Abstract
Objective
This study aims to assess the therapeutic efficacy and safety of nivolumab combined with chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, specifically comparing the outcomes of oxaliplatin-based versus albumin-bound paclitaxel (nab-paclitaxel)-based therapies.
Methods
We retrospectively analyzed 93 patients with advanced gastric cancer or gastroesophageal junction adenocarcinoma treated at the First Medical Center of Chinese PLA General Hospital from September 2017 to November 2022. Patients were categorized into the nivolumab + oxaliplatin (N-OX group) or nivolumab + nab-paclitaxel (N-AP group) based on the chemotherapy regimen. Progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and safety were evaluated as endpoints.
Results
At the end of the follow-up period on September 31, 2023, we reported an ORR of 65.6% and DCR of 95.7% across all patients. The median PFS was 8.4 months, with no significant difference between the N-OX and N-AP groups (median, 7.8 vs 9.5 months; P = 0.450). Notably, patients with diffuse gastric cancer in N-AP group showed a 44.7% reduction in tumor progression risk compared with the N-OX group (P = 0.046). The overall safety profile was acceptable in two groups.
Conclusions
Our study suggested that nivolumab combined with chemotherapy was effective and safe as a first-line intervention for advanced gastric cancer. While both oxaliplatin and nab-paclitaxel regimens showed similar efficacy, the nab-paclitaxel may offer additional benefits for patients with diffuse gastric cancer. Further research is encouraged to confirm these findings and refine treatment strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common malignant tumor and the fourth leading death cause of cancer [1]. It is a global healthcare challenge, and China contributes to respectively 43.9% of morbidity and 48.6% of mortality for gastric cancer in the world [1]. The incidence of gastric cancer and adenocarcinoma of the gastroesophageal junction are still on the rise [2], and the 5-year overall survival rate of patients with distant metastasis is less than 5% [3]. For patients with ERBB2 (formerly HER2)–negative advanced gastric cancer, cytotoxic chemotherapy is still the backbone of treatment, but the median overall survival (OS) is difficult to break through 1 year [4]. However, for patients with HER2-positive gastric cancer, the optimal selection is more effective targeted therapy. In the first-line setting, the anti-HER2 antibody trastuzumab showed benefit in patients with HER2-positive tumors enrolled in the phase III TOGA trial in 2010 [5]. Nevertheless, HER-2-positive patients only account for 10%–20% of advanced gastric cancer patients, and the effect of targeted therapy is limited [6]. In past decades, the development and universal application of immune-checkpoint inhibitors (ICIs) have greatly changed the model of cancer therapy. The effective rate of ICIs alone for gastric cancer is only 10%–15%, which has no advantage compared with chemotherapy, while the combination of ICIs and chemotherapy can increase the effective rate to more than 60% [7]. The combination of ICIs and chemotherapy has been recommended as the first-line treatment pattern of advanced gastric cancer. Based on the CheckMate-649 study [8, 9], FDA approved the indication of nivolumab combined with platinum and fluorouracil chemotherapy drugs for the first-line treatment of gastric in April 2021. The 2022 ESMO GI Conference further released the 2-year follow-up results of the study in Chinese subgroup, which showed that the Chinese population had a more obvious trend of benefit compared with the global population [9].
The influence of chemotherapy drugs on the immune microenvironment has been confirmed in many types of tumors [10]. Consistent with the findings from the CheckMate 649 study [9], previous research has demonstrated that adding immunotherapy to standard chemotherapy as a first-line treatment for gastric cancer significantly improves overall survival. These findings were further supported by the ORIENT-16 study, which reported a median OS of 15.2 months with the combination of Sintilimab and XELOX (capecitabine plus oxaliplatin) [7]. Additional evidence from the ATTRACTION-4 trial [11] and other clinical studies has consistently reinforced the efficacy of combining ICIs with platinum-based chemotherapy as first-line treatment.
While the efficacy of platinum-based combinations has been well established, the potential of paclitaxel-based regimens in conjunction with ICIs remains less explored. Preclinical and clinical evidence suggest promising therapeutic potential for paclitaxel in gastric cancer management. The AIO-FLOT3 trial [12] demonstrated the significant role of paclitaxel as neoadjuvant therapy, showing measurable improvements in both OS and progression-free survival (PFS). Furthermore, the ABSOLUTE study [13] established the non-inferiority of weekly nab-paclitaxel compared to solvent-based paclitaxel in terms of OS, leading to its inclusion in the National Comprehensive Cancer Network (NCCN) guidelines as a recommended second-line treatment for gastric cancer.
Given the established efficacy of paclitaxel-based therapies and the emerging evidence supporting ICIs in gastric cancer treatment, there is a compelling need to investigate the therapeutic potential of combining ICIs with nab-paclitaxel-based regimens as first-line treatment for advanced gastric cancer. This combination warrants further clinical evaluation to establish its efficacy, safety profile, and potential superiority over existing treatment paradigms.
Patients and Methods
Patients
The study was conducted at the First Medical Center of Chinese PLA General Hospital. It included 93 patients with pathologically confirmed advanced gastric or gastroesophageal junction adenocarcinoma treated between September 2017 and November 2022. Eligible patients were those who received either oxaliplatin-based or nab-paclitaxel based first-line therapy and had undergone at least one post-treatment efficacy evaluation.
Treatment
Nivolumab (360 mg) was administered intravenously in combination with oxaliplatin-based or nab-paclitaxel based chemotherapy every 3 weeks. Oxaliplatin was administered 130 mg/m2 intravenously on day 1 of each 21-day cycle in Oxaliplatin-based chemotherapy (XELOX [capecitabine and oxaliplatin] or SOX [S-1 and oxaliplatin]), with capecitabine 800–1000 mg/m2 or S-1 40 mg/m2 orally twice a day for days 1–14. Nab-paclitaxel was administered 130 mg/m2 intravenously on days 1 and 8 of each 21-day cycle in nab-paclitaxel based chemotherapy, with capecitabine 800–1000 mg/m2 or S-1 40 mg/m2 orally twice a day for days 1–14. Trastuzumab was administered intravenously for HER-2 positive patients with an initial loading dose of 8 mg/kg followed by a maintenance dose of 6 mg/kg every 3 weeks. All patients completed at least 2–3 cycles of the treatment regimen. Efficacy was assessed every 2–3 cycles according to the RECIST 1.1 criteria. After completing 6–8 cycles, patients transitioned to maintenance therapy, which included nivolumab alone or in combination with S-1/capecitabine ± trastuzumab, based on individual response and tolerability.
Efficacy Evaluation
The follow-up period concluded on September 31, 2023. For patients without documented disease progression at the end of the study, the last known follow-up date was used as the endpoint for analysis. The primary endpoint was progression-free survival (PFS), which was defined as the time from treatment initiation with nivolumab plus chemotherapy until disease progression, as determined by radiographic assessment using RECIST 1.1 criteria, or death from cancer-related causes. The secondary endpoints of this study were objective response rate (ORR) and disease control rate (DCR). The ORR is determined as the percentage of patients achieving either a confirmed complete response (CR) or partial response (PR) in accordance with the RECIST v1.1 guidelines. And the DCR is calculated as the percentage of patients who achieved either stable disease (SD), partial response (PR), or complete response (CR) relative to the total number of cases. The short-term efficacy evaluation was based on the classification into SD, progressive disease (PD), CR, and PR. Adverse events were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 5.0, to assess the safety and tolerability of the treatments.
Statistical Methods
The SPSS 22.0 software was used for statistical analysis; χ2 test was used to compare the remission rate of the two groups of patients. The median progression-free survival was estimated using the Kaplan–Meier method. Log-rank test was used for comparing between-group differences for progression-free survival. The between-treatment group HRs and corresponding two-sided 95% CIs were estimated using Cox proportional hazards model. P < 0.05 was considered statistically significant.
Results
Basic Information of Enrolled Patients
A total of 93 patients were enrolled with a median age of 59 years (ranging from 19 to 84 years), including 64 males (68.8%) and 29 females (31.2%). In 93 patients, gastric adenocarcinoma and gastroesophageal junction cancer accounting for 94.6% (n = 88) and 5.4% (n = 5), respectively. The expression of PD-L1, assessed using combined positive score (CPS), was evaluable in 51.6% of patients (n = 48), among whom 64.6% exhibited CPS ≥ 1. The positive expression of HER2 was identified in 6.5% of patients (n = 6), who subsequently received trastuzumab as part of their first-line treatment regimen.
The cohort was divided into two treatment groups: 49.5% (n = 46) received nab-paclitaxel plus cisplatin (N-AP) chemotherapy, while 50.5% (n = 47) were treated with oxaliplatin-based (N-OX) chemotherapy. Comparative analysis revealed no significant differences between the two groups in key demographic and clinical characteristics, including gender distribution, age, primary tumor location, histological subtype, Lauren classification, metastatic patterns, number of metastatic sites, PD-L1 expression status, and trastuzumab administration (all P > 0.05). However, significant baseline differences were observed in surgical history and prior adjuvant chemotherapy exposure (P < 0.05), which were subsequently adjusted for in the multivariate analysis. The details were included in Table 1.
Efficacy
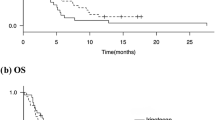
The follow-up period ended on September 30, 2023. A total of 93 patients received first-line nivolumab combined with chemotherapy, the following treatment responses were observed: four patients (4.3%) achieved CR, 57 patients (61.3%) achieved PR, 28 patients (30.1%) achieved SD, and four patients (4.3%) achieved PD. This resulted in an ORR of 65.6% and a DCR of 95.7% in this cohort. The median progression-free survival (mPFS) was 8.4 months (95% CI 6.464–10.336 m), while the median overall survival (mOS) had not been reached at the time of analysis (Fig. 1).
In the N-AP group, the following treatment responses were observed: CR in two patients (4.3%), PR in 30 patients (65.2%), SD in 13 patients (28.3%), PD in one patient (2.2%), and the ORR and DCR were 69.6% and 97.8%, respectively, while the mPFS was 9.5 m [95%CI 6.301–12.699]. In the N-OX group, the following treatment responses were observed: CR in two patients (4.3%), PR in 27(57.4%) patients, SD in 15 patients (31.9%), PD in three patients (6.4%), and the ORR and DCR were 61.7% and 93.6%, respectively, while the mPFS was 7.8 m [95% CI 5.308–10.292]. There was no significant difference of mPFS between the two groups (P = 0.450) (Fig. 2 and Tables 1 and 2).
Subgroup Analysis
Subgroup analysis demonstrated differential efficacy between above two groups, with the N-AP group showing a 15.9% reduction in tumor progression risk compared to the N-OX group (P = 0.452). However, in the subset of patients with diffuse-type gastric cancer, N-AP treatment was associated with a statistically significant reduction of 44.7% in progression risk relative to N-OX (P = 0.046). Notably, a consistent trend toward prolonged mPFS with N-AP treatment was observed across several clinically relevant subgroups, including female patients, those aged < 65 years, patients without prior adjuvant chemotherapy, cases with ≥ 2 metastatic organs, individuals with liver or peritoneal metastases, and patients with PD-L1 CPS score ≥ 1, suggesting potential differential treatment effects based on specific patient characteristics (Table 3).
Safety
There were no treatment-related deaths in the whole population in the combination of nivolumab and chemotherapy. The incidence of peripheral neurotoxicity was 46.8% higher in the combination of the N-OX group, but no neurotoxicity was greater than grade 3. In this study, the incidence of immune-related adverse reactions in the whole population was 14.0% for hypothyroidism, followed by immune-related rash (4.3%). The incidence of rash in the N-AP group was higher than that in the N-OX group, but the incidence of rash in grade ≥ 3 was the same in the two groups. One case of immunotherapy-related enteritis and one case of hypophysitis occurred in the N-OX group, and nivolumab therapy was discontinued due to grade ≥ 3 adverse reactions (see Table 4 for details).
Discussion
Gastric cancer is one of the most important malignant tumors in China, with the highest morbidity and mortality [14]. Globally, gastric cancer mainly occurs in China, Japan, South Korea, and other countries. In 2020, almost two-thirds (n = 696,112) of gastric cancer diagnosed were diagnosed in East and Southeast Asia [1]. In China, about 260,400 patients die of gastric cancer every year, ranking the third cancer death [14]. The prognosis of gastric cancer is very poor; the 5-year OS is less than 10% [15]. For several decades, the standard first-line treatment for advanced gastric cancer has consisted of platinum-based chemotherapy in combination with fluorouracil. However, this conventional therapeutic approach has demonstrated limited clinical efficacy with mPFS ranging from 4.9 to 6.0 months [4]. Immunotherapy has also drawn the treatment of gastric cancer into a new era, and the first-line treatment for gastric cancer has shifted to immunotherapy combined with chemotherapy. CheckMate-649 [9] is the first global phase III study to confirm a significant survival benefit of immunotherapy combined with chemotherapy versus chemotherapy alone in first-line patients with advanced gastric cancer. OS, PFS, and ORR were significantly improved with nivolumab plus chemotherapy compared with chemotherapy, and the effect of immunotherapy is more durable. ORIENT-16 [16] was the first randomized, double-blind phase III study in China to confirm the significant population-wide benefit of first-line immunotherapy combined with chemotherapy for advanced gastric cancer. The primary study endpoint showed a significant OS benefit, raising the OS to 15.2 months for the first time [7]. In this study, we retrospectively analyzed the efficacy of first-line nivolumab combined with chemotherapy in 93 cases of advanced gastric cancer and gastroesophageal junction adenocarcinoma.
Compared with CheckMate-649 [9] Chinese subgroup, the proportion of patients with one metastatic site in our study was higher, the proportion of liver metastasis was lower, but the proportion of peritoneal metastasis was higher. In our study, 48.4% patients were not tested for PD-L1 expression and only 33.3% patients had PD-L1 CPS score ≥ 1. In terms of efficacy, including four cases (4.3%) of CR, 57 cases (61.3%) of PR, 28 cases (30.1%) of SD, four cases (4.3%) of PD, 65.6% of ORR, 95.7% of DCR, and 8.4 m mPFS. The ORR in this study (65.6%) was comparable to that of Chinese subgroup (CPS ≥ 5) and that of all randomized patients (68% and 66%, respectively) in CheckMate-649 study [9]. The ORR in this study was slightly higher than that in Attraction-4 study (62.7%) and that of patients with CPS ≥ 5 in ORIENT-16 (63.6%) [16].
The median PFS of 8.4 months in this study is higher than that of patients with PD-L1 CPS ≥ 5 (7.7 months) and that of all randomized patients (7.1 months) in ORIENT-16 [16]. Then, the median PFS is lower than that in Attraction-4 [17] (10.45 months), which was comparable to that of Chinese populations with PD-L1 CPS ≥ 5 (8.5 months) and that of all populations (8.3 months) in CheckMate-649 [9].
However, the current mainstream clinical trials are all based on platinum-based doublet chemotherapy. There is no adequate clinical data to support the effect of paclitaxel as first-line treatment combined with immunotherapy. Paclitaxel is widely used in various tumors due to its broad anticancer activity, such as lung cancer, breast cancer, and ovarian cancer [18,19,20]. Paclitaxel is different to solute in water, currently using polyoxyethylene castor oil and ethanol as solvent, but the solvent system will cause serious side effects, such as allergic reactions and pharmacokinetic properties change [21]. Nab-paclitaxel is the first targeted chemotherapy drug based on nanotechnology; high concentrations of paclitaxel can be produced locally in the tumor to enhance antitumor activity. It solves the problem of solubility and solution stability of paclitaxel; thus, no longer requires the use of toxic co-solvents (such as polyethylene castor oil), while increasing efficiency and improving convenience and economy [22]. Emerging evidence suggests that nab-paclitaxel exerts immunomodulatory effects within the tumor immune cycle, potentially enhancing the efficacy of immunotherapy [23]. The nab-paclitaxel eliminates the need for corticosteroid premedication and demonstrates unique clinical advantages in specific patient populations, particularly in individuals with comorbid diabetes mellitus or those presenting with high tumor burden.
In this study, the ORR of the N-AP group was 69.6%, which was better than the results of clinical trial of nab-paclitaxel combined with S-1 in the first-line treatment of advanced gastric cancer [22, 24]. mPFS was better than that of GAPSO study and similar to that of phase II clinical trial [24]. In this study, the ORR and mPFS of the N-AP group were significantly better than that of sintilimab combined with nab-paclitaxel as second-line treatment of advanced gastric cancer [25]. Moreover, the median PFS of the N-AP group was 1.7 months longer than that of the N-OX group, and the risk of disease progression was 15.9% lower. The results revealed non-inferior efficacy of the N-AP treatment compared to the N-OX treatment in the first-line therapy. Subgroup analysis showed that for diffuse gastric cancer, nivolumab combined with nab-paclitaxel could reduce the risk of tumor progression by 44.7% compared with oxaliplatin-based regimen (P = 0.046). GAPSO, PHOENIX-GC, and ABSOLUTE clinical trials also showed that patients with undifferentiated, poorly differentiated, signet ring cell carcinoma or diffuse type of gastric cancer may benefit more from paclitaxel [13, 24, 26]. In our study, patients younger than 65, the N-AP group reduced the risk of tumor progression by 42.7% compared with the N-OX group, but difference was not statistically significant (P = 0.058), which was similar to the results of GAPSO [24]. Therefore, the first-line application of nivolumab combined with nab-paclitaxel may be more beneficial for patients younger than 65 years old with diffuse gastric cancer.
In terms of safety, the incidence of neurotoxicity and vomiting of grade 3 or higher was lower in the N-AP group than in the N-OX group. No immune-related death occurred in the nab-paclitaxel group after combined immunotherapy, and immune-related adverse reactions were similar to those in the N-OX group. Immune-related rash of any grade was slightly more common with oxaliplatin than with oxaliplatin, but rash of grade ≥ 3 occurred with equal frequency in the two groups.
However, this retrospective study has certain limitations. First, the number of patients is relatively small and the adverse reactions may not be recorded and collected sufficiently. Second, due to the short follow-up time, the long-term survival has not been reached, and the effects of different treatment regimens on subsequent treatment and long-term survival are unknown. Third, the optimal combination cycle number of immunotherapy combined with nab-paclitaxel and the mechanism of drug assistance need to be further explored and refined by large-scale prospective clinical trials.
Conclusions
Our study suggested that nivolumab combined with chemotherapy was effective and safe as a first-line intervention for advanced gastric cancer. While both oxaliplatin and nab-paclitaxel regimens showed similar efficacy, the nab-paclitaxel may offer additional benefits for patients with diffuse gastric cancer. Further research is encouraged to confirm these findings and refine treatment strategies.
Data Availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Yang S, Lin S, Li N, Deng Y, Wang M, Xiang D, et al. Burden, trends, and risk factors of esophageal cancer in China from 1990 to 2017: an up-to-date overview and comparison with those in Japan and South Korea. Journal of hematology & oncology. 2020;13(1):146. https://doi.org/10.1186/s13045-020-00981-4.
Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020;18(3):534–42. https://doi.org/10.1016/j.cgh.2019.07.045.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London, England). 2020;396(10251):635–48. https://doi.org/10.1016/s0140-6736(20)31288-5.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010;376(9742):687–97. https://doi.org/10.1016/s0140-6736(10)61121-x.
Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Jr., et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(22):3030–6. https://doi.org/10.1200/jco.2010.33.6313.
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study. Annals of Oncology. 2021;32. https://doi.org/10.1016/j.annonc.2021.08.2133.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England). 2021;398(10294):27–40. https://doi.org/10.1016/s0140-6736(21)00797-2.
Liu T, Bai Y, Lin X, Li W, Wang J, Zhang X, et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. International journal of cancer. 2023;152(4):749–60. https://doi.org/10.1002/ijc.34296.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021 Jun;21(6):345–359. https://doi.org/10.1038/s41568-021-00347-z.
Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Annals of oncology : official journal of the European Society for Medical Oncology. 2019;30(2):250–8. https://doi.org/10.1093/annonc/mdy540.
Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA oncology. 2017;3(9):1237–44. https://doi.org/10.1001/jamaoncol.2017.0515.
Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. The lancet Gastroenterology & hepatology. 2017;2(4):277–87. https://doi.org/10.1016/s2468-1253(16)30219-9.
Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024 Mar 23;46(3):221–231. Chinese. https://doi.org/10.3760/cma.j.cn112152-20240119-00035.
Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center. 2022;2(1):1–9. https://doi.org/10.1016/j.jncc.2022.02.002.
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. Jama. 2023;330(21):2064–74. https://doi.org/10.1001/jama.2023.19918.
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2022;23(2):234–47. https://doi.org/10.1016/s1470-2045(21)00692-6.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355(24):2542–50. https://doi.org/10.1056/NEJMoa061884.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. The New England journal of medicine. 2007;357(26):2666–76. https://doi.org/10.1056/NEJMoa072113.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(17):3194–200. https://doi.org/10.1200/jco.2003.02.153.
Ahmed Khalil A, Rauf A, Alhumaydhi FA, Aljohani ASM, Javed MS, Khan MA, et al. Recent developments and anticancer therapeutics of paclitaxel: an update. Current pharmaceutical design. 2022;28(41):3363–73. https://doi.org/10.2174/1381612829666221102155212.
He MM, Wang F, Jin Y, Yuan SQ, Ren C, Luo HY, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer science. 2018;109(11):3575–82. https://doi.org/10.1111/cas.13813.
Chen Y, Liu R, Li C, Song Y, Liu G, Huang Q, et al. Nab-paclitaxel promotes the cancer-immunity cycle as a potential immunomodulator. American journal of cancer research. 2021;11(7):3445–60..
Dai YH, Yu XJ, Xu HT, Zhuang L, Zhang MS, Zou YM, et al. Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study). Therapeutic advances in medical oncology. 2022;14:17588359221118020. https://doi.org/10.1177/17588359221118020.
Wang J, He Y, Zhang B, Lv H, Nie C, Chen B, et al. The Efficacy and Safety of Sintilimab combined with nab-paclitaxel as a second-line treatment for advanced or metastatic gastric cancer and gastroesophageal junction cancer. Frontiers in oncology. 2022;12:924149. https://doi.org/10.3389/fonc.2022.924149.
Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(19):1922–9. https://doi.org/10.1200/jco.2018.77.8613.
Author information
Authors and Affiliations
Contributions
Juanli and shuman li wrote the main manuscript text, all authors prepared figures and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Li, S., Zhang, Y. et al. The Efficacy and Safety of Nivolumab Combined with Nab-Paclitaxel or Oxaliplatin as a First-Line Treatment for Advanced or Metastatic Gastric Cancer and Gastroesophageal Junction Cancer. J Gastrointest Canc 56, 109 (2025). https://doi.org/10.1007/s12029-025-01211-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12029-025-01211-1