We provide a methodologically focused overview of current methods for performing transcriptomics and proteomics, including their extensions for single-cell and spatial measurements. We discuss approaches to integrate data across modalities, resolutions and time. Key recent applications within the cardiac field are highlighted.
Summary
Each -omics modality can provide insight, yet each existing experimental method has technical or conceptual limitations. Integrating data across multiple modalities can leverage strengths and mitigate weaknesses, ultimately enhancing our understanding of cardiac pathophysiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease remains a leading cause of morbidity and mortality worldwide [1], necessitating innovative approaches to unravel its etiologies. -Omics approaches enable data driven strategies that can capture the multifaceted nature of disease drivers. Modern molecular biology has become a largely technologically driven endeavor [2] and with each generation of advancements in -omics technologies, science has achieved deeper insights into disease mechanisms.
Multi-omic approaches, which integrate data across different -omics modalities, offer a strategy to disentangle the processes driving cardiac conditions beyond what a single modality can achieve in isolation. This is especially the case when considering the relative strengths and weaknesses of current technological methods to measure different -omics modalities. This review discusses the information that can be gained from the various modalities of -omics data, with a primary focus on the transcriptome and the proteome, and the benefits of combining them. We highlight current advances in -omic technologies, data integration strategies, and their recent applications in cardiac research.
Moving Beyond the Genome
The initial wave of insights in heart disease from -omics methodologies emerged from the field of genomics, which significantly advanced our understanding of inherited cardiac diseases. Genomic studies have elucidated critical proteins and pathways involved in the etiology of cardiac diseases, providing valuable information from both monogenic disorders and association studies that identify genetic predispositions to various cardiac conditions. For instance, studies of monogenic disease have classified key genes encoding ion channels or their interactors as definitively causative of long QT syndrome [3]. Genome-wide association studies (GWAS) have increased our understanding of heritable disorders of complex etiologies. An early example was the association of variations in BAG3 with dilated cardiomyopathy [4], but there are now insights into many cardiac diseases including heart failure [5] and atrial fibrillation [6]. Many variants associated with cardiac disease however remain of unknown significance or are without annotated causal genes and thus are of limited clinical utility until we can determine their pathogenicity and characterise their mechanistic role.
For acquired or lifestyle-associated cardiac diseases, insights must be derived from molecular layers beyond the genome. In contrast to the approximately static inherited DNA sequence, the downstream transcriptome and thence proteome change according to the interaction between an individual’s genome and environment. These molecular modalities therefore provide more information with which to elucidate pathophysiological processes (Fig. 1A).
A. Research scientists commonly aim to explain the molecular mechanisms underpinning phenotypes. The “central dogma” of biology explains how information encoded in the genome is first transcribed and then translated into proteins that ultimately determine phenotypes. However, at each stage additional information is incorporated (with some exemplar additional mechanisms shown) that means measurements correspond more directly to phenotypic outcomes. B. The correlation between transcript and protein abundance measured in the heart tissue of a single donor by Wang et al. [13], where each datapoint corresponds to a single gene. For some genes, such as PKP2 in the upper inset, transcript levels strongly correlate with protein abundance (R2 = 0.78), indicating that most regulation occurs at the transcriptional level. For others, such as PRDX1 in the lower inset, transcript levels show little correlation with protein abundance (R2 = 0.04), suggesting that post-transcriptional regulation plays a larger role. C. From samples of cardiac tissue, -omics methods can be applied to bulk samples, or variants now allow measurements to be resolved by cell or within space
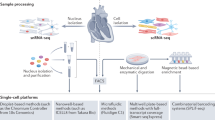
Bulk Transcriptomics and Proteomics
Bulk Transcriptomics
Transcriptomics technologies followed upon the back of genomic technologies and are likewise facilitated by the ability to amplify small amounts of starting material using polymerase chain reaction (PCR). RNAseq has become the mature technology of choice, being relatively affordable and now also widely accessible. Moreover, there are mature pipelines adequate for analysis of the data generated [7, 8]. Bulk approaches are used here to refer to those that measure the total sum of a given transcript within a sample, such as a tissue biopsy, in effect averaging gene expression across all constituent parts.
We can begin to assess cardiac dysregulation in various disease states through bulk transcriptomics data obtained from human cardiac biopsies of diverse patient backgrounds. RNAseq has been used to characterise subtypes within cardiomyopathies and has revealed distinct gene expression signatures unique to heart failure with preserved and reduced ejection fraction [9], as well as specific expression profiles in dilated and ischemic cardiomyopathy patient biopsies [10]. Analysing gene expression patterns associated with specific heart conditions enables us to characterise these diseases and gain insights into their potential underlying causes.
The underlying assumption of profiling mRNA to identify genes with altered expression is that mRNA abundance serves as a good predictor of the corresponding protein levels. However, correlation between mRNA expression levels and protein abundance is relatively weak, with typical Pearson correlation coefficients around 0.4 [11, 12]. Figure 1B shows the abundance of mRNA plotted against that of the corresponding protein, measured in heart tissue by Wang et al. [13]. For some genes, such as PKP2 in the upper inset, mRNA abundance is excellent at predicting protein abundance, whereas for other such as PRDX1 in the lower inset there is little predictive value of mRNA abundance. Whilst these measurements were taken in steady state conditions, there fortunately appears to be a better correlation between mRNA and protein levels when evaluating changes across conditions. Nonetheless, one way to address this problem is to measure protein abundance directly, rather than to utilise mRNA abundance as a proxy.
Bulk Proteomics
Proteomics presents a significantly greater technical challenge than transcriptomics. Firstly, there exists no current equivalent of PCR in order to amplify the amount of protein present in a sample. Quantification is then also much more difficult due to higher dynamic range of protein expression in comparison to transcripts, encompassing an additional ~ 3 orders of magnitude [14]. It is worth noting that the heart presents a particular challenge here, due to the specialisation of the tissue for contractile and supporting functions, meaning that just the 10 most abundant proteins comprise almost 20% of the total measured protein abundance.
Mass spectrometry (MS) has emerged as the method of choice for proteomics studies, allowing unbiased and (at least in theory) system wide identification and quantification of proteins. Recent improvements in MS technology have significantly increased the number of proteins that can be quantified, with current capabilities exceeding 10,000 protein groups in the heart [15] and quantification of 6–7,000 proteins in cardiac tissue is achievable using routine workflows by specialist groups [16,17,18,19].
Using MS-based proteomics on human cardiac biopsies, researchers can distinguish proteomic profiles of cardiomyopathies and track changes associated with disease progression. This approach has been employed to distinguish cardiac proteome profiles of ischemic from dilated cardiomyopathies [20] and to differentiate amyloidosis subtypes [21]. In the same manner as with transcriptomics data, altered molecular pathways can be identified based upon the relative changes in protein abundance between disease and control groups. For instance, this has led to insights into DNA damage processes being involved in arrhythmogenic cardiomyopathy [17] and of inflammation driving the electrical remodeling in sinus node dysfunction in heart failure [16].
Protein abundance is a correlate of function, but a proteins activity is also determined by both a suite of post-translational modifications and the other proteins that it interacts with. Further than quantifying protein abundance, proteomics can also be used to quantify changes in post translational modifications to proteins [22, 23]. In cardiac pathologies, quantitative phosphoproteomics has been used to outline how signaling networks in failing hearts are re-wired with beta-blocker and ACE inhibitor treatment [24] and to unveil a mechanism for exercise- or catecholaminergic induced arrythmias in arrhythmogenic cardiomyopathy [25]. The methodology also enables outlining of phosphorylation-mediated signaling networks in cardiac tissue or cells [26, 27]. Proteomics has also been used to outline protein–protein interaction networks of importance for cardiac physiology [28].
Biomarkers
Whilst measurement of molecular abundance within tissue is essential to the study of disease mechanisms, proteomics is increasingly employed in peripheral blood serum or plasma in order to identify biomarkers that are predictive of disease diagnosis or prognosis. Peripheral blood contains factors that are released by the heart, but this signal will be convoluted by the simultaneous release and uptake from other tissues. A canonical approach to biomarker discovery is therefore to identify factors that are secreted into the circulation whose expression is specific to the heart tissue, as was the case for example for cardiac troponins [29]. By integrating our existing knowledge in this manner to improve specificity for cardiac disease, we can help to avoid some of the issues of the low statistical power with which biomarker studies have historically been performed [30]. This is especially the case when studies are performed directly in humans, who exhibit higher variation than that seen in controlled experiments utilising model organisms.
Large scale biobanks are changing this paradigm, enabling biomarker discovery at population scale [31]. Analogies can be drawn to how the genomics field addressed the poor reproducibility of small scale genetic association studies [32] to pave the way for modern GWAS with their much greater statistical rigor [33]. The chief challenge of proteomics in the blood plasma is the even higher dynamic range than in heart tissue [30]. Again there have been technological advancements to enable performing proteomics at this scale, through either aptamer- (e.g. SomaScan (somalogic, USA); [34]) or antibody-based proteomics (e.g. Olink (Olink Proteomics, Sweden); [35]), or advances in sample preparation before mass spectrometry based proteomics (e.g. SEER Proteograph (SEER, USA); [36]). The UK Biobank plasma proteome project has led the way, measuring 1463 plasma proteins in 54,306 individuals [31]. Schuermans and colleagues leverage this dataset in order to calculate composite biomarker scores for 4 common cardiac diseases [37]. This approach can be particularly powerful with the integration of genetic sequence data, which allows the use of mendelian randomisation methods to assess which protein-disease relationships are supported by causal evidence. These technologies can also be employed in targeted patient populations, as exemplified by the examination of the impact of the SGLT2 inhibitor empagliflozin upon the circulating proteome during the EMPEROR heart failure clinical trials [38,39,40].
Beyond Assuming Tissue Homogeneity
Historically, bulk approaches as discussed so far have been necessary in order to gather enough input material for -omics methods. These continue to have notable advantages in data quality when quantifying species abundance, minimising technological noise and in particular in proteomics increasing measurement “depth”: the number of analytes that are quantified in an experiment.
However, bulk approaches make the (patently incorrect) assumption that species abundance is homogenous throughout a tissue sample, averaging signals from mixed cell populations and across different regions in space. Cell type specific changes may therefore be masked, particularly as the signal from rare but pathophysiologically relevant cell types or states is diluted. The interpretation of bulk data therefore benefits from complementary cell-specific approaches to provide this insight (Fig. 1C).
Cell-type Resolved Measurements
Single Cell Transcriptomics
Advances in multiplexing in transcriptomics methods have revolutionised cardiac research, enabling gene expression to be measured within single cells [41]. This has allowed refined characterisations of cardiac cell populations, such as outlining transitions in fibroblast states in failing hearts [42]. It has also provided insights beyond cardiomyocytes and fibroblasts in failing hearts [43], as well as expanded our understanding of the role of inflammatory cells in atrial fibrillation [44].
The progression in the field has involved first mapping the cell populations present in the heart, followed by detailed information on regional and anatomical niches, and then studying adaptations in specific cell populations in cardiac disease states. High-quality sample preparation is a prerequisite for single-cell transcriptomics measurements. Due in large to the fibrous nature of heart tissue, dissociating individual cells across all cell types using a single dissociation approach is challenging. An alternate approach is to isolate nuclei and sequence nuclear transcripts, which however results in the loss of all extranuclear RNA content. Each isolation process will lead to over- and under-representation of certain cell types. Several isolation approaches have been combined to create an atlas describing the cells of the healthy human heart [43, 45, 46]. These datasets represent a major technological achievement and comprise huge numbers of cells and nuclei (704,296 in the current version of the heart cell atlas [46]) which has vastly increased our understanding of the heterogeneity within heart regions and between different cell types. Atlas approaches are also being extended to include disease states in the heart. For example, Reichart et al. present a cellular atlas of dilated cardiomyopathy containing 880,000 nuclei from 61 patients with non ischaemic cardiomyopathy (with or without annotated pathogenic genetic variants) and 18 controls [47].
Currently, there remains a tradeoff that must be made between (very) high degrees of multiplexing allowing the analysis of many cells or nuclei in parallel on the one hand, and the introduction of technical artefacts in the data (such as “sparsity”: a disproportionate number of zeros in a dataset) on the other. The number of cells or nuclei sequenced and the number of patients are likely to increase with current technological developments focusing on more convenient sample preparation protocols. These advancements will allow these methods to scale towards bigger and more clinically relevant studies. While a higher number of cells or nuclei is desirable, it is important for statistical rigour that the relevant n remains the biological sample size, and not the number of cells or nuclei in an experiment [48].
Single Cell Proteomics
A generational change has recently occurred in mass spectrometers [49, 50], representing an “inflection point” [51] in our ability to perform proteomics with increasingly low amounts of input material. Coupled with sample preparation [52] and cell sorting technologies [53], this is beginning to make single cell proteomics feasible without severe compromises in measurement depth. Single cell proteomics has now for example been employed to characterise heterogeneity within induced pluripotent stem cells [54]. Although still emerging, these technologies are beginning to be applied in cardiac research, with Kreimer and colleagues measuring > 1000 protein groups in 92 single cardiomyocytes isolated from a single human donor at a throughput of 96 cells per day [55]. The major technical challenge lies in liberating single cells from cardiac tissue without introducing a selection bias. Further than the fibrous nature of cardiac tissue, the large size of cardiomyocytes adds a layer of complexity to isolating and sorting cells as they are too big for traditional FACS or MACS approaches. Downstream sample preparation before MS analysis is becoming increasingly automated, which is crucial to minimise technological variation that would otherwise be introduced during manual handing [53]. Quantifying protein changes at cellular resolution in cardiac disease states will be a next leap forward likely happening within the next couple of years.
Spatially Resolved Measurements
Spatial Transcriptomics
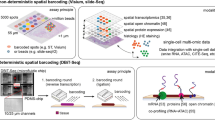
Spatial resolution provides additional value beyond the cellular context of gene expression and facilitates the generation and testing of hypotheses about how the local (micro)environment influences gene expression. This is done under the heuristic that proximity is a good proxy for interactions. The experimental possibilities that these methods have made tractable led to spatial transcriptomics being declared Nature method of the year in 2021 [56]. Spatial transcriptomics was first applied in the human heart by Kuppe et al., who used it in combination with single cell transcriptomics to evaluate changes in cell type and cell state composition after myocardial infarction, and how this varies with proximity to both other cell types and their proximity to the ischaemic zone [57]. Kanemaru and colleagues use a similar approach to map cells to microanatomical structures such as the sinoatrial node, providing new insight into the compartmentalisation of cells within it [46].
Spatial Proteomics
Spatial proteomics is an emerging field that enables quantitative measurement of protein distribution within tissue sections, analogous to approaches used in spatial transcriptomics. The potential value of this information is again underscored by spatial proteomics being declared Nature method of the year, this time in 2024 [58].
Just as the advancement of mass spectrometers and sample preparation workflows has enabled single cell proteomics, the suitability of these methods for low amounts of input material means they can equally be employed to resolve protein abundance in space. This is achieved most simply using the established method of laser microdissection [59] to dissect regions of interest from tissue sections before proceeding with MS based proteomic workflows [60]. Crucially, the increased sensitivity of modern methods means that extensive pooling of tissue areas or samples is no longer required, and that spatial resolution can be high enough to deliver genuine insight: even at cellular resolution. This enables for example the characterisation of distinct cell types while preserving their spatial relationships, offering valuable insights into cellular interactions within their native environment.
Other strategies towards spatial proteomics that build upon traditional imaging approaches utilising antibody based immunohistochemistry to detect specific proteins also exist. Current advancements are rapidly increasing the number of analytes that can be multiplexed within a single sample. Quantitation can then be performed by a growing range of methods that include cyclic immunofluorescence [61], co-detection by indexing [62], multiplexed ion beam imaging [63] and imaging mass cytometry (IMC), the latter feasible to perform even in 3 dimensions [64].
A recent development seeks to combine multiplexed imaging with mass spectrometry: coined as “deep visual proteomics” (DVP). This first employs a small panel of markers, enabling in silico segmentation of tissue into its constituent cells and microenvironments based upon image analysis using deep neural networks. Laser microdissection then allows subsequent proteomic analysis by MS proteomics as discussed previously [65]. DVP has to date only been employed outside of the cardiac field, where it has made previously intractable hypothesis generating experiments possible. Validated insights have so far been provided into primary melanoma using archived tissue samples [66], and in a single composite case of small lymphocytic and classical Hodgkin lymphoma [67] in order to inform personalised medicine approach. Having demonstrated proof-of-concept, it is ripe to be exploited within the cardiac field.
Approaches to Integrate Diverse Datasets
There are myriad different ways to integrate multiple -omics datasets, and as is often the case mature and consensus strategies to robustly analyse data lag behind our ability to generate the datasets themselves.
Principled Integration
The most common approaches to data integration are principled approaches that integrate different -omics modalities based upon our dogmatic knowledge of which information each modality can best espouse: what information for example does single cell transcriptomics provide, that is orthogonal and complimentary to protein bulk protein abundance? By integrating our prior knowledge in this manner, we are required to make fewest additional assumptions of our datasets.
Integrating RNAseq and proteomics provides a more comprehensive molecular picture than either modality alone. Due to the costs involved, -omics experiments typically utilise small sample sizes and so we can have increased confidence if findings are seen across multiple molecular modalities. We emphasise that the quantification of gene product abundance by transcriptomic and proteomics are not redundant and there is therefore a notable cost of a high false negative rate with this approach, as proteins whose abundance is not regulated primarily by transcription may not show concordant regulation (Fig. 1A-B).
Principled integration also allows flexibility to draw in information from external datasets, including across organisms. For example, we demonstrated that integrating bulk proteomics data with single cell transcriptomics data enables the prediction of the cell populations from which the measured proteins are most likely to originate: generating insight despite the current challenges in measuring cell resolved protein abundance in the heart directly [16, 28].
Genetic evidence can similarly be integrated via simple intersection [68, 69]. There is good evidence for the utility of this practice, with drugs developed with such supporting evidence from human genetics at least twice as likely to be approved than those without [70]. We utilised human GWAS in order to confirm relevance to the human electrocardiogram of proteins whose abundance is changed in a mouse model of heart failure with concurrent sinus node dysfunction [16], and to prioritise for functional importance among protein interactors of cardiac ion channels [28]. Major initiatives are underway in order to better curate such datasets into databases and so facilitate their use by researchers without the required technical expertise. Examples focus primarily upon genetic information [71], protein expression [72, 73] or molecular signaling pathways [74]. The advent of databases with such broad scope is beginning to reduce the domain expertise required by researchers to manually curate such information themselves, and efforts to integrate some of this analysis directly into analytic pipelines [75] will help overcome what is commonly a bottleneck in -omics experiments.
Data Driven Integration Strategies
An alternative approach to principled integration is to rely more heavily on the data themselves and employ data driven integration strategies. The principle strengths and drawbacks of these approaches are captured eloquently by Miao et al.: “In a sense, machine learning methods completely avoid the careful modelling of mechanisms and instead apply a generically complex model to a very large reference dataset to produce a well-performing model with unknown parts” [76].
These will become increasingly powerful as the costs of experimental methods reduce and larger, more statistically powerful studies emerge [77]. -Omics datasets already present a statistical challenge, since the number of analytes measured is vastly greater than the number of statistical replicates (often referred to as the “curse of dimensionality”). This is especially true at the technical bleeding edge, where experimental costs are greatest and hence sample sizes often smallest. Datasets of genuine size are already beginning to present computational challenges, with methods needing to scale for example to analyse millions cells and hence tens of billions of datapoints within a single project [76].
Across Modalities
Data driven approaches to integrate different -omics modalities are nascent, and parallels may be drawn to analytic pipelines for individual -omics datasets, where in many cases it has taken a period of time after technological innovations for us to understand the characteristics of the data produced well enough to analyse it in a manner that reaches real rather than spurious conclusions [33, 78, 79]. Data driven approaches may currently be thought of as generating hypotheses about interactions. These can broadly be categorised into those that integrate different molecular modalities early in the analytic strategy (which provide the most flexibility for models to uncover novel molecular interactions) and those that integrate datasets late in the pipeline [80]. The latter better consider technical variation, a majority of which is assay specific. A theoretical tradeoff must be made between the flexibility of a model and its tendency to “overfit” a dataset: a very flexible model may interpret the experimental data so closely that predictions do not generalise to external or future data. Commonly, these methods seek to interpret such highly dimensional input by embedding into a lower dimensional space. Argelaguet and colleagues introduce what they describe can be intuited as a “versatile and statistically rigorous generalization of principal component analysis”, in which the lower dimensional space identified using factor analysis [81]. Allesøe and colleagues use generative deep learning based models based upon variational autoencoders to embed multiple datasets into a low dimensional space [82]. The generative nature of these models however further allows in silico investigation of the impact of perturbations to help identify associations across -omics modalities.
Across Time
The studies discussed so far in large have a cross-sectional design, where two or more groups (such as disease and control) are compared. A cross-sectional design is useful for identifying molecules significantly associated with a disease state and can provide valuable knowledge on what biological processes are associated with the disease. In contrast, if an experiment is designed with repeated measurements over time (such as repeated protocol biopsies following heart transplantation) it is possible to identify how and when molecules change. It is then possible to define clusters of molecules that have the same dynamic behavior and by application of the principle of “guilt by association” infer likely functional relationships. By analysing trajectories over time we can address the order in which changes occur, and begin to disentangle cause and effect at the molecular level. Whilst interventional evidence remains the gold standard to infer causality, observing for example that process A occurs after process B means that it is impossible that A causes B. In this manner, time series data help to prioritise candidates for additional follow-up experiments.
The most prevalent approach to analyse -omics data is univariate: to analyse the trajectory of each analyte separately. This is typically done using regression models due to their flexibility to accommodate many experimental designs within a single framework [7, 8, 83]. The specification of the appropriate regression model depends on the study design. For time series data, it is especially important to determine whether there are repeated measurements of the same statistical unit (e.g. patient, animal) or whether each measurement is made independently. If measurements are taken serially from the same subject and this is not taken into account during analysis, the assumption of independence is violated and hence an ordinary regression model is invalid. A typical choice instead is to use linear mixed-effects models to account for within-subject correlation [84, 85]. However, for some experimental designs covariance pattern models might better capture typical covariance structures in time series data, with implementation such as lmmStar [86] or nlme [87] available for R [88]. If there are no repeated measurements in the study, it is advisable to use a weighted least squares fit, since variance tends to increase over time in longitudinal data [89].
Conclusion and Outlook
Here, we have reviewed recent technological developments in our ability to measure the proteome and the transcriptome in bulk, in single cells and across space, and highlighted their recent applications in the study of cardiac diseases. In addition to the focus here, other types of -omics data such as epigenomics and metabolomics and derivatives of all of the above each provide distinct insights into the biological system. Only by integrating multiple complimentary -omics modalities, and resolving their measurement across space, time and by cell type of origin, can we begin to understand pathophysiology at a systems level. As a greater number of datasets with increasingly large samples sizes are acquired, the next significant challenge lies in data analysis and interpretation. We must continue to develop strategies to most effectively utilise and integrate these diverse pieces of information in order to understand the dysregulation underlying cardiac disease states and hence develop new therapeutic strategies to ultimately benefit patients.
Key References
-
Schuermans A, Pournamdari AB, Lee J, Bhukar R, Ganesh S, Darosa N, et al. Integrative proteomic analyses across common cardiac diseases yield mechanistic insights and enhanced prediction. Nat Cardiovasc Res. 2024 Nov 21;1–15.
-
Utilising the UK Biobank to combine plasma proteomics with phenotypes, Schuermans and colleagues identify associations between plasma proteomics and 4 common cardiac diseases. Evidence of causality is assessed through the use of mendelian randomisation by combination with genomic data.
-
Kuppe C, Ramirez Flores RO, Li Z, Hayat S, Levinson RT, Liao X, et al. Spatial multi-omic map of human myocardial infarction. Nature. 2022 Aug;608(7924):766–77.
-
Provide the first use of spatial transcriptomics in the human heart, which is interpreted in concert with single cell gene expression and single cell chromatin accessibility data from the same samples. They provide new insight into interactions between cell types and states after myocardial infarction.
-
Kanemaru K, Cranley J, Muraro D, Miranda AMA, Ho SY, Wilbrey-Clark A, et al. Spatially resolved multiomics of human cardiac niches. Nature. 2023 Jul;619(7971):801–10.
-
Combine single cell and spatial transcriptomics to characterise microanatomic regions of the healthy heart, focusing upon the cardiac conduction system.
Data Availability
Data underlying Fig. 1B is available in the supplementary table EV6 of [13], and code to reproduce the figure available at: https://doi.org/10.5281/zenodo.14901617.
References
Institute for Health Metrics and Evaluation (IHME). Global burden of disease 2021: findings from the GBD 2021 study [Internet]. Seattle, WA: IHME; 2024. Available from: https://www.healthdata.org/research-analysis/library/global-burden-disease-2021-findings-gbd-2021-study. Accessed 8 Jan 2025.
Hood L. A Personal View of Molecular Technology and How It Has Changed Biology. J Proteome Res. 2002;1(5):399–409.
Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation. 2020;141(6):418–28.
Villard E, Perret C, Gary F, Proust C, Dilanian G, Hengstenberg C, et al. A Genome-Wide Association Study Identifies Two Loci Associated with Heart Failure Due to Dilated Cardiomyopathy. Eur Heart J. 2011;32(9):1065–76.
Levin MG, Tsao NL, Singhal P, Liu C, Vy HMT, Paranjpe I, et al. Genome-Wide Association and Multi-Trait Analyses Characterize the Common Genetic Architecture of Heart Failure. Nat Commun. 2022;13(1):6914.
Miyazawa K, Ito K, Ito M, Zou Z, Kubota M, Nomura S, et al. Cross-Ancestry Genome-Wide Analysis of Atrial Fibrillation Unveils Disease Biology and Enables Cardioembolic Risk Prediction. Nat Genet. 2023;55(2):187–97.
Law CW, Chen Y, Shi W, Smyth GK. Voom: Precision Weights Unlock Linear Model Analysis Tools for Rna-Seq Read Counts. Genome Biol. 2014;15(2):R29.
Love MI, Huber W, Anders S. Moderated Estimation of Fold Change and Dispersion for Rna-Seq Data with DESeq2. Genome Biol. 2014;15(12):550.
Hahn VS, Knutsdottir H, Luo X, Bedi K, Margulies KB, Haldar SM, et al. Myocardial Gene Expression Signatures in Human Heart Failure With Preserved Ejection Fraction. Circulation. 2021;143(2):120–34.
Verdonschot JAJ, Wang P, Derks KWJ, Adriaens ME, Stroeks SLVM, Henkens MTHM, et al. Clustering of Cardiac Transcriptome Profiles Reveals Unique: Subgroups of Dilated Cardiomyopathy Patients. JACCL Basic Trans Sci. 2023;8(4):406–18.
Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165(3):535–50.
Buccitelli C, Selbach M. mRNAs, Proteins and the Emerging Principles of Gene Expression Control. Nat Rev Genet. 2020;21(10):630–44.
Wang D, Eraslan B, Wieland T, Hallström B, Hopf T, Zolg DP, et al. A Deep Proteome and Transcriptome Abundance Atlas of 29 Healthy Human Tissues. Mol Syst Biol. 2019;15(2):e8503.
Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global Quantification of Mammalian Gene Expression Control. Nature. 2011;473(7347):337–42.
Doll S, Dreßen M, Geyer PE, Itzhak DN, Braun C, Doppler SA, et al. Region and Cell-Type Resolved Quantitative Proteomic Map of the Human Heart. Nat Commun. 2017;8(1):1469.
Kahnert K, Soattin L, Mills RW, Wilson C, Maurya S, Sorrentino A, et al. Proteomics Couples Electrical Remodelling to Inflammation in a Murine Model of Heart Failure with Sinus Node Dysfunction. Cardiovasc Res. 2024;120(8):927–42.
Pérez-Hernández M, van Opbergen CJM, Bagwan N, Vissing CR, Marrón-Liñares GM, Zhang M, et al. Loss of Nuclear Envelope Integrity and Increased Oxidant Production Cause DNA Damage in Adult Hearts Deficient in PKP2: A Molecular Substrate of ARVC. Circulation. 2022;146(11):851–67.
Linscheid N, Santos A, Poulsen PC, Mills RW, Calloe K, Leurs U, et al. Quantitative Proteome Comparison of Human Hearts with Those of Model Organisms. PLoS Biol. 2021;19(4):e3001144.
Linscheid N, Poulsen PC, Pedersen ID, Gregers E, Svendsen JH, Olesen MS, et al. Quantitative Proteomics of Human Heart Samples Collected In Vivo Reveal the Remodeled Protein Landscape of Dilated Left Atrium Without Atrial Fibrillation. Mol Cell Proteomics. 2020;19(7):1132–44.
Li M, Parker BL, Pearson E, Hunter B, Cao J, Koay YC, et al. Core Functional Nodes and Sex-Specific Pathways in Human Ischaemic and Dilated Cardiomyopathy. Nat Commun. 2020;11(1):2843.
Kourelis TV, Dasari SS, Dispenzieri A, Maleszewski JJ, Redfield MM, Fayyaz AU, et al. A Proteomic Atlas of Cardiac Amyloid Plaques. JACC CardioOncol. 2020;2(4):632–43.
Kim JC, Pérez-Hernández M, Alvarado FJ, Maurya SR, Montnach J, Yin Y, et al. Disruption of Ca2+i Homeostasis and Cx43 Hemichannel Function in the Right Ventricle Precedes Overt arrhythmogenic Cardiomyopathy in PKP2-Deficient Mice. Circulation. 2019;140(12):1015–30.
Phadke K, D’Anna S, Torres Vega E, Xiao J, Lin X, Zhang M, et al. Atrial cardiomyopathy resulting from loss of plakophilin-2 expression: response to adrenergic stimulation and implications for the exercise response. J Physiol. 2024. Available from: https://doi.org/10.1113/JP286985.
Sorrentino A, Bagwan N, Linscheid N, Poulsen PC, Kahnert K, Thomsen MB, et al. Beta-blocker/ACE Inhibitor Therapy Differentially Impacts the Steady State Signaling Landscape of Failing and Non-Failing Hearts. Sci Rep. 2022;12(1):4760.
van Opbergen CJM, Bagwan N, Maurya SR, Kim JC, Smith AN, Blackwell DJ, et al. Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2–Deficient Hearts. Circulation. 2022;145(19):1480–96.
Lundby A, Andersen MN, Steffensen AB, Horn H, Kelstrup CD, Francavilla C, et al. In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci Signal. 2013;6(278):ra11.
Achter JS, Vega ET, Sorrentino A, Kahnert K, Galsgaard KD, Hernandez-Varas P, et al. In-depth phosphoproteomic profiling of the insulin signaling response in heart tissue and cardiomyocytes unveils canonical and specialized regulation. Cardiovasc Diabetol. 2024;23(1):258.
Maurya S, Mills RW, Kahnert K, Chiang DY, Bertoli G, Lundegaard PR, et al. Outlining cardiac ion channel protein interactors and their signature in the human electrocardiogram. Nat Cardiovasc Res. 2023;2(7):673–92.
Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med. 2016;4(10):194.
Geyer PE, Holdt LM, Teupser D, Mann M. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol. 2017;13(9):942.
Sun BB, Chiou J, Traylor M, Benner C, Hsu YH, Richardson TG, et al. Genetic regulation of the human plasma proteome in 54,306 UK Biobank participants [Internet]. bioRxiv; 2022;2022.06.17.496443. https://doi.org/10.1101/2022.06.17.496443v1
Ioannidis JPA, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29(3):306–9.
The Wellcome Trust Case Control Consortium. Genome-Wide Association Study of 14,000 Cases of Seven Common Diseases and 3,000 Shared Controls. Nature. 2007;447(7145):661.
Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-Based Multiplexed Proteomic Technology for Biomarker Discovery. PLoS ONE. 2010;5(12):e15004.
Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE. 2014;9(4):e95192.
Blume JE, Manning WC, Troiano G, Hornburg D, Figa M, Hesterberg L, et al. Rapid, Deep and Precise Profiling of the Plasma Proteome With Multi-Nanoparticle Protein Corona. Nat Commun. 2020;11(1):3662.
Schuermans A, Pournamdari AB, Lee J, Bhukar R, Ganesh S, Darosa N, et al. Integrative Proteomic Analyses Across Common Cardiac Diseases Yield Mechanistic Insights and Enhanced Prediction. Nat Cardiovasc Res. 2024;21:1–15.
Zannad F, Ferreira JP, Butler J, Filippatos G, Januzzi JL, Sumin M, et al. Effect of Empagliflozin on Circulating Proteomics in Heart Failure: Mechanistic Insights Into the EMPEROR Programme. Eur Heart J. 2022;43(48):4991–5002.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451–61.
Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, et al. Massively Parallel Digital Transcriptional Profiling of Single Cells. Nat Commun. 2017;8(1):14049.
Chaffin M, Papangeli I, Simonson B, Akkad AD, Hill MC, Arduini A, et al. Single-Nucleus Profiling of Human Dilated and Hypertrophic Cardiomyopathy. Nature. 2022;608(7921):174–80.
Koenig AL, Shchukina I, Amrute J, Andhey PS, Zaitsev K, Lai L, et al. Single-Cell Transcriptomics Reveals Cell-Type-Specific Diversification in Human Heart Failure. Nat Cardiovasc Res. 2022;1(3):263–80.
Hulsmans M, Schloss MJ, Lee IH, Bapat A, Iwamoto Y, Vinegoni C, et al. Recruited Macrophages Elicit Atrial Fibrillation. Science. 2023;381(6654):231–9.
Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. Cells of the Adult Human Heart. Nature. 2020;588(7838):466–72.
Kanemaru K, Cranley J, Muraro D, Miranda AMA, Ho SY, Wilbrey-Clark A, et al. Spatially Resolved Multiomics of Human Cardiac Niches. Nature. 2023;619(7971):801–10.
Reichart D, Lindberg EL, Maatz H, Miranda AMA, Viveiros A, Shvetsov N, et al. Pathogenic Variants Damage Cell Composition and Single Cell Transcription in Cardiomyopathies. Science. 2022;377(6606):eabo1984.
Zimmerman KD, Espeland MA, Langefeld CD. A Practical Solution to Pseudoreplication Bias in Single-Cell Studies. Nat Commun. 2021;12(1):738.
Guzman UH, Martinez-Val A, Ye Z, Damoc E, Arrey TN, Pashkova A, et al. Ultra-Fast Label-Free Quantification and Comprehensive Proteome Coverage with Narrow-Window Data-Independent Acquisition. Nat Biotechnol. 2024;1:1–12.
Heil LR, Damoc E, Arrey TN, Pashkova A, Denisov E, Petzoldt J, et al. Evaluating the Performance of the Astral Mass Analyzer for Quantitative Proteomics Using Data-Independent Acquisition. J Proteome Res. 2023;22(10):3290–300.
Hendricks NG, Bhosale SD, Keoseyan AJ, Ortiz J, Stotland A, Seyedmohammad S, et al. An Inflection Point in High-Throughput Proteomics with Orbitrap Astral: Analysis of Biofluids, Cells, and Tissues. J Proteome Res. 2024;23(9):4163–9.
Kverneland AH, Harking F, Vej-Nielsen JM, Huusfeldt M, Bekker-Jensen DB, Svane IM, et al. Fully Automated Workflow for Integrated Sample Digestion and Evotip Loading Enabling High-Throughput Clinical Proteomics. Mol Cell Proteomics. 2024;23(7):100790.
Ctortecka C, Hartlmayr D, Seth A, Mendjan S, Tourniaire G, Mechtler K. An automated workflow for multiplexed single-cell proteomics sample preparation at unprecedented sensitivity. bioRxiv; 2022;2021.04.14.439828. https://doi.org/10.1101/2021.04.14.439828v2
Schoof EM, Furtwängler B, Üresin N, Rapin N, Savickas S, Gentil C, et al. Quantitative Single-Cell Proteomics as a Tool to Characterize Cellular Hierarchies. Nat Commun. 2021;12(1):3341.
Kreimer S, Binek A, Chazarin B, Cho JH, Haghani A, Hutton A, et al. High-Throughput Single-Cell Proteomic Analysis of Organ-Derived Heterogeneous Cell Populations by Nanoflow Dual-Trap Single-Column Liquid Chromatography. Anal Chem. 2023;95(24):9145–50.
Marx V. Method of the Year: Spatially Resolved Transcriptomics. Nat Methods. 2021;18(1):9–14.
Kuppe C, Ramirez Flores RO, Li Z, Hayat S, Levinson RT, Liao X, et al. Spatial Multi-Omic Map of Human Myocardial Infarction. Nature. 2022;608(7924):766–77.
Method of the Year 2024: spatial proteomics. Nat Methods. 2024;21(12):2195–6.
Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, et al. Laser-Capture Microdissection. Nat Protoc. 2006;1(2):586–603.
Makhmut A, Qin D, Hartlmayr D, Seth A, Coscia F. An Automated and Fast Sample Preparation Workflow for Laser Microdissection Guided Ultrasensitive Proteomics. Mol Cell Proteomics. 2024;23(5):100750.
Lin JR, Fallahi-Sichani M, Chen JY, Sorger PK. Cyclic Immunofluorescence (CycIF), a Highly Multiplexed Method for Single-cell Imaging. Curr Protoc Chem Biol. 2016;8(4):251–64.
Black S, Phillips D, Hickey JW, Kennedy-Darling J, Venkataraaman VG, Samusik N, et al. CODEX Multiplexed Tissue Imaging with DNA-Conjugated Antibodies. Nat Protoc. 2021;16(8):3802–35.
Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, et al. Multiplexed ion Beam Imaging (MIBI) of Human Breast Tumors. Nat Med. 2014;20(4):436–42.
Kuett L, Catena R, Özcan A, Plüss A, Schraml P, Moch H, et al. Three-Dimensional Imaging Mass Cytometry for Highly Multiplexed Molecular and Cellular Mapping of Tissues and the Tumor Microenvironment. Nat Cancer. 2022;3(1):122–33.
Mund A, Brunner AD, Mann M. Unbiased Spatial Proteomics with Single-Cell Resolution in Tissues. Mol Cell. 2022;82(12):2335–49.
Mund A, Coscia F, Kriston A, Hollandi R, Kovács F, Brunner AD, et al. Deep Visual Proteomics Defines Single-Cell Identity and Heterogeneity. Nat Biotechnol. 2022;40(8):1231–40.
Zheng X, Pedersen LM, Bzorek M, Mund A, Gjerdrum LMR, Mann M. Deep Visual Proteomics Unveils Precision Medicine Insights in Composite Small Lymphocytic and Classical Hodgkin Lymphoma. bioRxiv; 2024;2024.06.12.598635. https://doi.org/10.1101/2024.06.12.598635v1
Arking DE, Pulit SL, Crotti L, van der Harst P, Munroe PB, Koopmann TT, et al. Genetic Association Study of QT Interval Highlights Role for Calcium Signaling Pathways in Myocardial Repolarization. Nat Genet. 2014;46(8):826–36.
Lundby A, Rossin EJ, Steffensen AB, Acha MR, Newton-Cheh C, Pfeufer A, et al. Annotation of Loci from Genome-Wide Association Studies Using Tissue-Specific Quantitative Interaction Proteomics. Nat Methods. 2014;11(8):868–74.
Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, et al. The Support of Human Genetic Evidence for Approved Drug Indications. Nat Genet. 2015;47(8):856–60.
Ochoa D, Hercules A, Carmona M, Suveges D, Baker J, Malangone C, et al. The Next-Generation Open Targets Platform: Reimagined, Redesigned, Rebuilt. Nucleic Acids Res. 2023;51(D1):D1353–9.
Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, AitBlal H, et al. A subcellular map of the human proteome. Science. 2017;356(6340):eaal3321.
The UniProt Consortium. UniProt: the Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025;53(D1):D609–17.
Türei D, Valdeolivas A, Gul L, Palacio-Escat N, Klein M, Ivanova O, et al. Integrated Intra- and Intercellular Signaling Knowledge for Multicellular Omics Analysis. Mol Syst Biol. 2021;17(3):e9923.
Santos A, Colaço AR, Nielsen AB, Niu L, Strauss M, Geyer PE, et al. A knowledge Graph To Interpret Clinical Proteomics Data. Nat Biotechnol. 2022;31:1–11.
Miao Z, Humphreys BD, McMahon AP, Kim J. Multi-Omics Integration in the Age of Million Single-Cell Data. Nat Rev Nephrol. 2021;17(11):710–24.
Svensson V, Vento-Tormo R, Teichmann SA. Exponential Scaling of Single-Cell Rna-Seq in the Past Decade. Nat Protoc. 2018;13(4):599–604.
Ioannidis JPA, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, et al. Repeatability of Published Microarray Gene Expression Analyses. Nat Genet. 2009;41(2):149–55.
Gibson G. Perspectives on Rigor and Reproducibility in Single Cell Genomics. PLoS Genet. 2022;18(5):e1010210.
Zitnik M, Nguyen F, Wang B, Leskovec J, Goldenberg A, Hoffman MM. Machine Learning for Integrating Data in Biology and Medicine: Principles, Practice, and Opportunities. Information Fusion. 2019;1(50):71–91.
Argelaguet R, Velten B, Arnol D, Dietrich S, Zenz T, Marioni JC, et al. Multi-Omics Factor Analysis—a Framework for Unsupervised Integration Of Multi-Omics Data Sets. Mol Syst Biol. 2018;14(6):e8124.
Allesøe RL, Lundgaard AT, Hernández Medina R, Aguayo-Orozco A, Johansen J, Nissen JN, et al. Discovery of Drug–Omics Associations in Type 2 Diabetes with Generative Deep-Learning Models. Nat Biotechnol. 2023;41(3):399–408.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma Powers Differential Expression Analyses for Rna-Sequencing and Microarray Studies. Nucleic Acids Res. 2015;43(7):e47.
Huang T, Staniak M, da VeigaLeprevost F, Figueroa-Navedo AM, Ivanov AR, Nesvizhskii AI, et al. Statistical Detection of Differentially Abundant Proteins in Experiments with Repeated Measures Designs and Isobaric Labeling. J Proteome Res. 2023;22(8):2641–59.
Straube J, Gorse AD, Team PC of E, Huang BE, Cao KAL. A Linear Mixed Model Spline Framework for Analysing Time Course ‘Omics’ Data. PLOS ONE. 2015;10(8):e0134540.
Ozenne B, Forman J. LMMstar: repeated measurement models for discrete times [Internet]. 2024. Available from: https://cran.r-project.org/web/packages/LMMstar/index.html. Accessed 8 Jan 2025.
Pinheiro J, Bates D, DebRoy S, Sarkar D, EISPACK, Heisterkamp S, et al. nlme: linear and nonlinear mixed effects models [Internet]. 2024. Available from: https://cran.r-project.org/web/packages/nlme/index.html. Accessed 8 Jan 2025.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2022. Available from: https://www.r-project.org/. Accessed 8 Jan 2025.
Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2nd ed. Hoboken (NJ): John Wiley & Sons; 2011; pp. 758.
Funding
JFM is supported by the BRIDGE – Translational Excellence Programme, funded by the Novo Nordisk Foundation (grant agreement no. NNF20SA0064340). MSS is supported by a fellowship from the Danish Cardiovascular Academy, which is funded by the Novo Nordisk Foundation (NNF20SA0067242) and The Danish Heart Foundation. AL is supported by Independent Research Fund Denmark (https://doi.org/10.46540/4285-00115B).
Author information
Authors and Affiliations
Contributions
JFM, ELM, MSS and AL collectively conceptualised, wrote, and edited the manuscript. JFM and ELM prepared the figure. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
No animal or human subjects were used by the authors in this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mulvey, J.F., Meyer, E.L., Svenningsen, M.S. et al. Integrating -Omic Technologies across Modality, Space, and Time to Decipher Remodeling in Cardiac Disease. Curr Cardiol Rep 27, 74 (2025). https://doi.org/10.1007/s11886-025-02226-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-025-02226-7