Abstract
Purpose of Review
The pandemic of obesity preceded global spread of Inflammatory Bowel diseases by almost 2 decades. A pathogenic relationship has been described between obesity and inflammatory bowel diseases, but Crohn`s disease may be selectively impacted. The role of diet in pathogenesis has also gained significant support in the last few decades. This review explores dietary relationships to account for epidemiological observations.
Recent Findings and Summary
Quantifiable indices for diets have been described including a glycemic index, inflammatory indices and levels of food processing. Meta-analyses have been published which examine each for effects on obesity and co-morbidities as well as Crohn’s disease and ulcerative colitis. This review suggests that ultra-processed foods provide the best link between obesity and Crohn’s disease explaining epidemiological observations. However, the other 2 types of dietary indices likely contribute to ulcerative colitis as well as to co-morbidities related to both obesity and inflammatory bowel diseases.
The term ultra-processed foods cover a large number of additives and extensive work is needed to define individual or combined harmful effects. Furthermore, the interactions among the 3 main indices need clarification in order to precisely apply therapeutic diets to both diseases (obesity and inflammatory bowel disease).
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The last five decades has seen a pandemic of obesity [1], accompanied by type 2 diabetes (T2D) [2] and fatty liver disease (NAFLD), more recently renamed Metabolic Dysfunction- Associated Steatotic Liver Disease (MASLD) [3, 4].
Obesity (OB) was designated as a disease by the World Health organization in 1995 [5]. Many illnesses, labeled as civilization diseases [6] accompany obesity (OB) [7,8,9,10]. Among these the Inflammatory Bowel Diseases (IBD; consisting of ulcerative colitis UC and Crohn’s disease, CD) are the most recent [11, 12]. There is a hypothesis suggesting that obesity, because of the presence of low grade inflammatory state predisposes to IBD by setting up conditions for that disease [11]. Pathogenic relationships between OB and IBD have been described [13]. However, CD is reported to be more closely related than UC [14]. Nevertheless, co-morbidities associated with OB are also related with both forms of IBD [12, 13].
Obesity spread across the globe starting from North America in the mid1970 s [15]. While IBD has been initially confined to Europe and North America, recently, it has become more common in low incidence regions of the world [16,17,18]. On a global level (although not regionally) the correlation between CD and UC is strong [19]. The correlations between OB and CD based on data from around the beginning of the current century up to 2008, was found to be r = 0.34, and that of UC r = 0.37 [20]. Crude correlative studies suggest that there is a relatively low correlation between Gross Domestic Product (GDP) and IBD, a recognized marker of national wealth which is a potential correlate of industrialization. [20, 21]. When data were extended in available countries and time up to 2013, the correlations between OB, CD and UC were moderate (CD, r = 0.5-, UC, r = 0.51) [21]. While these results suggest a relationship between OB and IBD causality cannot be inferred.
Indeed, OB is attributed to multiple factors, exposomes [22], but the major hypothesis for the pandemic is spread of altered Western style dietary consumption [1]. Production of Western type diet required a degree of industrialization. This lead to inventions which modified basic foods through processing. Such modifications resulted in relatively cheaper foods with improved shelf lives. As a result less industrialized nations could relieve hunger by purchasing such products.
The pathogenesis of IBD is also thought to be related to multiple exposomes [23]. However, the same crude studies suggest that on a global level IBD is closer correlated with GDP and weakly with obesity [18, 20]. When another index of socioeconomic development, the HDI, is used, the pattern of correlations are similar except CD has a higher r value of 0.75 with HDI (unpublished observations).
The concept of exposomes explaining both diseases contains inherent examples of contributing variables including different genetic predisposition to both IBD and OB, early life exposure to antibiotics in IBD, smoking, birth control medications and other variables. Further, industrialization is associated with other factors which could impact on IBD development.
In the past several years the pathogenic role of diet in IBD has been gaining support. This narrative review will explore relationships between Western style diets, obesity and both forms of IBD. A review of the characteristics of Western diets and their potential effects on intestinal defenses is reviewed. We will then explore the relationships between diet, obesity, and IBD, through systematic reviews and meta-analyses focusing on the role of foods quantified by responses to carbohydrate ingestion, inflammatory responses and level of processing. By examining epidemiological patterns and mechanistic studies, we aim to clarify how diet may serve as a bridge linking obesity to IBD, particularly CD.
Quantifiable Characteristics of Western Diets
The Western diet is characterized by high fat, sugar, and salt, along with varying degrees of food processing and low fiber intake. The pandemic of obesity is also marked by low intensity of physical activity [1, 6, 15]. OB and many diseases are thought to be related to a process of chronic low-grade inflammation, and western diet is thought to promote inflammatory processes in the gut [24]. The pro-inflammatory effects of Western diets alter the microbiome/metabolome, intestinal mucus layer, and the intestinal epithelial barrier functions as further detailed below.
There are four characteristics of Western diet (all recognized in the last 3 decades). Addition of obesogens may contribute to the existing pandemic. These are compounds which cause weight gain independent of calorie content. They may increase number of volume of fat cells. Alternatively, they may alter appetite, metabolic rate, or energy balance. These include food additives and environmental pollutants which interfere with the normal feedback mechanisms of endocrine and metabolic functions [25,26,27]. These additives may contribute also to inflammation (for example sugary drinks quoted below). However, the addition of various food additives are usually measured in the context of other foods and are not by themselves quantifiable.
Three other type of diets use quantifiable features. Although, not mutually exclusive, these include the glycemic Index, Inflammatory indices (defined as diets that increase measurable markers of inflammation) and the level of food processing). These three type of diets can be used to compare dietary effects.
Glycemic Index (GI)
Excess sugars and carbohydrates in the Western diet leads to increased glycemia and stimulates insulin. The concept of glycemic index was first introduced by Jenkins and Wolover [28]. The index represents a comparison of the ratio expressed as a percentage of the glycemic response to a reference food such as glucose solution or white bread. (GI = (iAUCtest food/iAUCreference food) × 100: AUC = Area Under the Curve) [29]. The glycemic index was developed originally based on the notion that more complex carbohydrates are digested differently in health and diabetes and do not result in as rapid rise in blood sugar. In general it would be better for diabetics to have a diet rich in complex carbohydrates with a low glycemic index which releases glucose slowly into the blood stream [30]. Overall, however, the glycemic response depends on the intake amount whether a low or high glycemic food is ingested. The glycemic index is then applicable to designating different foods and how they impact on the rise of blood glucose, insulin and is related to the glycemic load. The rise of blood glucose and associated insulin response is associated with insulin resistance, oxidative stress, and induction of inflammation [31]. The glycemic index has been evaluated in the context of cardiovascular risks. Bullo et al. studied 511 elderly people and followed them for a year with a Food Frequency Questionnaire. These participants were part of a 6 year study evaluating cardiovascular risks. They measured leptin and adiponectin as well as some markers of inflammation. At baseline TNFα levels were significantly elevated (15.36, CI; 13.14–18.4, p = 0.046) in participants in the 4 th quartile of high glycemic food intake. After a year, the highest quartile intake of foods with a high glycemic index was associated with lower leptin (Delta; − 0.39, CI:− 0.87- − 0.26, p = 0.019) and adiponectin (Delta; − 15.14,CI: − 27.3- − 0.29, p = 0.027) differences from baseline. This suggested a higher risk for CVD with high glycemic intake [32]. However, the glycemic index is somewhat more controversial when applied to other Western diseases. First, not every study consistently shows a relationship of the glycemic index with obesity and second, very few studies show any relationship with IBD. These will be further discussed below.
Inflammatory Indices
The notion of quantifying inflammatory effects of diet was put forth by Cavicchia et al. in 2009 [33]. In this article, different food nutrients were evaluated for their ability to affect C- reactive protein (CRP), a marker of inflammation. Shivappa et al. in 2014 [34] refined this concept. The important change was that the inflammatory index was based on the literature consisting of about 2000 articles where individual food items were related to an expanded measurement of pro-inflammatory and anti-inflammatory cytokines (IL- 1b, IL- 4, IL- 6, IL- 10, and TNF-α) as well as CRP. Based on this review they evaluated an algorithm which assessed 45 individual nutrients and food items and how they affected measured parameters. Foods which raised IL 4 and IL10 and/or lowered IL- 1b, IL- 6, TNF-α and CRP were deemed anti-inflammatory while those that did the opposite were deemed pro-inflammatory. The index range extended from − 8.87 (anti-inflammatory) to a maximum of 7.98 (pro-inflammatory). A modification of the original Shivappa index was published later [35]. The application of combining single food items to derive an overall effect is quite daunting in practice. To make this concept easier to use, a somewhat simplified algorithm was developed by Tabung et al. This index called the Estimated Dietary Index (EDII) measures three markers (IL- 6, TNF-αR2 and CRP) based on 18 food groups with 9 anti and 9 pro-inflammatory scores [36]. Several other variations on the DII were published [37, 38]. One of these was used to assess an estimated DII in IBD and is further described in the section on indexed diets below in the section on ‘The Western diet and Inflammatory Bowel Diseases’.
On a more practical level diets consisting of pro or anti-inflammatory food groups have generally been assigned. In this paradigm, the Western diet is generally considered as pro-inflammatory [39], while diets from the Mediterranean area [40] and more recently, southern countries bordering the Atlantic Ocean [41] are considered anti-inflammatory. Both the Mediterranean and Atlantic diets consist of foods (more fish, nuts and more fiber) with less potential production of pro-inflammatory cytokines. It is of note that an anti-inflammatory index was developed for the Mediterranean diet which was inversely correlated with the dietary inflammatory index (r was – 0.45) [42]. Examples of anti and pro-inflammatory foods are shown in Table 1, based on references [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
Ultra-Processed Foods (UPFs)
An important attribute of Western diet is the recognition of the extent of food processing. Industrialization has led to the modification of basic foods to various extents. The original purpose of food processing was to make foods safer and enhance shelf life at a cheaper cost. However, Monteiro questioned the aggressive marketing of ultra-processed foods and questioned its health benefits, particularly since by the time he published it was recognized that processed foods contributed to obesity. The degree of food processing has been classified into four levels based on NOVA classification [58, 59] (this stands for the Portuguese term, nova classificação,'new classification’). These are shown with examples in Table 2.
It is estimated that the Western diet contains up to 50% UPFs [59, 61]. The impact of UPFs has been reviewed in general [62] and for obesity and associated complications specifically [63]. These UPFs have been associated with a high inflammatory potential as well [64,65,66]. However, a question remains whether diets based on inflammatory indices predict the same level of effects. The largest study by Minogna et al. [64] evaluated the relationship between an energy dependent DII diet and UPFs against a known marker of high DII related score (INFLA –score) in a population numbering 24,325 men and women. A positive correlation was noted between a higher EDII and UPFs, and a lower association with minimally processed foods. When the relationship was assessed with a multivariable model, DII explained 88.5% of the INFLA – score. However, UPFs explained only 32.6% of the INFLA – score. This suggested that the DII may not capture all the effects of UPFs. The authors hypothesized that additional factors were likely affected by processing (e.g. impact on food matrix). This difference may be relevant because it raises an imprecision in evaluating effects of diets for IBD (as well as other diseases). Furthermore, specific effects of individual UPFs need to be assessed for interfering with different foods and diets given for disease. However, this under assessment of the DII of diets may be more specific for CD since as described later some studies using the DII do relate more positively with UC.
Although, the impacts of UPFs have been studied extensively on risks for diseases, the actual causality has not yet been universally accepted. The reasons include the arguments that UPFs improve food safety, have lower costs and longer shelf lives. A recent publication challenged the role of UPFs as a cause of obesity based on statistical specificity, clarity, and inconsistency [67]. Similar arguments may be raised for other diseases as well. In the case of IBD, Fitzpatrick et al. questioned the possible variability in the NOVA classification. Some of these limitations involve the vagueness of what is meant by UPF. The author uses the example of flavoured yogurt and suggests that while plain yogurt may be relatively free of processing, the addition of sugar, and food coloring makes for increased processing. Second, because UPFs are cheaper,it may correlate with poor diets in general. As well there is no specific data on how much UPFs are necessary for poor effects on health (including dose or threshold effects) [60]. This criticism may also echo questions raised for effects of inflammatory indices above.
Effects of Diet on the Intestine
Major determinants of the impact of different food content are their effects on intestinal microbiome/metabolome, intestinal mucus layer, and the intestinal epithelial barrier function.
The intestinal microbiome consists of bacteria, archaea, fungi, and viruses [68]. While all may be important for function, the main thrust of research and knowledge at this time is based on changes in bacterial populations and metabolism. The microbiome is malleable and responds to many stimuli, particularly diets. The 2 main bacterial phyla making up human microbiome (85 − 90%) are the gram + ive Firmicutes and the gram – ive Bacteroidetes. About 10- 15% are made up of Proteobacteria, Fusobacteria and Verrucomicrobia [69]. Western diet consisting of high fat and low fiber results in disruption of relationships among normal microflora (dysbiosis), increased intestinal immune responses and increased intestinal epithelial damage with altered barrier function [70, 71].
An encompassing study from the Netherlands by Bolte et al. evaluated 173 food items consumed long term by 1425 participants who were divided into four cohorts. These included patients with Crohn`s disease, ulcerative colitis, irritable bowel syndrome and healthy controls [72]. Diet was assessed by a semi-quantitative food frequency questionnaire and metagenomic profiling of bacteria was used to compare food groups and bacterial clusters. Metabolomic characteristics of clusters were also identified. The main findings were that similar bacterial responses occurred across the 4 cohorts to diets. Inflammatory diets resulted in similar groups of bacteria. Animal derived diets and processed foods consistently led to increased species of Firmicutes, Ruminococcus and the genus Blautia, as well as increased pathways for endotoxin synthesis. High fiber and diets rich in plant-based diets with low processed foods and absence of sugary drinks prevented intestinal inflammatory response. High fiber diets resulted in increased short chain fatty acids (SCFA) which in turn improved the immune response. Poor adherence to anti-inflammatory diets increased risks for IBD. Similar to many studies on microbiome the outcomes could not yet establish causation between observations and risks of IBD.
Part of the protection of the host against invasion by potential pathogens is the mucus layer overlying the mucosa, which gets progressively thicker distally. Moreover, the colon is covered by a second layer of mucus that is more superficial and coherent while the deeper layer is more adherent [73]. These layers are re-enforced by proteins that are antibacterial, secreted, by Paneth cells from the epithelial layer, IgA secreting Plasma cells and a number of enzyme inhibitors [74,75,76]. The layer of mucus is kept in balance by synthetic rate and the presence of mucin degrading bacteria such as Akkermansia muciniphila and Bacteroides thetaiotaomicron. The degradation of mucus leads to formation of other oligosaccharides which promotes the cross-feeding of other bacteria [73]. The impacts of Western and low fiber diets have shown reducing effects on intestinal mucus. The loss of fiber in diet leads to expansion of mucin degrading bacteria which metabolize host glycans and results in loss of this barrier function. Loss of mucus allows potential pathogenic bacteria (pathobionts) or subsequent parts of bacteria to contribute to trans epithelial (usually paracellular) migration as elaborated further below. On the other hand, high fat diets reduce Akkermansia muciniphila which in turn has been reported to be beneficial for gut barrier function [77].
Below the mucus layer rests the primary barrier consisting of a single epithelial layer of cells. A number of specialized cells are interspersed (e.g., goblet, Paneth, enterochromafin and M cells). These secrete anti-bacterial and other agents like mucin from goblet cells to prevent trans-epithelial invasion and translocation of harmful bacteria derived endotoxins [74]. The cells are important because they have different functions, whether they are in the small bowel or large bowel. In the upper intestine, cells absorb nutrient either through trans-cellular or para-cellular routes. The second function is to protect the host from invasion by organisms and bacterial metabolites. The epithelial layer is joined by the apical junctional complex, consisting of tight junctions’ proteins (occludins, claudins and adhesion –protein molecule A) and adherence junctions. Flux across the epithelium is governed by molecular size. Small molecules generally cross trans- epithelium while larger molecules are regulated by IL- 13 and TNF-α [61, 78]. The epithelial membrane can become leaky via inflammatory cytokines and result in bacterial and endotoxin translocations which compound inflammation and potential disease developments. Diets which are anti-inflammatory may be able to reduce some of these abnormalities. For example a recent study of 53 patients with UC showed reduced levels of fecal calprotectin ≤ 150 µg/g (69% anti – inflammatory diet vs 37% on regular diet, p = 0.02). This suggested to the authors that the anti –inflammatory diet reduced subclinical inflammation (but did not prevent clinical relapse) [79]. The epithelial barrier is affected by high fat diets (increase leakiness) and reduced by SCFA through increased fiber consumption. Below the single layer of epithelium are cells which produce cytokines in response to various stimuli.
UPFs with multiple additives can contribute to disruption at anyone of the levels which contribute to host protection. Vissers et al. reviewed the effects of various food additives: emulsifiers and thickeners (carrageenan and carboxylmethylcellulose), food colorings (red dye 40, yellow dye 6 and titanium dioxide), artificial sweeteners, salt and phosphates. All of these can alter the microbiome and increase intestinal permeability leading to induction of inflammation and bacterial/endotoxin translocations [61] In addition it is also not clear whether UPFs promote poor nutrition by reducing NOVA 1 class foods [80]. Interestingly, and contrary to expectations, a recent, albeit small (N24) randomized 4 week study compared food containing high and low emulsifiers in patients with active ileal CD. At the end of the period no significant effects were noted between the high or low emulsifier consuming groups [81]
Therefore, the general effect of a Western diet is the promotion of a low grade inflammatory state with increased exposure of the host to intraluminal bacterial products and toxins.
Altered microbiomes and leaky intestinal membranes are thought to play roles in both IBD and obesity pathogenesis. In CD there is at best moderate evidence for the Mediterranean diet which reduces risk for disease. In active disease total enteral diet or a partial enteric diet with CD exclusion diet may be the best for induction of remission. In UC in any stage at this time a weak evidence suggests a healthy diet may be beneficial [82]
How Does the Western Diet Impact on Obesity and IBD?
Obesity and the Western Diet
While the explanation that obesity is a condition dependent on excess energy intake with insufficient expenditure, other factors are involved in contributing to obesity [1, 6]. The concept of exposomes attempts to explain the interactions of several environmental variables resulting in obesity [20]. These include genetics with over 300 polymorphs involved [83]. As well the role of epigenetics in altering genes via several mechanisms has been recognized. Stress and socioeconomic status (especially poverty) affects eating behavior and raises affordability as a potential barrier to good nutrition [15]. In addition as pointed out above obesogens which interfere with appetite and hormonal communications of regulation of energy intake contribute [3, 25]. Diet also impacts on the microbiome (which may affect barrier functions). Additional variables hypothesized include ambient temperatures, and even the recent Covid − 19 pandemic (with stress, mental effects and long haul impact on the intestine with weight loss and possible diarrhea) [20].
The relationships of the three quantifiable Western diets with OB and IBD are reviewed and Table 3 and 4 respectively show published systematic reviews and meta-analyses.
Pro-Inflammatory Diets
The inflammatory potential of the Western diet has been extensively studied. Five meta-analyses in the last 6 years looked at inflammatory diets and various derivatives of the inflammatory indices. Namazi et al., evaluated dietary studies up to 2017 for risks of cardiovascular disease, metabolic syndrome, all cause and cardiovascular disease (CVD) and mortality. Although obesity was not specifically evaluated, these co-morbidities are strongly associated with other parameters evaluated. In the 17 studies, all cause mortallty, cancer and CVD mortalities were modestly increased. In this analysis. however,metabolic syndrome (MetS) was not significantly affected, but only 5 studies were included for this part of the analysis. Therefore the sample size for statistical significance for MetS may not have been enough [84]
Farhangi et al. evaluated 36 observational studies on various Western diet related co-morbidities including systolic, diastolic hypertension, blood glucose, insulin and Hemoglobin A1c. Higher dietary scores were associated with increased risks for these co-morbidities [85].
A recent meta-analysis of 32 observational studies comprising 103,071 participants by Farhangi et al. found an almost twofold increased risk in waist circumference with a higher DII [86]. Another meta-analysis of 14 cross-sectional and 4 cohort studies reported a positive association between a higher inflammatory index and MetS. This study also reported positive effects (ie. a higher odds ratio with confidence intervals that do not cross below 1, implying increased risks) with hypertension, central obesity, hyperglycemia, and hypertriglyceridemia [87]. Finally, a small meta-analysis of the relationship between T2D and a higher DII by Tan et al. found a statistically significant increased risk for T2D [88].
These analyses generally report modest but significantly increased risks for obesity and its co-morbid state. Since these studies were observational they infer associated risk without assigning causation.
Ultra-Processed Foods
The impact of UPFs also have been evaluated in relationship to obesity and its complications. An earlier meta- analysis of a variety of non-communicable disease risks from UPFs was reported by Pagliai et al. The group reported on 183,491 participants and found increased risks for CVD, stroke and overall mortality with higher intake of UPFs. They also reported a 39% increased risk for obesity and 79% increased risk for MetS [90].
A systematic review of 17 studies evaluated obesity (515,048 participants) with an average follow up of 6.1 years an Odds Ratio or Relative Risk of 1.37 with CI 95%, 1.11–179. They also evaluated for T2D (9,216,918 participants) and found a mean increased risk of approximately 34%. In all these analyses UPFs were variably reported as a percentage of daily intake, weight/g of food intake or servings/d. Higher UPF intake was associated with higher energy intakes and may change eating patterns. Similarly modest but statistically significant increased risks were found for hypertension, dyslipidemia and MetS [91].
A meta-analysis of 14 studies (1 cohort and 13 cross-sectional) up to 2019 by Askari et al. also found a small but statistically significant pooled effect of high UPFs and being over-weight or obese [89].
Subsequently a 9 study meta- analysis of the relationship between higher UPFs and MASLD by Henney showed a statistically significant dose effect. Results were significant both at moderate and higher intake of UPFs [92].
The most recent umbrella meta-analysis by Lv published 6 systematic reviews and 13 meta-analyses. The mean number of participants was 66,235 and cases were 15,152. The authors divided studies based on statistical significance which was then classified as convincing to suggestive evidence. Based on their assessment the highest intake of UPFs was associated with obesity and T2D compared to the lowest intake [93].
Although there is overlap of included studies, the analyses measure different outcomes and do not include the exact same studies. These suggest a positive albeit modest to moderate association between intake of UPFs and obesity with their comorbidities. Given the variability of methods used do define amount of UPF intake it is difficult at this time to determine whether there is a linear relationship or a threshold. The study by Henney which examined the UPF effect on MASLD did show a dose effect with moderate exposure being lower (but still statistically significant) and highest intake showing a greater effect [92]. Also some studies showed that a 10% increased intake of UPFs increased risk for metabolic syndrome [93]. A similar estimate of each 10% increase in UPF consumption increased risk of diabetes 25% [91]. Additional studies on dose effects would help define the impacts of UPFs.
Glycemic Index Relation with Obesity and Comorbidities
The impact of the glycemic index on obesity is somewhat controversial. The relationship may rely more on addiction induced by carbohydrates, increasing calorie intake [99]. A large Finnish study however, showed an inverse association between BMI and intake of foods with a high glycemic index [100]. Several confounding variables were noted in this study. The article did not specify participants with diabetes. The definition of obesity was here defined by BMI and these participants may have under-reported carbohydrate intake. In addition the glycemic index according to the authors does not correlate with a healthy outcome.
Another systematic review of 73 publications (cross-over and RCTs) for glycemic index and glycemic response failed to find any relationships with diseases [29]. An analysis of 14 randomized controlled studies by Schwinglshakl et al. found no association of the glycemic index with anthropometric measurements, fasting lipids, fasting blood sugar or hemoglobin A1-C, However, this study was a therapeutic assessment and the authors concluded that a food with a low glycemic index is helpful in controlling for obesity with a reduction of measured markers of inflammation and glycemic control [94].
There is some evidence for a high glycemic index of foods increasing risk for CVD as alluded to above [32]. A meta- analysis to assess risks for cardiovascular disease with total sugar, added sugar and fructose intake showed a modest increase [101]. Another meta-analysis of 12 studies found a modest but statistically significant relationship between MetS and the glycemic index but not with the glycemic load [102]. Although, not strictly evaluating the glycemic index to glycemic response ratio, a meta-analysis of fructose in sugary drinks on increasing lipids in the liver was published. This study reviewed 51 studies and found a significant increase in hepatic lipids with increasing sugar intake. The standardized mean difference in intake in those with increased hepatic lipid was (1.72 95% CI, 1.08 to 2.36, p < 0.001) [103].
In relationship to markers of the inflammatory response, however, a meta-analysis of 28 RCTs by Milarejdi found no effect of the glycemic index on any markers of inflammation [95]. There is a suggestion that addition of NOVA 4 UPFs to diets increases the glycemic index when these are compared to NOVA 1 foods [104]
Of the 3 quantitatively analyzed diets, inflammatory indices and addition of UPFs exert increased risk. Studies with UPFs may be more consistent and pronounced toward promoting obesity. Additional studies could shed more insight on this. Nevertheless, it is clear that all three descriptors of diets are inter-related and contribute to obesity (and associated co-morbidities). Given the multitude of studies it would be important to relate these 3 quantitative elements so different outcomes could be better defined by each method. For instance how does the UPF content of food relate to inflammatory indices used to analyze effects? As the study of Minogna et al., suggests DII and UPFs could have some different effects which may impact on outcomes [64].
The Western Diet and Inflammatory Bowel Diseases
As noted above, IBD has its own causes and there are over 200 genes (not including mono-genetic risks for very early onset IBD as well the numbers are increasing) which predispose to either CD or UC explaining perhaps 20–25% of cases [105, 106]. These genes control the body`s defense against bacterial invasion (e.g.NOD 2, ATG16L1 autophagy), Others, regulate responses to both innate and adaptive immunity and barrier functions through control of claudins which are responsible partly for maintaining intercellular functions. Still other like the one for IL23 – Receptor have several variants which reduce risk for both Crohn`s disease and ulcerative colitis (e.g., G149R, V326I, R381Q). Since diet as a pathogenic agent has gained momentum, the emphasis in this analysis is on pre disease diets which connect obesity with IBD and offers a potential explanation to geographic spread and timing of IBD to the latter.
There appears to be a variable clinical effect of diet on CD and UC. In consideration of CD, pre-disease is different from diets for active CD and even for maintaining remission. In the case of UC pre-disease, active disease and maintaining remission are less variable.
While early childhood nutrition is probably relevant, pre-diagnosis diet refers to long-term diet, several years before the diagnosis is established. Alluded to above an excellent review of this organization of diet for both CD and UC is provided [82]. In general total protein and sugary drinks are consistently shown to increase risk for CD. Increased total dairy and increased fiber decrease risk for CD. In UC increased animal protein, red meat, high sugars with low intake of vegetables predisposes to UC. It is of interest to note that while UPFs may be the ultimate pre-disease contributor for CD development, an enteral liquid diet or partial enteral diet (the ultimate UPF) is found to induce remission in CD but not UC, in contrast, a recent small study found that oral UPFs during active disease were made symptomatically worse in the highest tertile consumption in UC but not CD [107]
These will not be further discussed here because management of active IBD is beyond the scope of the review.
Some pre-disease studies are mentioned here to emphasize some of the deficiencies which reduce ability to compare diets which may link IBD with obesity.
The role of pre illness low vitamin D levels have been associated with increased rates of hospitalization and increased rates of surgery in CD [108, 109]. A review of vitamin B12 deficiency in IBD suggested that deficiency of this vitamin is restricted to patients with extensive ileal-resections and less to extensive small bowel disease [110]. Besides vitamins, iron deficiency is common, as well as several micronutrients [111, 112]. While such deficiencies may be present with obesity, they are more often seen in IBD.
Patterns of diets that predate disease have been sought. In a large study of more than 67,000 French women, using a validated questionnaire, Jantchou et al. reported that a diet high in animal fat (meat and fish but not eggs or dairy) was associated with a higher risk of IBD [113]. A subsequent meta –analysis of 19 studies comprising over 6600 patients with CD, UC and healthy controls “intake of saturated fats, monounsaturated fatty acids, total polyunsaturated fatty acids (PUFAs), total omega- 3 fatty acids, omega- 6 fatty acids, mono- and disaccharides, and meat increased subsequent CD risk “. Increased intake of fiber and fruits decreased risk for CD. In UC “High intakes of total fats, total PUFAs, omega- 6 fatty acids, and meat were associated with an increased risk. High vegetable intake decreased risk of UC” [114].
In terms of relapse, higher intake of animal protein and carbohydrates and lower intake of unsaturated fats and fiber increased risks [115]. A Dutch study with 724 IBD patients also suggested that traditional Dutch pattern diet (a Western diet) consisting of meats, sauces, condiments, oils and grain products were associated with flares within a 2 year follow up [116].
Interestingly consumption of dairy products as outlined may be protective for IBD [117]. Some salient features that could account for these observations may relate to the effects of calcium on the extra cellular (calcium sensing receptor) CaSR in the intestines. This multi molecule sensor can reduce both the inflammatory and neoplastic responses [118]. Other potential mechanisms could relate to the functions of conjugated linoleic acids which also reduce an inflammatory response possibly via activation of peroxisome proliferator-activated receptors which in turn regulate lipid and sugar metabolism in cells [119].Finally in persons unable to digest lactose a potential induction of Bifidobacteria species which have probiotic properties and could also participate in this reduction of risk [120].
A recent umbrella meta-analysis of individual foods in IBD supports these findings as well as a modest protective effect of dairy foods [121].
While these studies suggest relationships with both obesity and inflammation and likely contain UPFs they are difficult to compare with diets associated with obesity.
Index Based Diets
As stated, foods included in the Western diet tend to be pro-inflammatory. There have been studies examining inflammatory indices and IBD.
Two studies found no clear impact. A study using a FFQ on 238 IBD, 261 IBS and 195 healthy controls compared diet quality based on the Dutch healthy diet index and adapted dietary inflammatory index. This study found that possibly in IBD, abdominal pain alone correlated with increased inflammatory diet [122]. In a small study of 143 participants a high DII or (empirical dietary index pattern) EDIP, was not associated with increased severity of either CD or UC [123].
Three additional studies did show a relationship between DII and IBD. A case–control study from Iran by Shivappa showed that with UC, a higher DII increased the risk of disease by 55% [124]. A more recent study from China showed that a higher DII was associated with sarcopenia in patients with CD. Again, there were relatively few participants [125]. A large study on almost 4 X 105 persons followed for 13.6 years showed that a high inflammatory score of diet increased risk for CD (HR: 1.88, 95% CI: 1.14–3.10; p-trend < 0.01) but not UC (Not Significant). This was evident only in women [126]. Therefore a clear distinction between the effects of high DII foods from those described below for UPFs may still be lacking.
There have also been studies evaluating adherence to an anti-inflammatory Mediterranean diet. A meta-analysis of 14 observational studies evaluated index-based diet intake (5 of these, used DII and variations). Five studies evaluated an adherence score to a Mediterranean diet. They found an inverse risk of IBD progression with this diet. They also noted a probable increased risk of disease aggravation in diets with high DII [96].
An extensive study on 3 cohorts of populations (Nurses Health Study 1 and 2 and the Health Professionals Follow-up Study) comprising 166,903 women and 41,931 men, had 328 cases of CD and 428 cases of UC. This study comprises a large number of participants. There are also enough cases followed prospectively for almost 5 million person years giving a strong statistical validity. A diet frequency questionnaire was repeated at baseline and 8 years later. Based on the EDIP (empirical dietary inflammatory pattern), diet was assessed by quartiles In the highest quartile of EDIP intake, there was a HR of 1.51 increase in CD (CD, 1.10–2.07), and significant difference in BMI. Interestingly, if EDIP was high at baseline and switched to low EDIP the risk of CD normalized. Similarly, it increased if the base was low and then high after the 8 year follow up. The EDIP had no effect on risk of UC [37].
UPF Intake and IBD
As discussed, the UPF (NOVA 4 classification) diets have been related to increased inflammatory markers. The multiple additives for cosmetics and preservation may not be captured in currently developed indices. There are many potential reasons for questioning the role of UPFs in abetting IBD [61]
A single study comprising over 105,000 participants followed for almost two and a half years could not assign increased risk to any pattern of diet or the addition of UPFs. However, there were only 75 who developed IBD and as well, follow up may have been too short [127].
Subsequent six publications supported the findings that UPFs posed an increased risk for IBD and particularly CD. Four were single studies and 2 were meta-analyses. Of the single studies Narula et al. followed 116,087 participants from 21 countries for over 9.7 years. Of these 467 developed IBD. Higher intake of UPFs was associated with a hazard ratio of 1.82 (CI 95 % 1.22 to 2.72) for both forms of IBD with more than 5 servings/day compared with 1–4 servings/d, p = 0.006 [128].
A smaller international study by Trakman et al. found in 531 participants (234 patients with CD, their relatives, and unrelated healthy controls) that patients with CD were exposed to more UPFs than family or controls [129].
A study of 3 cohorts comprising of 245,112 participants followed for several decades found a significant increased risk for CD (HR, 1.70; CI, 1.23–2.35; p = 0.0008). There was no consistent relationship with UC [130].
Another study with 187,854 participants in the UK Biobank followed for 10 years found an increased incidence risk for CD (HR 2.00 95% CI: 1.32, 3.03, p = 0.001), but not UC. This study also found an increased risk between UPF and need for surgery in IBD (HR 4.06 CI: 1.52, 10.86, p = 0.005) [131].
Subsequently two meta-analyses evaluated the association of UPFs and IBD. Twenty-four studies comprising over 4, 035, 000 participants from 20 countries with risks increased for IBD (RR, 1.13; CI, 1.06–1.21; P = 0.001). The risk was more for CD (RR, 1.19; CI, 1.00–1.41; P = 0.046) than for UC (RR = 1.11; CI, 0.99–1.26; P = 0.085) [97].
A meta – analysis of 5 studies (4/5 high quality) comprising 1,068,425 participants (followed for 13,594,422 person-years) reported 916 patients with CD and 1934 developed UC. Those who consumed the highest UPFs were in a higher risk for CD incidence ((HR, 1.71; 95% CI, 1.37–2.14; I2 = 0%). Those in the lower consumption categories had lower risks of CD (HR, 0.71; CI, 0.53–0.94). In the case of UC there was no significant increased risk with increased consumption of UPFs [98].
Glycemic Index and IBD
A search for studies evaluating the relationship between GI and IBD did not turn up any publications. Since high glycemic indices related foods contribute to complications shared with obesity and IBD more studies examining impact would be of interest in IBD.
A review expressing a viewpoint opinion suggested that the common link defining risk for IBD was consumption of carbohydrates [132]. Furthermore, a study which evaluated the impact of low, moderate or high consumption of sugar sweetened drinks on existing CD and UC found more than 50% increased risk for hospital visits and almost twofold risks for increased markers of inflammation [133]. This study could qualify under a UPF effect since addition of sugar or artificial sweeteners constitute some UPFs.
Of these 3 quantifiable diets UPFs seem to explain a link between obesity and a selective predisposition to CD. Hypothesized explanations for this discrepancy is discussed below.
However, all three dietary indices likely contribute to some aspects related to IBD and likely account for similar co-morbidities encountered between obesity and IBD.
Discussion
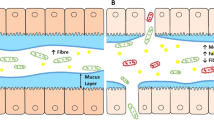
Obesity is a disease which promotes multiple other diseases. Among these the most recent addition is IBD. Pathogenic mechanisms postulated to predispose to IBD in obesity are outlined in Fig. 1. However, the association seems to affect mainly Crohn`s disease [14].
A simplified summary is shown of pathogenic relationships between obesity, some comorbidities and inflammatory bowel diseases (IBD). Obesogens are compounds of environmental pollutants which may impact metabolic homeostasis, and food additives which alter metabolic homeostasis (e.g., polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers, food additives and others [25,26,27]) and promote and inflammatory state. Pro-inflammatory cytokines, TNFα (tumor necrosis –alpha) inhibits tyrosine kinase at the insulin receptor leading to insulin resistance and formation of reactive oxygen species, IL- 6 (interleukin- 6), VCAM- 1 (vascular cell adhesion molecule- 1), MCP- 1(monocyte chemoattractant protein- 1), CES2 deficiency (carboxylesterase- 2, a triglyceride hydroxylase) promotes fatty liver in IBD and possibly in obesity, TMAO (trimethylamine) is a bacterial metabolic product from choline and carnitine which is oxidized in the liver to trimethylamine-N oxide and promotes platelet adhesiveness leading to CVD risks in obesity and possibly IBD and T2D. (figure taken from ref [13], available under the terms of the Creative Commons Attribution-Non Commercial 4.0 License.)
This review was undertaken to elaborate a relationship between these 2 diseases. The question posed centers around the observation that the pandemic of obesity predated the global expansion of IBD by about 1 or 2 decades. If obesity predisposes to IBD why/how did it take longer time for IBD to globalize and follow OB? Moreover, independent studies suggest that OB predisposes mainly to CD. Yet on a global scale CD and UC are closely correlated (although the relationship may vary in different geographic location). In addition co-morbidities of OB also are found in both forms of IBD [12].
In the last 2–3 decades diet as a potential pathogenic variable has come to the forefront for many diseases. The reasons for this include the recognition that the intestinal fauna plays a major role in pathogenesis. The intestinal anatomy consisting of a mucus layer and the function of the intestine as barrier to invasions and the impact of diet on these, was developed in parallel.
It has also been recognized that diet can affect the microbiome/metabolome and the protective functions of the intestine. It was also noted that different foods can alter internal milieu such that anti and pro-inflammatory cytokines are stimulated. In addition processing of food alters structures of foods. In this paradigm Western diets have been found to promote a low grade inflammation in obesity. This state is hypothesized to contribute to many obesity associated diseases.
The harmful potential of Western diets have been quantified, permitting a more objective way to assess effects of diets on disease outcomes [33, 34]. Using these methods we assessed individual diets on Obesity and IBD to generate a hypothesis which could account for epidemiological observations.
We noted previously that OB was less strongly correlated with GDP, a recognized marker of industrialization. On the other hand IBD was more closely correlated with GDP [20]. Assessing studies using these dietary methods we found that while the 3 main assignments to diet all impacted to some extent on both OB and IBD and mutually associated co-morbidities, UPFs seemed to be the best dietary assessment which connected OB to IBD.
Importantly, the independent assessment of the different dietary assignments, the glycemic index, dietary inflammatory indices and the NOVA classification of food processing found that so far, support findings of this connection for both. That is, OB is apparently related mainly with CD, and pre- clinical disease, UPFs appear to selectively favour CD also. However, overwhelming evidence for pathogenic role of diet in UC and obesity associated co-morbidities nevertheless, links these two conditions as well.
This differential impact of diet on risks for CD and UC is also reported in a recent meta—analysis of therapeutic benefits in either inducing remission or maintaining remission in CD. Although, these conclusions were of low or very-low GRADE of evidence they also found no significant benefits in UC [134]. In light of this and supported by studies listed herein, the American Association of Gastroenterology recommends the use of the anti-inflammatory Mediterranean diet for patients with IBD [135].
This literature review raises two questions. First, how is the geographic and time delay relationship between OB and IBD explained? UPFs were introduced into western diets after the Second World War and production increased progressively [136]. These products were cheaper, high-energy foods made into snacks and sugary drinks. Although they were produced in countries with high incomes and more advanced industrialization, they could be purchased by poorer countries. In a poignant study by Monteiro et al., 79 countries were assessed for sales of UPFs between 1998–2012 and correlated with gross national income (GNI). There was an inverse relationship between the GNI and growth of UPF national sales [137]. This could then explain a weaker correlation between obesity and national wealth as depicted by GDP. That is the availability of cheap high caloric UPFs led to development of obesity first and as industrialization followed, IBD rates climbed. This could then explain a weaker correlation between obesity and national wealth as depicted by GDP. Another plausible contribution to the delay between obesity followed by IBD is supported by findings from the GEM project where microbial dysbiosis can precede clinical IBD by several years delaying clinical disease and diagnosis [74]
The second, is how to account for the differential impact of UPFs in diets on CD and UC. Several possibilities are entertained. The simplest explanation questions whether there is a true differential impact of diets on CD and UC, or could this be due to statistical bias? However, this possibility is unlikely due to the multiple studies showing the effect and as well the impact shown with therapeutic trials. It is emphasized that this analysis does not clearly distinguish effects of inflammation from UPF containing diets. Although the effects of UPFs seem to be more on CD pathogenesis. This will be further elaborated below.
Another explanation likely relates to the anatomical differences in intestinal and colonic functions. In general UPFs are part of ingested foods and as a result are metabolized or absorbed mainly in the small bowel with unclear quantities reaching the colon.
Amounts of UPFs arriving at the colon also takes into consideration the quantity of ingested additives and for now there is a relative lack of information about this. In addition, there is a likelihood that different UPFs exert different effects. An example of this possibility is provided by a recent study examining the 30-year impact of UPF consumption on all-cause mortality. In an evaluation of two long term US studies: the National Health and Nutrition Examination Survey (NHANES) and Health Professionals Follow-Up Study (HPFS), UPFs were classified into 9 groupings and the highest intake was compared to the lowest intake. A small but statistically significant 4% increased mortality was reported in those with the highest UPF intake. Among the UPFs meat/poultry/seafood ready to eat products stood out most, however, the findings selectively excluded mortality from cancers and CVD [138].
The apparent differential effect of UPFs on CD and UC, could emphasize that these two diseases although, related are different. Similar paradigm is observed with the effects of smoking, where it is protective against UC and detrimental in CD [139]. Other remaining dichotomous effects may relate to the therapeutic effects of probiotics which seem to work more favorably in maintaining remission in UC but less so in CD [140,141,142,143]. In both instances there is no clear explanations for the opposite effects. Smoking has not been shown to be causative for either CD or UC [144]. In the case of probiotics there may be too few randomized controlled trials in CD to clearly state an advantage for UC [145]. Furthermore, a meta-analysis of over 550 patients found that 21% of patients with UC and 30% of patients with CD achieved remission with the ultimate potential probiotic, fecal microbial transplant [146].
Strengths of this review are that despite the lack of granularity in defining effects of multiple food additives, there appears to be a consensus that the dietary factor which explains the relationship between obesity and Crohn’s disease lies within UPFs. There is less consensus with IBD for application of pro-inflammatory diets or diets with high glycemic indices.
Some limitations need to be considered. Firstly the findings are not based on a systematic review, rather it is an examination of retrospective patterns. In order to reduce this weakness available meta-analyses were included. Secondly, although, all the characteristics mentioned in this review (addition of obesogens, glycemic index, dietary inflammatory indices and the levels of food processing) impact on Western diets, they assess different aspects. Obesogens including food additives make up part UPFs. There is no uniform consensus on how much of these quantifiable variables impact on each food and what quantities are needed to incur harm. Table 5 outlines some knowledge gaps which limits conclusions in this review.
Conclusions
In conclusion this assessment of the literature suggests that the epidemiological links between OB and IBD are best exemplified by the introduction of UPFs into Western diet.
In other words neither an inflammatory diet nor foods with a high glycemic index seem to describe the apparent close association of CD with OB. However, all three quantifiable aspects of Western diets could account for the connections between obesity with its associated other co-morbidities seen in both OB and IBD. The term UPF encompasses a wide array of food additives which may have individual or combined effects on gut defense mechanisms. Much work is needed still to tease out granularity of different aspects which make up UPFs.
Key References
-
Kopp W. How the Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes.Metabolic Syndrome and Obeitys. 2019;12:2221–2236. https://doi.org/10.2147/DMSO.S216791.
-
This paper summarizes the global spread of obesity and many of its accompanying comorbidities.
-
This paper names obesity related comorbidities and diseases as `diseases of civilization
-
-
Szilagyi A. Interactions Between obesity and Inflammatory Bowel Diseases: The pandemic promoting ‘civilization’ diseases Eeuropean Medical Journal- Gastroenterol. 2021;10[1]:78–87.`
-
This paper summarizes pathogenic relationships between obesity and inflammatory bowel diseases
-
The paper discusess proinflammatory drivers and also describes some microbial similarities previously described between obesity and inflammatory bowel diseases
-
-
Rahmani J., Kord-Varkaneh H., Hekmatdoost A., Thompson J, Clark C, Salehisahlabadi A, et al. Body mass index and risk of inflammatory bowel disease: A systematic review and dose–response meta-analysis of cohort studies of over a million participants. Obes Rev. 2019;20(9):1312–1320. https://doi.org/10.1111/obr.12875.
-
The paper evaluates the relationship between body mass index and inflammatory bowel disease in a large cohort of people. The paper concludes that Crohn`s disease has a closer link with obesity than ulcerative colitis.
-
-
Ng S.C., Shi H.Y., Hamidi N., Underwood FE, Tang W, Benchimol EI,et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21 st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769 - 2778. https://doi.org/10.1016/S0140-6736(17)32448-0.
-
These two papers describe the global spread of inflammatory bowel diseases.
-
-
Ng S.C., Kaplan G.G., Tang W., Banerjee R, Adigopula B, Underwood FE,et al. Population Density and Risk of Inflammatory Bowel Disease: A Prospective Population-Based Study in 13 Countries or Regions in Asia-Pacific. American Journal of Gastroenterology. 2019;114(1):107–115. https://doi.org/10.1038/s41395-018-0233-2.
-
In addition to describing Inflammatoery Bowel diseases in Asia; previously low incidence areas the paper supports the impact of industrialization and population growth as epidemiological variables promoting these inflammatory diseases.
-
-
Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.;. et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutrition. 2019;22(5):936-941. https://doi.org/10.1017/S1368980018003762.
-
This paper was instrumental in introducing the concept of ultra- processed foods and also includes the rationale for their production but as well as the spread of its popularity.
-
-
Fitzpatrick J.A., Halmos E.P., Gibson, P.R., Machado P.P. Ultraprocessed foods and risk of Crohn`s disease:How much is too much? Clinical Gastroenterology and Hepatology. 2023;21:2478 - 2480. https://doi.org/10.1016/j.cgh.2023.03.009.
-
this is a recent editorial exposing the potential impact of ultra-processed foods on inflammatory bowel diseases
-
It also points out a number of remaining questions as to the need to evaluate quantity and precision of type of foods involved.
-
-
Bolte L.A., Vich Vila A., Imhann F., Imhann F, Collij V, Gacesa R, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70(7):1287 - 1298. https://doi.org/10.1136/gutjnl-2020-322670.
-
This large study evaluated the impact of food patterns on the microbiome The study found processed foods and derived from animals, induced similar and consistent groups of bacteria across healthy, irritable bowel syndrome and inflammatory bowel diseases patients.
-
-
Halmos EP, Godny L, Vanderstappen J, Sarbagili-Shabat C, Svolos V. Role of diet in prevention versus treatment of Crohn's disease and ulcerative colitis. Frontline Gastroenterol. 2024;15(3):247-257. https://doi.org/10.1136/flgastro-2023-102417. eCollection 2024
-
Limketkai B.N., Godoy-Brewer G., Parian A., Krishna M, Shah ND, White J, et al. Dietary intervention for the treatment of inflammatory bowel diseases: An updated systematic review and meta-analysis, Clinical Gastroenterology and Hepatology 2023; 21: 2508 - 2525. https://doi.org/10.1016/j.cgh.2022.11.026.
-
This interesting systematic review tends to support the overall findings of the current review of diets for inflammatory bowel disesaes while the evidences are not robust theer is a suggestion that to date current dietary interventions also favor Crohn`s disease over ulcerative colitis.
-
-
Monteiro C.A., Moubarac J.C., Cannon G., Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obesity Reviews. 2013 Nov;14 Suppl 2:21 - 8. https://doi.org/10.1111/obr.12107.
-
This is the key paper which suggests that the current global spread of obesity is related largely to the cheap availability of ultra processed foods, snacks and sugary drinks.
-
The gist of the current review points out that while obesity may be dependent largely on the use of ultra-processed foods, inflammatory bowel diseases needed the arrival of accompanying industrialization as well as the use of ultra-processed foods.
-
As such the paper may account for the decade long delay in the noted association of Crohn`s disease with obesity.
-
Data Availability
No datasets were generated or analysed during the current study.
References
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. https://doi.org/10.1111/j.1753-4887.2011.00456.x.
Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world-a growing challenge. N Engl J Med. 2007;356(3):213–5. https://doi.org/10.1056/NEJMp068177.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98. https://doi.org/10.1038/s41574-019-0176-8.
Allen AM, Pose E, Reddy KR, Russo MW, Kamath PS. Nonalcoholic Fatty Liver Disease Gets Renamed as Metabolic Dysfunction-Associated Steatotic Liver Disease: Progress But With Challenges. Gastroenterology. 2024;166(2):229–34. https://doi.org/10.1053/j.gastro.2023.11.007.
James WPT. WHO recognition of the global obesity epidemic. Int J Obes (Lond). 2008;32(Suppl 7):S120-126. https://doi.org/10.1038/ijo.2008.247.
Kopp W. How the Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221–36. https://doi.org/10.2147/DMSO.S216791.
Mensah GA, Mokdad AH, Ford E, Narayan KM, Giles WH, Vinicor F, et al. Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol Clin. 2004;22(4):485–504. https://doi.org/10.1016/j.ccl.2004.06.005.
Kinlen D, Cody D, O’Shea D. Complications of obesity. Q J Med. 2018;111(7):437–43. https://doi.org/10.1093/qjmed/hcx152.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. https://doi.org/10.1056/NEJMsr1606602.
Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12:1347–52. https://doi.org/10.1097/00042737-200012120-00015.
Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011;56(3):837–44. https://doi.org/10.1007/s10620-010-1541-6.
Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22(35):7868–81. https://doi.org/10.3748/wjg.v22.i35.7868.
Szilagyi A. Interactions Between obesity and Inflammatory Bowel Diseases: The pandemic promoting ‘civilization’ diseases. Eur Med J Gastroenterol. 2021;10(1):78–87.
Rahmani J, Kord-Varkaneh H, Hekmatdoost A, Thompson J, Clark C, Salehisahlabadi A, et al. Body mass index and risk of inflammatory bowel disease: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes Rev. 2019;20(9):1312–20. https://doi.org/10.1111/obr.12875.
Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34(4):717–32. https://doi.org/10.1016/j.psc.2011.08.005.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. https://doi.org/10.1053/j.gastro.2011.10.001.
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. https://doi.org/10.1016/S0140-6736(17)32448-0.
Ng SC, Kaplan GG, Tang W, Banerjee R, Adigopula B, Underwood FE, et al. Population Density and Risk of Inflammatory Bowel Disease: A Prospective Population-Based Study in 13 Countries or Regions in Asia-Pacific. Am J Gastroenterol. 2019;114(1):107–15. https://doi.org/10.1038/s41395-018-0233-2.
Szilagyi A, Leighton H, Burstein B, Xue X. Latitude, sunshine, and human lactase phenotype distributions may contribute to geographic patterns of modern disease: the inflammatory bowel disease model. Clin Epidemiol. 2014;6:183–98. https://doi.org/10.2147/CLEP.S59838.eCollection2014.
Szilagyi A, Smith BE, Sebbag N, Xue X. Global associations of national economic wealth are more robust with inflammatory bowel diseases than with obesity. Med Hypotheses. 2021;148:110505. https://doi.org/10.1016/j.mehy.2021.110505.
Szilagyi A, Smith BE, Sebbag N, Xue X. Changing Patterns of Relationships Between Geographic Markers and IBD: Possible Intrusion of obesity. Crohn’s, Colitis 360. 2020;2(2):otaa044. https://doi.org/10.1093/crocol/otaa044.
Catalán V, Avilés-Olmos I, Rodríguez A, Becerril S, Fernández-Formoso JA, et al. Time to Consider the “Exposome Hypothesis” in the Development of the obesity Pandemic. Nutrients. 2022;14(8):1597. https://doi.org/10.3390/nu14081597.
Rogler G, Vavricka S. Exposome in IBD: recent insights in environmental factors that influence the onset and course of IBD. Inflamm Bowel Dis. 2015;21(2):400–8. https://doi.org/10.1097/MIB.0000000000000229.
Adolph TE, Zhang J. Diet fuelling inflammatory bowel diseases: preclinical and clinical concepts. Gut. 2022;71(12):2574–86. https://doi.org/10.1136/gutjnl-2021-326575.
Shahnazaryan U, Wójcik M, Kuryłowicz A. Role of obesogens in the pathogenesis of obesity. Medicina (Kaunas). 2019;55(9):515. https://doi.org/10.3390/medicina55090515.
Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11(11):653–61. https://doi.org/10.1038/nrendo.2015.163.
Iughetti L, Lucaccioni L, Predieri B. Childhood obesity and environmental pollutants: a dual relationship. Acta Biomed. 2015;86(1):5–16.
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6. https://doi.org/10.1093/ajcn/34.3.362.
Vega-López S, Venn BJ, Slavin JL. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients. 2018;10(10):1361. https://doi.org/10.3390/nu10101361.
Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266S-273S. https://doi.org/10.1093/ajcn/76/1.266S.
de CarvalhoVidigal F, GuedesCocate P, Gonçalves Pereira L, de CássiaGonçalvesAlfenas R. The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutr Hosp. 2012;27(5):1391–8. https://doi.org/10.3305/nh.2012.27.5.5917.
Bulló M, Casas R, Portillo MP, Basora J, Estruch R, García-Arellano A, et al. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2013;23(5):443–50. https://doi.org/10.1016/j.numecd.2011.09.009.
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139(12):2365–72. https://doi.org/10.3945/jn.109.114025.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. https://doi.org/10.1017/S1368980013002115.
Pawlow X, Ott R, Winkler C, Ziegler AG, Hummel S. A new mathematical approach to improve the original dietary inflammatory index (DII) calculation. PLoS One. 2021;16(11):e0259629. https://doi.org/10.1371/journal.pone.0259629.
Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560–70. https://doi.org/10.3945/jn.115.228718.
Lo CH, Lochhead P, Khalili H, Song M, Tabung FK, Burke KE, Richter JM, Giovannucci EL, Chan AT, Ananthakrishnan AN. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2020;159(3):873-883.e1. https://doi.org/10.1053/j.gastro.2020.05.011.
van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, Ferreira I, van Greevenbroek MM, van der Kallen CJ, et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr. 2013;98(6):1533–42. https://doi.org/10.3945/ajcn.112.056333.
Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. Systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10(11):3164. https://doi.org/10.3390/cells10113164.
Davis C, Bryan J, Hodgson J, Murphy K. Definition of the mediterranean diet: a literature review. Nutrients. 2015;7(11):9139–53. https://doi.org/10.3390/nu7115459.
Cambeses-Franco C, Gude F, Benítez-Estévez AJ, González-García S, Leis R, Sánchez-Castro J, Moreira MT, et al. Traditional Atlantic Diet and Its Effect on Health and the Environment: A Secondary Analysis of the GALIAT Cluster Randomized Clinical Trial. J Am Med Assoc Netw Open. 2024;7(2):e2354473. https://doi.org/10.1001/jamanetworkopen.2023.54473.
Hodge AM, Bassett JK, Shivappa N, Hébert JR, English DR, Giles GG, et al. Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control. 2016;27(7):907–17. https://doi.org/10.1007/s10552-016-0770-1.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. https://doi.org/10.1126/science.1110591.
Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13(4):1067. https://doi.org/10.3390/nu13041067.
Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014;13:5. https://doi.org/10.1186/1475-2891-13-5.
Ricker MA, Haas WC. Anti-inflammatory diet in clinical practice: a review. Nutr Clin Pract. 2017;32(3):318–25. https://doi.org/10.1177/0884533617700353.
Shafiee NH, Manaf ZA, Mokhtar NM, Raja Ali RA. Anti-inflammatory diet and inflammatory bowel disease: what clinicians and patients should know? Intest Res. 2021;19(2):171–85. https://doi.org/10.5217/ir.2020.00035.
O’Connor L, Imamura F, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin Nutr. 2018;37(4):1313–22. https://doi.org/10.1016/j.clnu.2017.05.030.
Ma X, Nan F, Liang H, Shu P, Fan X, Song X, et al. Excessive intake of sugar: An accomplice of inflammation. Front Immunol. 2022;13:988481. https://doi.org/10.3389/fimmu.2022.988481.
Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function- A systematic review and meta-analysis. Nutrients. 2015;7(9):7651–75. https://doi.org/10.3390/nu7095356.
Papaioannou K-G, Kadi F, Nilsson A. Consumption of vegetables is associated with systemic inflammation in older adults. Nutrients. 2022;14(9):1765. https://doi.org/10.3390/nu14091765.
Manchal S, Murthy KNC, Patil BS. Crucial facts about health benefits of popular cruciferous vegetables. J Funct Foods. 2012;4(1):94–106. https://doi.org/10.1016/j.jff.2011.08.004.
Rajaram S, Damasceno NRT, Braga RM, Martinez R, Kris-Etherton P, Sala-Vila A. Effect of nuts on markers of inflammation and oxidative Stress: A Narrative review. Nutrients. 2023;5(5):1099. https://doi.org/10.3390/nu15051099.
Nieman KM, Anderson B, Cifelli CJ. The Effects of dairy product and dairy protein intake on inflammation: A Systematic Review of the literature. J Am Coll Nutr. 2020;40(6):571–82. https://doi.org/10.1080/07315724.2020.1800532.
Bordoni A, Danesi F, Di Nunzio M, Taccari A, Valli V. Ancient wheat and health: a legend or the reality? A review on KAMUT khorasan wheat. Int J Food Sci Nutr. 2016;68(3):278–86. https://doi.org/10.1080/09637486.2016.1247434.
Lail H, Feresin R, Hicks D, Stone B, Price E, Wanders D. Berries as a Treatment for obesity-Induced Inflammation: Evidence from Preclinical Models. Nutrients. 2021;13(2):334. https://doi.org/10.3390/nu13020334.
Miles EA, Calder PC. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: a narrative review. Front Immunol. 2021;12:712608.
Monteiro CA. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009;12(5):729–31. https://doi.org/10.1017/S1368980009005291.
Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. https://doi.org/10.1017/S1368980018003762.
Fitzpatrick JA, Halmos EP, Gibson PR, Machado PP. Ultraprocessed foods and risk of Crohn`s disease: How much is too much? Clin Gastroenterol Hepatol. 2023;21:2478–80. https://doi.org/10.1016/j.cgh.2023.03.009.
Vissers E, Wellens J, Sabino J. Ultra-processed foods as a possible culprit for the rising prevalence of inflammatory bowel diseases. Front Med. 2022;9:1058373. https://doi.org/10.3389/fmed.2022.1058373.
Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022;7(12):1128–40. https://doi.org/10.1016/S2468-1253(22)00169-8.
Poti JM, Braga B, Qin B. Ultra-processed Food Intake and obesity: What Really Matters for Health-Processing or Nutrient Content? Curr Obes Rep. 2017;6(4):420–31. https://doi.org/10.1007/s13679-017-0285-4.
Mignogna C, Costanzo S, Di Castelnuovo A, Ruggiero E, Shivappa N, Hebert JR, et al. The inflammatory potential of the diet as a link between food processing and low-grade inflammation: An analysis on 21,315 participants to the Moli-sani study. Clin Nutr. 2022;41(10):2226–34. https://doi.org/10.1016/j.clnu.2022.08.020.
Silva CA, Santos IDS, Shivappa N, Hebert JR, Crivellenti LC, Sartorelli DS. The role of food processing in the inflammatory potential of diet during pregnancy. Rev Saude Publica. 2019;53:113. https://doi.org/10.11606/S1518-8787.2019053001154. (eCollection 2019).
Mohamadi A, Shiraseb F, Mirzababaei A, Barekzai AM, Clark CCT, Aali Y, et al. Inflammatory markers may mediate the relationship between processed meat consumption and metabolic unhealthy obesity in women: a cross sectional study. Sci Rep. 2023;13(1):9261. https://doi.org/10.1038/s41598-023-35034-6.
Valicente VM, Peng CH, Pacheco KN, Lin L, Kielb EI, Dawoodani E, et al. Ultraprocessed foods and obesity risk: a critical review of reported mechanisms. Adv Nutr. 2023;14(4):718–38. https://doi.org/10.1016/j.advnut.2023.04.006.
Ruan W, Engevik MA, Spinler JK, Versalovic J. Healthy human gastrointestinal microbiome: composition and function after a decade of exploration. Dig Dis Sci. 2020;65(3):695–705. https://doi.org/10.1007/s10620-020-06118-4.
Bibbo S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20:4742–9.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. https://doi.org/10.1038/nature09944.
Tanaka S, Nemoto Y, Takei Y, Morikawa R, Oshima S, Nagaishi T, et al. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelia damage. Biochem Biophys Res Commun. 2020;522(4):971–7. https://doi.org/10.1016/j.bbrc.2019.11.158.
Bolte LA, Vich VA, Imhann F, Imhann F, Collij V, Gacesa R, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70(7):1287–98. https://doi.org/10.1136/gutjnl-2020-322670.
Johansson ME, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68(22):3635–41. https://doi.org/10.1007/s00018-011-0822-3.
Neustaeter A, Leibovitzh H, Turpin W, Croitoru K, CCC GEM consortium. Understanding Predictors of Crohn’s Disease: Determinants of Altered Barrier Function in Pre-Disease Phase of Crohn’s Disease. J Can Assoc Gastroenterol. 2024;7:68–77.
Suriano F, Nyström EEL, Serg D, Gustafsson JK. Diet, microbiota, and the mucus layer: The guardians of our health. Front Immunol. 2022;13:953196. https://doi.org/10.3389/fimmu.2022.953196.
Dupont A, Heinbockel L, Brandenburg K, Hornef MW. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes. 2014;5(6):761–5. https://doi.org/10.4161/19490976.2014.972238.
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–103. https://doi.org/10.1038/s41591-019-0495-2.
Raygoza Garay JA, Turpin W, Lee SH, Smith MI, Goethel A, Griffiths AM, et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology. 2023;165(3):670–81. https://doi.org/10.1053/j.gastro.2023.05.032.
Keshteli AH, Valcheva R, Nickurak C, Park H, Mandal R, van Diepen K, et al. Anti-Inflammatory diet prevents subclinical colonic inflammation and alters metabolomic profile of ulcerative colitis patients in clinical remission. Nutrients. 2022;14(16):3294. https://doi.org/10.3390/nu14163294.
Marion-Letellier R, Amamou A, Savoye G, Ghosh S. Inflammatory bowel diseases and food additives: To add fuel on the flames! Nutrients. 2019;11(5):1111. https://doi.org/10.3390/nu11051111.
Fitzpatrick JA, Gibson PR, Taylor KM, Anderson EJ, Friedman AB, Ardalan ZS, Smith RL, et al. Clinical Trial: the effects of emulsifiers in the food supply on disease activity in Crohn’s disease: an exploratory double-blinded randomised feeding trial. Aliment Pharmacol Ther. 2025;61(8):1276–89. https://doi.org/10.1111/apt.70041.
Halmos EP, Godny L, Vanderstappen J, Sarbagili-Shabat C, Svolos V. Role of diet in prevention versus treatment of Crohn’s disease and ulcerative colitis. Frontline Gastroenterol. 2024;15(3):247–57. https://doi.org/10.1136/flgastro-2023-102417.eCollection2024.
Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6(3):223–36. https://doi.org/10.1016/S2213-8587(17)30200-0.
Namazi N, Larijani B, Azadbakht L. Dietary Inflammatory Index and its association with the risk of cardiovascular diseases, metabolic Syndrome, and morta lity: A Systematic Review and Meta-Analysis. Horm Metab Res. 2018;50(5):345–58. https://doi.org/10.1055/a-0596-8204.
Farhang MA, Nikniaz L, Nikniaz Z, Dehghan P. Dietary inflammatory index potentially increases blood pressure and markers of glucose homeostasis among adults: findings from an updated systematic review and meta-analysis. Public Health Nutr. 2020;23(8):1362–80. https://doi.org/10.1017/S1368980019003070.
Farhangi MA, Vajdi M. The association between dietary inflammatory index and risk of central obesity in adults: An updated systematic review and meta-analysis. Int J Vitam Nutr Res. 2020;90(5–6):535–52. https://doi.org/10.1024/0300-9831/a000648.
Yi Q, Li X, He Y, Xia W, Shao J, Ye Z, et al. Associations of dietary inflammatory index with metabolic syndrome and its components: a systematic review and meta-analysis. Public Health Nutr. 2021;24(16):5463–70. https://doi.org/10.1017/S1368980021000288.
Tan QQ, Du XY, Gao CL, Xu Y. Higher dietary Inflammatory index Scores Increase the risk of diabetes mellitus: A meta-analysis and systematic review. Front Endocrinol (Lausanne). 2021;12:693144. https://doi.org/10.3389/fendo.2021.693144.
Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (London). 2020;44(10):2080–91. https://doi.org/10.1038/s41366-020-00650-z.
Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. J Nutr. 2021;125(3):308–18. https://doi.org/10.1017/S0007114520002688.
Mambrini SP, Menichetti F, Ravella S, Pellizzari M, De Amicis R, Foppiani A, et al. Ultra-processed food consumption and incidence of obesity and cardiometabolic risk factors in adults: a systematic review of prospective studies. Nutrients. 2023;15(11):2583. https://doi.org/10.3390/nu15112583.
Henney AE, Gillespie CS, Alam U, Hydes TJ, Cuthbertson DJ. Ultra-processed food intake is associated with non-alcoholic fatty liver disease in adults: a systematic review and meta-analysis. Nutrients. 2023;15(10):2266. https://doi.org/10.3390/nu15102266.
Lv J-L, Wei Y-F, Sun J-N, Shi YC, Liu FH, Sun MH, et al. Ultra-processed food consumption and metabolic disease risk: an umbrella review of systematic reviews with meta-analyses of observational studies. Front Nutr. 2024;11:1306310. https://doi.org/10.3389/fnut.2024.1306310.
Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. https://doi.org/10.1016/j.numecd.2013.04.008.
Milajerdi A, Saneei P, Larijani B, Esmaillzade A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: a systematic review and meta-analysis of randomized clinical trials. Am J Clin Nutr. 2018;107(4):593–606. https://doi.org/10.1093/ajcn/nqx042.
Tian Z, Zhuang X, Zhao M, Zhuo S, Li X, Ma R, et al. Index-based dietary patterns and inflammatory bowel disease: a systematic review of observational studies. Adv Nutr. 2021;12(6):2288–300. https://doi.org/10.1093/advances/nmab069.
Babaei A, Pourmotabbed A, Talebi S, Mehrabani S, Bagheri R, Ghoreishy SM, et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: a systematic review and dose-response meta-analysis of 4 035 694 participants. Nutr Rev. 2023;82(7):861–71. https://doi.org/10.1093/nutrit/nuad101.
Narula N, Chang NH, Mohammad D, Wong ECL, Ananthakrishnan AN, Chan SSM, et al. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2023;21(10):2483–95. https://doi.org/10.1016/j.cgh.2023.01.012.
Lennerz B, Lennerz JK. Food Addiction, High-Glycemic-Index Carbohydrates, and obesity. Clin Chem. 2018;64(1):64–71. https://doi.org/10.1373/clinchem.2017.273532.
Kaartinen NE, Knekt P, Kanerva N, Valsta LM, Eriksson JG, Rissanen H, et al. Dietary carbohydrate quantity and quality in relation to obesity: A pooled analysis of three Finnish population-based studies. Scand J Public Health. 2016;44(4):385–93. https://doi.org/10.1177/1403494815622860.
Khan TA, Tayyiba M, Agarwal A, Mejia SB, de Souza RJ, Wolever TMS, et al. Relation of total sugars, sucrose, fructose, and added sugars with the risk of cardiovascular disease: a systematic review and dose response meta-analysis of prospective cohort studies. Mayo Clin Proc. 2019;94(12):2399–414. https://doi.org/10.1016/j.mayocp.2019.05.034.
Askari M, Dehghani A, Abshirini M, Raeisi T, Alizadeh S. Glycemic index, but not glycemic load, is associated with an increased risk of metabolic syndrome: Meta-analysis of observational studies. Int J Clin Pract. 2021;75(10):e14295. https://doi.org/10.1111/ijcp.14295.
Lee D, Chiavaroli L, Ayoub-Charette S, Khan TA, Zurbau A, Au-Yeung F, et al. Important food sources of fructose-containing sugars and non-alcoholic fatty liver disease: a systematic review and meta-analysis of controlled trials. Nutrients. 2022;14(14):2846. https://doi.org/10.3390/nu14142846.
Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultraprocessed foods: a preliminary study with 98 ready-to-eat foods. Food Function. 2016;7(5):2338–46. https://doi.org/10.1039/C6FO00107F.
Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology. 2004;126:1504–17. https://doi.org/10.1053/j.gastro.2004.01.063.
El Hadad J, Schreiner P, Vavricka S, Greuter T. The genetics of infammatory bowel disease. Mol Diagn Ther. 2024;28:27–35. https://doi.org/10.1007/s40291-023-00678-7.
Vagianos K, Dolovich C, Witges K, Graff LA, Bernstein CN. Ultra-processed food, disease activity, and inflammation in ulcerative colitis: the manitoba living with IBD study. Am J Gastroenterol. 2024;119(6):1102–9. https://doi.org/10.14309/ajg.0000000000002667.
Ananathakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–9. https://doi.org/10.1053/j.gastro.2011.11.040.
Olmedo Martín RV, González-Molero I, OlveiraFuster G, AmoTrillo V, Jiménez Pérez M. Vitamin D deficiency in outpatients with inflammatory bowel disease: prevalence and association with clinical-biological activity. Rev Esp Enferm Dig. 2019;111(1):46–54. https://doi.org/10.17235/reed.2018.5714/2018.
Battat R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, et al. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20(6):1120–8. https://doi.org/10.1097/MIB.0000000000000024.
Lee TW, Kolber MR, Fedorak RN, van Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis. 2012;6(3):267–75. https://doi.org/10.1016/j.crohns.2011.09.010.
Waśko-Czopnik D, Paradowski L. The influence of deficiencies of essential trace elements and vitamins on the course of Crohn’s disease. Adv Clin Exp Med. 2012;21(1):5–11.
Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105(10):2195–201. https://doi.org/10.1038/ajg.2010.192.
Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73. https://doi.org/10.1038/ajg.2011.44.
Opstelten JL, de Vries JHM, Wools A, Siersema PD, Oldenburg B, Witteman BJM. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin Nutr. 2019;38(4):1892–8. https://doi.org/10.1016/j.clnu.2018.06.983.
Peters V, Spooren CEGM, Pierik MJ, Weersma RK, van Dullemen HM, Festen EAM, et al. Dietary Intake Pattern is Associated with Occurrence of Flares in IBD Patients Jonkers DMAE. J Crohns Colitis. 2021;15(8):1305–15. https://doi.org/10.1093/ecco-jcc/jjab008.
Opstelten JL, Leenders M, Dik VK, Chan SS, van Schaik FD, Khaw KT, et al. Dairy products, dietary calcium, and risk of inflammatory bowel disease: results from a european prospective cohort investigation. Inflamm Bowel Dis. 2016;22(6):1403–11. https://doi.org/10.1097/MIB.0000000000000798.
Iamartino L, Elajnaf T, Kallay E, Schepelmann M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24(36):4119–31. https://doi.org/10.3748/wjg.v24.i36.4119.
Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated Linoleic Acid Ameliorates Inflammation-Induced Colorectal Cancer in Mice through Activation of PPARγ1, 2, 3. J Nutrition. 2010;140:515–21.
JanssenDuijghuijsen L, Looijesteijn E, van den Belt M, Gerhard B, Ziegler M, Ariens R, et al. Changes in gut microbiota and lactose intolerance symptoms before and after daily lactose supplementation in individuals with the lactase nonpersistent genotype. Am J Clin Nutr. 2024;119(3):702–10. https://doi.org/10.1016/j.ajcnut.2023.12.016.
Christensen C, Knudsen A, Arnesen EK, Hatlebakk JG, Sletten IS, Fadnes LT. Diet, food, and nutritional exposures and inflammatory bowel disease or progression of disease: an umbrella review. Adv Nutr. 2024;15(5):100219. https://doi.org/10.1016/j.advnut.2024.100219.
de Graaf MCG, Spooren CEGM, Hendrix EMB, Hesselink MAM, Feskens EJM, Smolinska A, et al. Diet quality and dietary inflammatory index in dutch inflammatory bowel disease and irritable bowel syndrome patients. Nutrients. 2022;14(9):1945. https://doi.org/10.3390/nu14091945.
Mirmiran P, Moslehi N, Morshedzadeh N, Shivappa N, Hébert JR, Farsi F, et al. Does the inflammatory potential of diet affect disease activity in patients with inflammatory bowel disease? Nutr J. 2019;18(1):65. https://doi.org/10.1186/s12937-019-0492-9.
Shivappa N, Hébert JR, Rashvand S, Rashidkhani B, Hekmatdoost A. Inflammatory potential of diet and risk of ulcerative colitis in a case-control study from Iran. Nutr Cancer. 2016;68(3):404–9. https://doi.org/10.1080/01635581.2016.1152385.
Bian D, Liu X, Wang C, Jiang Y, Gu Y, Zhong J, et al. Association between dietary inflammatory index and sarcopenia in Crohn’s disease patients. Nutrients. 2022;14(4):901. https://doi.org/10.3390/nu14040901.
Meyer A, Chan SSM, Touvier M, Julia C, Tjønneland A, Kyrø C. Inflammatory potential of the diet and risk of Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2025;61:1032–42. https://doi.org/10.1111/apt.18497.
Vasseur P, Dugelay E, Benamouzig R, Savoye G, Lan A, Srour B, Hercberg S, et al. Dietary patterns, ultra-processed food, and the risk of inflammatory bowel diseases in the nutrinet-santé cohort. Inflamm Bowel Dis. 2021;27(1):65–73.
Narula N, Wong ECL, Dehghan M, Mente A, Rangarajan S, Lanas F, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. Br Med J. 2021;374:n1554. https://doi.org/10.1136/bmj.n1554.
Trakman GL, Lin WYY, Hamilton AL, Wilson-O’Brien AL, Stanley A, et al. Processed food as a risk factor for the development and perpetuation of Crohn’s disease-the ENIGMA study. Nutrients. 2022;14(17):3627. https://doi.org/10.3390/nu14173627.
Lo CH, Khandpur N, Rossato SL, Lochhead P, Lopes EW, Burke KE, et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin Gastroenterol Hepatol. 2022;20(6):e1323–37. https://doi.org/10.1016/j.cgh.2021.08.031.
Chen J, Wellens J, Kalla R, Fu T, Deng M, Zhang H, et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J Crohn’s Colitis. 2023;17(4):535–52. https://doi.org/10.1093/ecco-jcc/jjac167.
Britto S, Kellermayer R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease. J Crohn’s Colitis. 2019;13(7):942–8. https://doi.org/10.1093/ecco-jcc/jjz011.
Ahsan M, Koutroumpakis F, Rivers CR, Wilson AS, Johnston E, Hashash JG, et al. High sugar-sweetened beverage consumption is associated with increased health care utilization in patients with inflammatory bowel disease: a multiyear, prospective analysis. J Acad Nutr Diet. 2022;122(8):1488-1498.e1. https://doi.org/10.1016/j.jand.2022.01.001.
Limketkai BN, Godoy-Brewer G, Parian A, Noorian S, Krishna M, Shah ND, et al. Dietary intervention for the treatment of inflammatory bowel diseases: An updated systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21:2508–25. https://doi.org/10.1016/j.cgh.2022.11.026.
Hashash JG, Elkins J, Lewis JD, Binion DG. AGA Clinical practice update on diet and nutritional therapies in patients with inflammatory bowel disease: Expert review. Gastroenterology. 2024;166(3):521–32.
Logan AC, D’Adamo CR, Pizzorno JE, Prescott SL. “Food faddists and pseudoscientists!”: Reflections on the history of resistance to ultra-processed foods. Explore (NY). 2023;20(4):470–6. https://doi.org/10.1016/j.explore.2023.12.014.
Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(Suppl 2):21–8. https://doi.org/10.1111/obr.12107.
Fang Z, Rossato SL, Hang D, Khandpur N, Wang K, Lo CH, et al. Association of ultra-processed food consumption with all cause and cause specific mortality: population based cohort study. Br Med J. 2024;385:e078476. https://doi.org/10.1136/bmj-2023-078476.
Lakatos PL, Szamosi T, Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly? World J Gastroenterol. 2007;13(46):6134–9.
Heilpern D, Szilagyi A. Manipulation of intestinal microbial flora for therapeutic benefit in inflammatory bowel diseases: review of clinical trials of probiotics, pre-biotics and synbiotics. Rev Recent Clin Trials. 2008;3(3):167–84. https://doi.org/10.2174/157488708785700302.
Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, et al. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53(9):2524–31. https://doi.org/10.1007/s10620-007-0171-0.
Losurdo G, Iannone A, Contaldo A, Ierardi E, Di Leo A, Principi M. Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J Gastrointest Liver Dis. 2015;24(4):499–505. https://doi.org/10.15403/jgld.2014.1121.244.ecn.
Vakadaris G, Stefanis C, Giorgi E, Papazoglou AS, Papadakos SP, Karniadakis I, et al. The role of probiotics in inducing and maintaining remission in Crohn’s disease and ulcerative colitis: a systematic review of the literature. Biomedicines. 2023;11(2):494. https://doi.org/10.3390/biomedicines11020494.
Pedersen KM, Çolak Y, Vedel-Krogh S, Kobylecki CJ, Bojesen SE, Nordestgaard BG. Risk of ulcerative colitis and Crohn’s disease in smokers lacks causal evidence. Eur J Epidemiol. 2022;37(7):735–45. https://doi.org/10.1007/s10654-021-00763-3.
Limketkai BN, Akobeng AK, Gordon M, Adepoju AA. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2020;7(7):CD006634.
Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:8941340. https://doi.org/10.1155/2018/8941340. (eCollection 2018).
Author information
Authors and Affiliations
Contributions
A Sz- conceived project, review of literature, writing manuscript; J W – Conceptual discussions of topic, review of literature, revision and editing of manuscript; JA – Conceptual discussions of topic, preparing table on pro and anti-inflammatory diets, review of pertinent literature, editing final manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
This article does not contain any studies on human or animal subjects performed by any of the authors. Disclosure; There was no specific funding for this project.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Szilagyi, A., Wyse, J. & Abdulezer, J. Dietary Relationships between Obesity and Inflammatory Bowel Diseases: A Narrative Review of Diets Which May Promote Both Diseases. Curr Gastroenterol Rep 27, 29 (2025). https://doi.org/10.1007/s11894-025-00980-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11894-025-00980-w