Abstract
Introduction
Although some factors associated with asthma symptom deterioration and risk of exacerbation have been identified, these are not yet fully characterised. We conducted a clinical modelling and simulation study to understand baseline factors affecting symptom control, reliever use and exacerbation risk in patients with moderate–severe asthma during follow-up on regularly dosed inhaled corticosteroid (ICS) monotherapy, or ICS/long-acting beta2-agonist (LABA) combination therapy.
Methods
Individual patient data from randomised clinical trials (undertaken between 2001 and 2019) were used to model the time course of symptoms (n = 7593), patterns of reliever medication use (n = 3768) and time-to-first exacerbation (n = 6763), considering patient-specific and extrinsic factors, including treatment. Model validation used standard graphical and statistical criteria. Change in symptom control scores (Asthma Control Questionnaire 5 [ACQ-5]), reduction in reliever use and annualised exacerbation rate were then simulated in patient cohorts with different baseline characteristics and treatment settings.
Results
Being a smoker, having higher baseline ACQ-5 and body mass index affected symptom control scores, reliever use and exacerbation risk (p < 0.01). In addition, low forced expiratory volume in 1 s percent predicted, female sex, season and previous exacerbations were found to contribute to a further increase in exacerbation risk (p < 0.01), whereas long asthma history was associated with more frequent reliever use (p < 0.01). These effects were independent from the underlying maintenance therapy. In different scenarios, fluticasone furoate (FF)/vilanterol was associated with greater reductions in reliever use and exacerbation rates compared with FF or fluticasone propionate (FP) alone or budesonide/formoterol, independently from other factors (p < 0.01).
Conclusions
This study provided further insight into the effects of individual baseline characteristics on treatment response and highlighted significant differences in the performance of ICS/LABA combination therapy on symptom control, reliever use and exacerbation risk. These factors should be incorporated into clinical practice as the basis for tailored management of patients with moderate–severe asthma.
Plain Language Summary
In this study we quantified how individual baseline patient characteristics at the start of treatment influence the response to regular maintenance medication. Specifically, using computer modelling and simulations based on data from individual patients enrolled into clinical trials in moderate–severe asthma, we predicted how much reliever inhaler they need, how well they rate their asthma control, and how likely an asthma attack (exacerbation) is to occur within the next 12 months. Simulation scenarios were then implemented to evaluate opportunities to improve and personalise real-life management of patients in clinical practice. Considering symptom control level, reliever use and other patient-specific factors at the start of treatment, we assessed how well maintenance therapy with inhaled corticosteroids/bronchodilators contributes to symptom improvement and/or reduction in the risk of asthma attacks. These scenarios show that current smokers, people with higher asthma symptom scores, who are obese, and have a longer history of asthma tend to use their reliever inhalers more often. Moreover, this was linked to a higher risk of having an asthma attack and worse symptom control. This pattern appears to compensate in most cases for the effect of the same baseline factors on symptom control. Switching patients who are not responding well to initial treatment with the inhaled corticosteroid, fluticasone propionate, to fluticasone furoate/vilanterol resulted in a significantly greater reduction in reliever inhaler use and risk of asthma attack, compared with those switched to budesonide/formoterol. These findings highlight the importance of tailored choices for optimal management of patients with moderate–severe asthma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Our study shows that patient characteristics contribute to interindividual differences in symptom control, reliever medication use and risk of exacerbation in moderate–severe asthma, independently from treatment choices. |
In line with previous findings, baseline Asthma Control Questionnaire-5, body mass index and smoking habit were found to affect symptom control scores, reliever use and exacerbation risk; low forced expiratory volume in 1 s percent predicted, female sex, winter/spring season and previous exacerbation events were associated with an increased risk of future exacerbations, whereas asthma duration (disease history) contributed to more frequent reliever use. |
Simulation scenarios describing clinical management decisions in a real-life setting, i.e. dose increase, and step-up, indicate that fluticasone furoate/vilanterol (FF/VI 100/25 μg o.d.) results in a significantly lower exacerbation risk, compared to FF (100 μg o.d.), fluticasone propionate (FP, 250 μg b.i.d.) or regular dosing budesonide/formoterol (BUD/FOR 320/9 μg b.i.d.), regardless of differences in baseline characteristics; the effect of FF/VI on exacerbation is accompanied by greater symptom improvement (p < 0.01) compared to BUD/FOR. |
Achieving symptom control status while on treatment with ICS or ICS/LABA does not imply comparable exacerbation risk reduction, as shown by the lower exacerbation rates in FF/VI versus BUD/FOR-treated patients; these factors should be considered as a basis for personalised management of patients with moderate–severe asthma. |
Introduction
The level of treatment required to control a patient’s symptoms and prevent exacerbations is fundamental to successful asthma control, according to Global Initiative for Asthma (GINA) recommendations [1]. Assessing asthma severity requires consideration of (1) the burden of symptoms, lung function and exacerbations and (2) the concomitant level (i.e. GINA step) of treatment [1]. Thus, disease severity is distinct from symptom severity, which can exist as a range within a classification of the underlying disease (e.g. moderate-severe) that in turn is also affected by treatment and time.
On the other hand, asthma severity is also influenced by individual patient characteristics. Patient characteristics, including clinical and demographic factors, have been associated with exacerbation risk and partly reflect interindividual differences in the underlying disease processes, e.g. type 2 inflammation and airway hyperresponsiveness [2,3,4]. For instance, persistent poor symptom control, which can be measured using validated instruments such as the Asthma Control Questionnaire-5 (ACQ-5) [5], is a key factor associated with increased asthma exacerbation risk [6]; however, other features are independently associated with exacerbations even in the presence of apparently good asthma control [7]. Asthma duration and medical history, including comorbidities and reduced lung function, as assessed by forced expiratory volume in 1 s (FEV1) are associated with increasing asthma severity [8,9,10,11,12]. In fact, recurrent exacerbations contribute to faster lung function decline, in particular in young adults [13,14,15,16].
Another important point to consider when assessing symptom control is the degree of bronchoprotection achieved during treatment. Lasting bronchoprotection implies reduced airway hyperresponsiveness, and, therefore, less reliance on reliever medication [3]. Consequently, accurate management of individual patients and prediction of treatment response, i.e. achievement of symptom control and exacerbation-risk reduction should account for the role of the concurrent factors that have been identified to affect symptom severity and exacerbation risk. Nonetheless, limited quantitative research has been undertaken to characterise the magnitude of the effect of patient-specific factors driving symptom worsening and/or risk increase in moderate–severe asthma (e.g. occurrence of exacerbations, need for additional therapy to control symptoms or accelerated lung function decline) at an individual patient level [13,14,15].
In addition to patient-specific factors, differences exist in the potency, selectivity, bioavailability, systemic clearance and formulation of inhaled corticosteroids (ICS), which influence their therapeutic dose range and frequency. Fluticasone furoate (FF) has the longest duration of action within the available ICS molecules, as a consequence of high lung retention, glucocorticoid receptor binding affinity and potency, which enables once daily (o.d.) dosing [17, 18]. Other commonly used ICS molecules for asthma treatment require twice-daily (b.i.d.) dosing due to, for example, differences in affinity or lower lung glucocorticoid receptor occupancy after dosing than FF (100 μg o.d., 98.6%) or mometasone furoate [18]. How these differences translate in terms of asthma outcomes and treatment response in various subpopulations is largely unknown: some may be equally effective in achieving immediate symptom control in certain patient groups but more or less effective than others in maintaining bronchoprotection or reducing future risk. Moreover, broad-ranging comparisons have not been systematic in the context of clinical trials, making it difficult to disentangle the effect of patient- or disease-specific factors from drug-specific properties on the treatment response.
Recently, we have developed a series of drug-disease models using individual-level patient data from large clinical trials to explore the influence of clinical and demographic baseline characteristics on treatment response in moderate–severe asthma [2,3,4]. The primary goal was to quantify which factors alter individual ACQ-5 score trajectories, how much they affect individual patterns of reliever medication use and alter exacerbation risk, independently from the underlying maintenance therapy. During this initial evaluation, we assessed the effect of sex, smoking, body mass index (BMI), ACQ-5 symptom score, FEV1 percent predicted (FEV1% pred) at baseline on exacerbation risk in patients on regular dosing with fluticasone propionate (FP), FP/salmeterol (SAL) or budesonide/formoterol (BUD/FOR) treatment [2,3,4].
Here, we have modelled the effect of clinical and demographic baseline characteristics and treatment not only on long-term (1 year) exacerbation risk but also on asthma symptom control and reliever use in a population of patients with moderate–severe asthma receiving regular dosing of FF o.d., FF/vilanterol (VI) o.d. or BUD/FOR b.i.d. Using simulations, our aim was to evaluate the contribution of maintenance treatment choice in the management of moderate–severe asthma in addition to the other risk factors identified from the previous drug–disease models [2,3,4].
Methods
The use of drug–disease modelling and simulation as a tool for evidence synthesis and optimisation of the therapeutic use of medicines continues to expand in clinical research and across therapeutic areas [19,20,21,22]. The approach is of particular interest when confounding cannot be controlled in a clinical setting due to practical or ethical reasons. It has also been very useful in understanding the implications of treatment (e.g. dose, dosing regimen) as well as disease progression and severity in groups who are under-represented in clinical protocols [23, 24]. Moreover, simulation scenarios using in silico protocols shed light on specific features of an intervention in a controlled or in a real-life setting. Here, we describe the clinical trial population data available for this study, and summarise the main steps required for model development and subsequent evaluation of treatment performance across different simulation scenarios.
Study Patients
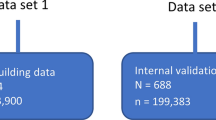
Our study was possible thanks to the availability of (1) individual level baseline patient data that reflect the heterogeneity of clinical and demographic baseline characteristics of the adult population with moderate–severe asthma and (2) drug–disease models describing the time course of symptoms (ACQ-5), reliever medication use (puffs/24 h) and the time to first moderate or severe exacerbation. The models implemented here were based on data from the maintenance phase of 10 randomised controlled Phase IIIIV studies (n = 10456; HZA106829 [25], 106837 [26], 106839 [27], 113091 [28], 116492 [29], 115150 [30], SAM40040 [31], SAM40056 [32], 201378 [33], and 205715 [34]), with a duration of at least 24 weeks in patients receiving ICS monotherapy or ICS/long-acting beta2-agonist (LABA) combination therapy. This pool of patients, who had individual clinical and demographic baseline details, treatment, dose and dosing regimens, was identified within GSK’s clinical data repository. Further selection criteria included the measurement of asthma symptom scores during the course of treatment. Studies which had ACQ-5 or Asthma Control Test (ACT) were prioritised, and, if necessary, ACT scores were converted to ACQ-5, as previously reported [2]. Finally, patients should have detailed information on maintenance therapy and self-reported reliever medication use (frequency, timing of administration). These data were integrated with records on the occurrence of exacerbation events. The definition of moderate or severe exacerbation was the one included in the individual study protocols (Table S3_5). The patient population used for model development and evaluation was part of a wider group of patients with moderate–severe asthma (n = 24,292) for whom baseline characteristics were available. This wide pool of patients was used for the implementation of the different simulation scenarios (Table 1). For completeness, an overview of the clinical trial data sources used for the development and validation of each drug-disease model is included in the Supplementary Material (Supplement 1: Table S1_1; Supplement 2: Table S2_1; Supplement 3: Table S3_1).
All clinical data used for the development and validation of the different models, as well as those required for re-sampling of the baseline characteristics of the virtual patient cohorts which were generated for the evaluation of the different simulation scenarios described in this study, were derived from clinical trials that have been performed according to the Declaration of Helsinki and were approved by the required ethics committee(s) and/or ethics review board(s). Re-use of the data for the purpose of the current investigation is in alignment with the terms of informed consent.
Modelling Approach, Data Source and Analysis Population
As indicated above, the studies included in the current analyses were multicentre trials undertaken between 2001 and 2019, which recruited patients with moderate or severe asthma, who had accurately measured symptom control scores, daily reliever medication use (albuterol/salbutamol 100 µg, as needed) and occurrence and time of exacerbations [25,26,27,28,29,30,31,32,33,34]. The studies lasted ≥ 24 weeks and included treatment with FF only, FF/VI or BUD/FOR. Per current GINA track 2 criteria [1], asthma severity in these patients at the time of enrolment corresponded to maintenance therapy ranging between steps 3–4 and 3–5. Given the duration of the studies, placebo interventions were not available. Therefore, FF monotherapy was selected as reference treatment, taking into account its potency relative fluticasone propionate.
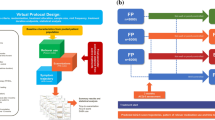
Parametric drug–disease models were used to describe the influence of interindividual differences in clinical and demographic baseline characteristics and treatment choices on asthma outcomes. The analysis took into account the availability of previously developed models using data from a similar population of patients receiving regular dosing ICS or ICS/LABA combination therapy, and comprised: (1) a longitudinal model of the individual time course of asthma symptom scores (ACQ-5) [4, 5], (2) a Poisson model of reliever medication use [3], and (3) a time-to-event model of the time and risk of a first exacerbation [2]. Patient-specific/disease-related parameter estimates from the previous analyses were used as prior distributions in the current study. An outline of the workflow, including data source, model development, evaluation and subsequent assessment of their predictive performance, including internal and external validation steps, is depicted in Fig. 1A. Further details on the implementation steps for each model are summarised in the Methods section of the Supplementary Material (Supplements 1–3).
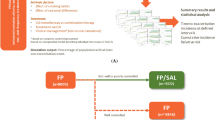
Drug–disease-modelling and simulation. a Workflow describing the main steps from data source to model development and evaluation to final model; b schematic of the simulation scenarios describing the individual trajectories of ACQ-5, patterns of reliever medication use and time to first exacerbation in patients with moderate–severe asthma*; c outline of the scenarios describing randomised clinical trial protocols stratified by baseline characteristics (clinical trial simulations) and clinical management of asthma patients in a real-life setting (not-in-trial simulations). ACQ-5 Asthma Control Questionnaire-5, BMI body mass index, BUD/FOR budesonide/formoterol, FF/VI fluticasone furoate/vilanterol, FP fluticasone propionate, ICS inhaled corticosteroid, LABA long-acting beta2-agonist. *For clarity, moderate asthma is defined as disease/symptom severity that requires regular ICS + LABA therapy, whereas severe asthma is defined as uncontrolled asthma or deterioration of asthma control despite regular use of high-dose ICS therapy, and treating aggravating factors
Finally, heat maps were created to visualise the overall impact of the different baseline characteristics on treatment performance. While heat maps do not allow more than four factors to be depicted at a time, they are a useful tool to quantify the magnitude and potential clinical implications of the effect interindividual differences in baseline characteristics (i.e. treatable traits) on asthma outcome. Separate heat maps were created for each endpoint, including data over a clinically relevant range to allow the identification of patient groups who are less likely to achieve symptom control or those who are exposed to a higher risk of exacerbation.
Clinical Trial Simulations
To further understand the clinical implications of interindividual differences in baseline characteristics, including potential treatable traits in patients with moderate–severe asthma, as well as assess the impact of step-up procedures and treatment choices on the overall response to interventions in clinical practice, clinical trial simulation scenarios were implemented, including randomised controlled conditions and real-life settings (i.e. not-in-trial simulation) [35] (full details, Supplement 4). Fixed, regular dosing was used throughout all scenarios, and treatment arms included both ICS monotherapy and ICS/LABA combination therapy, administered o.d. or b.i.d., as per the summary of product characteristics of each medicinal product. In the scenario describing step-up procedures in a real-life setting (e.g. dose increase, treatment switch), only non-responders to ICS monotherapy had their treatment changed. An outline of the clinical trial simulation workflow is shown in Fig. 1B, C. Full details of the protocol design characteristics, including statistical considerations and key assumptions used for the evaluation of the effect of baseline characteristics and treatment choices on reliever medication use, symptom control level and exacerbation risk, are summarised in Supplement 4, Table S4_2.
In each scenario, an asthma exacerbation was defined as either (1) deterioration of symptoms requiring treatment with oral corticosteroids (> 2 consecutive days), or a clinical deterioration assessed by the investigating physician as requiring oral steroid treatment, or (2) deterioration in asthma which required hospital admission. These criteria reflect the definitions mostly used to determine moderate or severe exacerbations in the selected clinical studies and correspond to the data used for the development of the model describing the time to first exacerbation.
In Silico Protocol (Controlled Setting)
For the simulations, baseline characteristics were randomly sampled from 24,292 patients in the pooled population of adults with moderate–severe asthma. The use of baseline data from real patients with moderate–severe asthma ensured accurate representation of the range of values and correlations between demographic and clinical characteristics.
The key simulation scenarios are outlined below:
-
Scenario 1: effect of symptom control at baseline (ACQ-5); three strata corresponding to patients with well-controlled asthma (ACQ-5 ≤ 0.75), not well-controlled asthma (ACQ-5 > 0.75 to ≤ 1.5) and poorly controlled asthma (ACQ-5 > 1.5).
-
Scenario 2: effect of BMI at baseline; four strata corresponding to patients with normal weight (BMI 18.5 to < 25 kg/m2), overweight (BMI 25 to < 30 kg/m2), obese (BMI 30 to < 35 kg/m2) and extremely obese (BMI ≥ 35 kg/m2).
-
Scenario 3: effect of exacerbation history (in the previous 12 months); three strata corresponding to patients with a history of 0, 1 or > 1 asthma exacerbations.
-
Scenario 4: effect of sex differences; male or female.
-
Scenario 5: effect of smoking habit at baseline; three strata corresponding to patients who have never smoked, stopped smoking (former smokers) and currently smoke.
-
Scenario 6: effect of asthma history at baseline; three strata corresponding to patients with a disease history of < 5 years, 5–10 years and > 10 years.
For each simulation scenario, changes in the number of reliever medication actuations (puffs) during the prior 24 h, together with ACQ-5 symptom scores at the end of the treatment period, and Kaplan–Meier estimated annualised exacerbation rates, were assessed. Statistical significance was evaluated, considering the effect of baseline characteristics and treatment choices on changes in ACQ-5 symptom scores, reliever medication use and annualised exacerbation rates over 12 months (further details, Supplement 4).
Treatment was assumed to be independent of baseline characteristics and was assigned randomly to each patient (Fig. 1C, left panel). All scenarios included treatment for the period of 1 year. Treatment doses and regimens were limited to those used during the maintenance phases of the clinical trials, (FF: 100 and 200 μg o.d.; FF/VI: 100/25 and 200/25 μg o.d.; BUD/FOR: 100/6, 200/6, 400/12, 160/4.5 and 320/9 μg b.i.d.). Parameter estimates describing treatment effect correspond to that of the mean dose during the maintenance phase used in the original study protocols.
Each simulation scenario included 1500 virtual patients per treatment arm or stratum investigated and were replicated with 500 iterations, as previously described [2,3,4]. The number of puffs over the last 24 h, along with ACQ-5 symptom scores and Kaplan–Meier estimates of the simulated exacerbation events, were summarised per scenario. Reduction in reliever use (canisters/year) and symptom improvement at the end of the treatment period were reported along with the change in annualised exacerbation rate.
Not-in-Trial Simulations (Real-Life Setting)
As in a sensitivity analysis [36, 37], not-in-trial simulations were carried out to illustrate potential real-world occurrences during the clinical management of adult patients with moderate–severe asthma, considering commonplace issues faced by healthcare professionals. In particular, this approach aimed to identify at-risk patients who are at a higher risk of exacerbation, as well as those reliant on reliever medication for short-term symptom management, who may benefit from treatment changes to facilitate long-term symptom control, reductions in morbidity and preservation of lung function. These scenarios comprised random treatment allocation and virtual cohorts of patients whose baseline characteristics were re-sampled from the available pooled population described above:
-
Scenario 7: effect of smoking cessation at 3 months after start of treatment.
-
Scenario 8: effect of ICS/LABA treatment switch at 3 and 6 months after start of therapy, irrespective of symptom control level.
-
Scenario 9: effect of treatment switch from ICS monotherapy to ICS/LABA combination therapy. Only patients not achieving adequate symptom control switched to ICS/LABA combination therapy at 3 months after treatment initiation.
For scenario 9, all patients were assumed to initiate treatment at a stage of asthma severity corresponding to GINA step 3, track 2 [1], i.e. ICS monotherapy with FP (Fig. 1C, right panel). In this real-life setting, patients who did not achieve control after 3 months on monotherapy were switched to regular fixed-dose maintenance therapy with FF/VI or BUD/FOR for up to 12 months (i.e. GINA step 3–5) [1]. At 3 months after treatment initiation, responders were considered to be patients achieving symptom control (ACQ-5 ≤ 0.75); non-responders were those with ACQ-5 scores remaining at > 0.75. The starting dose was: FP 250 μg b.i.d., with non-responders switching to treatment. Median doses simulated during the maintenance phase were: 100/25 μg o.d. for FF/VI, 500 μg b.i.d. for FP, or 320/9 μg b.i.d. for BUD/FOR.
For each simulation scenario, the predicted individual trajectories of ACQ-5 symptom scores, individual patterns of reliever use and asthma exacerbations were summarised numerically and graphically.
These scenarios aimed to describe the clinical management of adult patients with moderate-severe asthma in a real life setting. Scenarios 7 and 8 included 1500 virtual patients per treatment arm or stratum investigated. Scenario 9 comprised virtual cohorts of 8000 patients per treatment arm, and each scenario was repeated 500 times. Patients baseline characteristics were re-sampled from the pooled population during each iteration. Treatment was assumed to be independent of baseline characteristics and was randomly assigned at the start of the intervention (Fig. 1C, right panel).
All modelling and subsequent simulation scenarios were performed using a nonlinear mixed effects approach, as implemented in NONMEM 7.5 (ICON Development Solutions, MD, USA) using PsN 5.3.0 in an in-house Modelling Application Platform (MAP). Data formatting, graphical and statistical summaries were conducted in R version 4.1.3. The matching methods comparison was undertaken using R package, MatchIt, using “Optimal” pair matching in a 1:1 ratio (i.e. each BUD/FOR patient was matched with one FF or FF/VI patient). Distances, measured by propensity scores, were estimated with logistic regression. The statistical significance of the effect of baseline characteristics and treatment choices on changes in symptom scores, reliever medication use, and annualised exacerbation rates over the period of 12 months was evaluated in each scenario (further details on the statistical methods, Supplement 4).
Results
Analysis Population and Modelling Results
Data sources, model development and validation details for the three models are described in the Supplementary Materials (Supplement 1; Supplement 2; Supplement 3). Clinical and demographic baseline characteristics of the population included in each of the three models are shown in Tables S1_2 (n = 7593), S2_3 (n = 3768) and S3_2 (n = 6765).
The parameter estimates of the final models describing the time course of symptoms, reliever medication use and time to first exacerbation model are described in Tables S1_3, S2_5 and S3_3, respectively. Briefly, individual ACQ-5 trajectories were highly variable during the course of treatment and stabilisation of symptom scores required time, indicating that maximum treatment responses may not be reached within a period of 12 weeks. For the second model, the percentage change in reliever use for a 1 unit increase in ACQ-5 (relative to the mean ACQ-5 score of 1.8) was 62.5%. Notably, smokers on average used 75.4% more reliever, compared to patients who had never smoked, while reliever use in former smokers was 42.3% higher than in patients who had never smoked. For the time-to-first exacerbation model, relative to no exacerbation history, every previous exacerbation added a predicted 53.5% change in hazard; > 30% increased hazards were predicted for both current and former smokers (vs. patients who had never smoked), female patients (vs. males) and in winter (vs. summer).
Treatment effects relative to FF observed in the models included differences in time to reach half of the maximum shift in ACQ-5 scores (T50) as well as the final ACQ-5 score; treatment with BUD/FOR led to a significant, strong reduction in T50 but had a minor effect on the final AQC-5 score, while FF/VI reduced T50 and produced a large shift in the final ACQ-5 scores.
Significant reductions in reliever use following ICS/LABA combination were also found relative to FF monotherapy. In addition, compared to FF alone, patients receiving FF/VI had a 22.9% lower risk of exacerbation while patients receiving BUD/FOR had a 61.7% higher risk of exacerbation.
To visualise the contributions of BMI, smoking, baseline ACQ-5 score and treatment (FF, FF/VI or BUD/FOR) to asthma symptoms, reliever use and exacerbation probability within 12 months, these are illustrated as heat maps in Fig. 2. In all three endpoints, the colour gradients indicate that current smokers are mostly affected, with poorer outcomes. Increases in BMI were associated with probabilities of worse ACQ-5 symptom scores and increased asthma exacerbation rates across treatments. After 12 months, patients treated with FF/VI had a lower risk of exacerbation or lower probability of poor symptom control than those treated with FF alone or BUD/FOR [Fig. 2; across all variables the plotted risk estimates for ACQ-5 scores > 1.5 (poorly controlled asthma) or ≥ 1 exacerbation were lower for FF/VI than FF or BUD/FOR]. The simulation scenarios described below aim to unravel the effect of these factors from the effect of FF, FF/VI and BUD/FOR treatment by accounting for each at a time.
Heat maps showing the effect of treatment choices and three key baseline characteristics which were identified as covariates on immediate symptoms and long-term exacerbation risk, as assessed by drug–disease models describing individual patterns of reliever medication use, time course of asthma symptom (ACQ-5) score and probability of at least one exacerbation. Heat map depicting how clinical and demographic characteristics at baseline contribute to differences in ACQ-5 symptom score (top panel, symptom score after 12 months), reliever use (middle panel, canisters per year) and exacerbation risk (bottom panel, probability of at least one event/year) following treatment with FF 100 μg o.d., FF/VI 100/25 μg o.d. or regular dosing BUD/FOR. 320/9 μg b.i.d. Figures within each cell represent the estimates within 12 months from the start of treatment. Covariates on the time course of ACQ-5 scores: BMI, FEV1% pred, smoking status, sex, baseline ACQ-5 and treatment. The dotted lines represent actual data used in model development while surrounding areas depict the wider, clinically relevant range of patients with moderate-severe asthma in a real-world practice. The colour gradient (green to red) reflects increase in reliever use, symptom scores or probability of an exacerbation. All estimates are relative to ICS monotherapy (FF). ACQ-5 Asthma Control Questionnaire-5, BMI body mass index, BUD/FOR budesonide/formoterol, FEV1%pred forced expiratory volume in 1 s percent predicted, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol, SABA short-acting beta2-agonist
Simulation Scenarios
The pooled population of 24,292 patients used for simulation scenarios is described in Table 1. Overall, the mean age was 48 years old and the mean BMI was 29.2 kg/m2, 35% of patients were female and 67% had never smoked. Patients with available data had a mean ACQ-5 score and FEV1% pred. of 2.02 and 73.6%, respectively. The most common duration of previous ICS treatment and history of asthma ranged between ≥ 1– < 10 years (n = 839) and ≥ 25 years (n = 3883), respectively. Approximately half of patients had a history of asthma exacerbation in the prior year.
For each scenario presented below, population characteristics are described in Supplement 4, with simulation outputs reported and corresponding significance of differences between conditions and treatment, taking into account multiplicity for statistical evaluations.
The effect of baseline ACQ-5 was explored as shown in Fig. 3 (and Supplement 4, scenario 1). Asthma control and corresponding reliever use remained relatively stable for patients with well-controlled (ACQ-5 score ≤ 0.75) and not well-controlled symptoms (ACQ-5 > 0.75 to ≤ 1.5), but worsened for patients with poorly controlled symptoms (ACQ-5 > 1.5) at baseline. Despite similarities in initial ACQ-5 symptom control scores between treatments for patients with well-controlled asthma, long-term exacerbation rates were different between treatments. Cumulative exacerbation frequency was 6–12.6% lower in patients with well-controlled and not well-controlled versus poorly controlled symptoms at baseline.
Scenario 1: The effect of baseline ACQ-5 on ACQ-5 score, reliever use (canisters/year) and exacerbation risk. Graphs show the predicted change in asthma control (ACQ-5 change from baseline, left panels), reliever use (cumulative number of canisters, middle panels) and percentage of patients having ≥ 1 exacerbation within 12 months (right panels) in a cohort of 1500 patients randomly sampled from the pooled population (replicated 500 times) representing the effect of treatment (FF 100 μg o.d. monotherapy, FF/VI 100/25 μg o.d. or BUD/FOR 320/9 μg b.id.). Plots are shown stratified by asthma control status: well-controlled (ACQ-5 scores ≤ 0.75, green), not well controlled (ACQ-5 scores > 0.75 to ≤ 1.5, yellow) and poorly controlled (ACQ-5 scores > 1.5, red) asthma. Solid lines represent the median simulated curve, shaded areas depict the 95% confidence intervals of all simulated curves. The table shows the median and 95% CIs for the effect sizes of ACQ-5 change from baseline and annualised exacerbation rates for each treatment, and the mean and 95% CI for total reliever use. ACQ-5 Asthma Control Questionnaire-5, BUD/FOR budesonide/formoterol, CI confidence interval, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol
The effect of BMI was explored as shown in Fig. 4 (and Supplement 4, scenario 2). Exacerbation rates and reliever use increased across higher BMI categories. All patients had improvements in symptom control regardless of treatment, but FF/VI was associated with greater reductions in reliever canister use and exacerbation rates versus FF and BUD/FOR in obese patients than those of normal weight.
Scenario 2: The effect of BMI on ACQ-5 score, reliever use (canisters/year) and exacerbation risk. Graphs show the predicted change in asthma control (ACQ-5 change from baseline, left panels), reliever use (cumulative number of canisters, middle panels) and percentage of patients having ≥ 1 exacerbation within 12 months (right panels) in a cohort of 1500 patients randomly sampled from the pooled population (replicated 500 times) representing the effect of treatment (FF 100 μg o.d. monotherapy yellow, FF/VI 100/25 μg o.d. green or BUD/FOR 320/9 μg b.i.d. red). Plots are shown stratified by BMI category: normal weight (BMI 18.5 to < 25 kg/m2), overweight (BMI 25 to < 30 kg/m2), obese (BMI 30 to < 35 kg/m2) and extremely obese (BMI ≥ 35 kg/m2). Solid lines represent the median simulated curve, shaded areas depict the 95% confidence intervals of all simulated curves. The table shows the median and 95% CIs for the effect sizes of ACQ-5 change from baseline and annualised exacerbation rates for each treatment, and the mean and 95% CI for total reliever use. ACQ-5 Asthma Control Questionnaire-5, BUD/FOR budesonide/formoterol, CI confidence interval, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol
The effect of asthma exacerbation history was explored as shown in Fig. 5 (and Supplement 4, scenario 3). A history of ≥ 1 asthma exacerbation in the prior year was generally associated with reduced asthma control, increased reliever use and markedly higher annualised exacerbation rates versus no asthma exacerbation history.
Scenario 3: Effect of exacerbation history on ACQ-5 score, reliever use (canisters/year) and exacerbation risk. Graphs show the predicted change in asthma control(ACQ-5 change from baseline, left panels), reliever use (cumulative number of canisters, middle panels) and percentage of patients having ≥ 1 exacerbation within 12 months (right panels) in a cohort of 1500 patients randomly sampled from the pooled population (replicated 500 times) representing the effect of treatment (FF 100 μg o.d. monotherapy yellow, FF/VI 100/25 μg o.d. green or BUD/FOR 320/9 μg b.i.d. red). Plots are shown stratified by asthma exacerbation history: zero, one and more than one previous exacerbations. Solid lines represent the median simulated curve, shaded areas depict the 95% confidence intervals of all simulated curves. The table shows the median and 95% CIs for the effect sizes of ACQ-5 change from baseline and annualised exacerbation rates for each treatment, and the mean and 95% CI for total reliever use. ACQ-5 Asthma Control Questionnaire-5, BUD/FOR budesonide/formoterol, CI confidence interval, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol.
The effect of sex differences is shown in Supplement 4 (scenario 4). Males had lower reliever use and exacerbation rates than females.
The effect of smoking status is shown in Fig. 6 (and Supplement 4, scenario 5). There were differences in all three endpoints depending on smoking status; patients who never smoked had lower reliever use and exacerbation rates than former and current smokers. Current smokers on average used almost twice as many reliever canisters compared to patients who had never smoked; former smokers used approximately 1.4 times more reliever canisters than never smokers.
Scenario 5: The effect of smoking status on ACQ-5 score, reliever use (canisters/year) and exacerbation risk. Graphs show the predicted change in asthma control (ACQ-5 change from baseline, left panels), reliever use (cumulative number of canisters, middle panels) and percentage of patients having ≥ 1 exacerbation within 12 months (right panels) in a cohort of 1500 patients randomly sampled from the pooled population (replicated 500 times) representing the effect of treatment (FF 100 μg o.d. monotherapy yellow, FF/VI 100/25 μg o.d. green or BUD/FOR 320/9 μg b.i.d. red). Plots are shown stratified by patients’ smoking status: never smoked, former smoker and current smoker. Solid lines represent the median simulated curve, shaded areas depict the 95% confidence intervals of all simulated curves. The table shows the median and 95% CIs for the effect sizes of ACQ-5 change from baseline and annualised exacerbation rates for each treatment, and the mean and 95% CI for total reliever use. ACQ-5 Asthma Control Questionnaire-5, BUD/FOR budesonide/formoterol, CI confidence interval, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol
The effect of asthma history is shown in Supplement 4 (scenario 6). There were no clear differences in symptom control or exacerbation rates according to asthma history, however reliever use followed a trend of increasing with length of asthma duration.
The effect of smoking cessation at 6 months after the start of treatment is shown in Supplement 4 (scenario 7). Former smokers had improvements in asthma control and reduced reliever usage versus current smokers, however exacerbation rates were similar regardless of smoking cessation.
The effect of ICS/LABA treatment switch at 3 and 6 months after start of therapy, irrespective of symptom control level is shown in Supplement 4 (scenario 8). Switching from BUD/FOR to FF/VI treatment was accompanied by a marked decrease in reliever usage and an increase in the proportion of non-exacerbating patients. The converse was associated with switching from FF/VI to BUD/FOR treatment.
The effect of treatment step-up in non-responders to FP monotherapy was investigated, as shown in Fig. 7 (and Supplement 4, scenario 9). Regardless of asthma control status, the lowest proportion of exacerbating patients and the lowest reliever use were in patients who switched to FF/VI treatment, versus switching to BUD/FOR or higher FP dosage. Overall, the reduction in exacerbation risk with FF/VI was significantly greater compared with FP 500 µg (+ 4.9%, p < 0.01) or BUD/FOR (+ 8.9%, p < 0.01). Relative to BUD/FOR 320/9 μg b.i.d., switching to FF/VI 100/25 μg o.d. resulted in a reduction of 35.3% in the number of moderate–severe exacerbations over the period of 12 months. For patients with poor asthma control, switching therapy reduced reliever reliance and improved the risk of exacerbation over time.
Scenario 9: Effect of treatment switch (FP non-responders) on ACQ-5 score, reliever use (canisters/year) and exacerbation risk predictions. Graphs show the predicted change in asthma control (ACQ-5 change from baseline, left panels), reliever use (cumulative number of canisters, middle panels) and percentage of patients having ≥ 1 exacerbation within 12 months (right panels) in a cohort of virtual patients who initiate treatment with ICS monotherapy (FP 250 μg b.i.d.) and subsequent step-up of treatment in FP non-responders [FP (NR) switched to higher dose FP 500 μg yellow; FP (NR) switched to FF/VI 100/25 μg o.d. green; FP (NR) switched to BUD/FOR 320/9 μg b.i.d. red], compared with FP-responsive patients [FP (R) blue, continued on FP 250 μg]. Plots are shown stratified by asthma control status: well-controlled (ACQ-5 ≤ 0.75), not well-controlled (ACQ-5 > 0.75 to ≤ 1.5) and poorly controlled (ACQ-5 > 1.5) asthma. Solid lines represent the median simulated curve, shaded areas depict the 95% confidence intervals of all simulated curves. The table shows the median and 95% CIs for the effect sizes of ACQ-5 change from baseline and annualised exacerbation rates for each treatment, and the mean and 95% CI for total reliever use. ACQ-5 Asthma Control Questionnaire-5, BUD/FOR budesonide/formoterol, CI confidence interval, FF fluticasone furoate, FF/VI fluticasone furoate/vilanterol, NR non-responder [i.e. a patient not achieving symptom control (ACQ-5 > 0.75) at 3 months after treatment initiation with ICS monotherapy (FP)], R responder [i.e. a patient achieving symptom control (ACQ-5 ≤ 0.75) at 3 months after treatment initiation with ICS monotherapy (FP)]
Discussion
The current study comprised two distinct steps. First, parametric drug–disease models were developed using pooled patient data from clinical trials. This step provided estimates of patient-/disease-related factors and drug-specific properties. Subsequently, these estimates were used to explore and evaluate the effect of interindividual differences in baseline characteristics on treatment performance, disentangling it from the pharmacological effect itself (i.e. treatment response). Here, we discuss both steps and the implications of our findings for personalised management of patients with moderate–severe asthma.
Individual Patient Level Model-Based Analysis of Baseline Characteristics and Treatment Choices
Our analysis of the immediate symptom status, as assessed by ACQ-5 scores and reliever use over time along with long-term (1 year) exacerbation risk in moderate–severe asthma, showed that various individual clinical and demographic baseline characteristics were associated with different outcomes, partly explaining some of the heterogeneity in patient response on maintenance therapy. In line with previous findings, patients with higher ACQ-5 scores at baseline (i.e. with not well-controlled and poorly controlled asthma symptoms) showed less asthma symptom control, higher reliever use and higher risk of experiencing ≥ 1 asthma exacerbation within the first year [38]. Male patients, who were non-smokers, with a BMI < 25 kg/m2, and no exacerbation history (i.e. no exacerbation events over the last 12 months) had a lower risk of exacerbation (p < 0.01) compared with female smokers, with a BMI ≥ 25 kg/m2 and prior exacerbation history. Notably, smokers on average used 75.4% more reliever compared to a never-smoker patient. Likewise, reliever use in former smokers was 42.3% higher than in patients who never smoked. Age and geographical ancestry were not found to significantly affect reliever use.
From the evaluation of all three endpoints, ACQ-5, BMI, and smoking status at baseline were found to influence both immediate symptoms and exacerbation risk irrespective of treatment choice, whilst other baseline characteristics were associated with only one or two endpoints. Most importantly, the use of a model-based approach allowed us to distinguish patient-/disease-specific features from drug-specific properties. The separation of the effects associated with patient and drug factors provided insight into the intricate interplay between reliever use and varying symptom control status, which is apparently compensated by additional reliever use.
It is worth mentioning that we have also explored biomarkers (e.g. eosinophil count, FeNO) and other clinical variables that were available in the different studies. Eosinophil counts in blood and FeNO did not show a significant effect on the response measures of interest and therefore were not considered as covariates in the models. With regard to FEV1%pred, it was identified as a covariate on exacerbation risk. However, interindividual differences in lung function at baseline did not show a significant effect on ACQ-5 trajectories or in individual patterns of reliever medication use. Similarly, season was identified as a covariate only for the risk of exacerbation. Therefore, we have not evaluated specific scenarios with these variables.
Treatment differences were also apparent; patients treated with FF/VI had a lower risk of exacerbation than those treated with FF or BUD/FOR. This effect was also evident after evaluating propensity score-matched patients with moderate to severe asthma symptoms (p < 0.001). It also became evident that stabilisation of symptom scores requires time and that maximum treatment response may not be reached within 12 weeks, which represents the duration of many studies in moderate–severe asthma [39, 40]. Here, we have shown that, while treatment with BUD/FOR shows a faster decrease in ACQ-5 at the start of treatment, it does not reach the same final (maximum) effect on AQC-5 score. By contrast, FF/VI, produces a large shift (− 0.251) in the final ACQ-5 scores, which is detectable only after 12 months. Moreover, combination therapy (i.e. FF/VI and BUD/FOR) was found to produce a significantly higher reduction in reliever use than ICS monotherapy.
Simulation Scenarios and Implications of Baseline Characteristics and Treatment Choices for the Clinical Management of Patients with Moderate-Severe Asthma
The availability of model parameter estimates, including covariates describing the effect of clinical and demographic baseline characteristics along with the effect of different pharmacological interventions, provided an opportunity to evaluate the impact of interindividual differences, i.e. patient- and disease-related factors on symptom control, reliever use and exacerbation risk, independently from that of the underlying maintenance therapy with ICS/LABA. In addition, the use of virtual cohorts allowed us to look in parallel at the different features, controlling one at a time, which is not possible in a prospective or retrospective observational study, or in randomised controlled trials. Hence, simulation scenarios shed light into the implications of treatable traits in a way that no other approach allows, without confounding or interference from multiple concurrent factors, which cannot be fixed or controlled in a real-life setting. Yet, these are the exact factors which will determine heterogeneity in response to treatment in real patients.
The different simulation scenarios indicate that stepping-up patients uncontrolled on FP 250 µg to combination therapy with FF/VI may offer a significantly greater reduction in exacerbation risk than FP 500 µg (+ 4.9%, p < 0.01) or BUD/FOR (+ 8.9%, p < 0.01). Reasons for the long-term benefits observed with FF/VI may include higher adherence to once-daily dosing, compared with the twice-daily dosing for the BUD/FOR regimen, reducing the risk of periods of no bronchoprotection with suboptimal adherence [41,42,43]. This effect appears to be accompanied by a greater (mean) effect of FF/VI on reliever use compared with BUD/FOR, as shown by the lower number of canisters over the period of 12 months in patients with ACQ-5 > 0.75 (p < 0.01). It may also be explained by differences between the molecules within these formulations [17, 18], and correlates with findings from a short-term (3-month) comparison of switching from FP/SAL or BUD/FOR to FF/VI in a real-world setting that found improvements in lung function and asthma control with FF/VI [44].
There are various statistical methods and techniques to perform treatment or group comparisons [45,46,47,48]. Among these techniques, network meta-analysis [49] has been widely used, but it relies on mean estimates of treatment response. In contrast, the use of parametric models describing the time course of individual response, as implemented here, allows identification and separation of the effect of individual differences in baseline from that of the treatment. Unfortunately, there are no other reports on indirect comparisons between treatments in moderate–severe asthma that could be used as benchmark for our findings.
Our results suggest that the effects of ICS-containing treatments on long-term (1 year) outcomes are influenced by patients’ individual characteristics, highlighting the potential role for personalised interventions to choose the optimal therapy for patients and to identify those who would benefit from escalation to o.d. dual therapy earlier in their disease course. These results are in line with previous model-based analyses of pooled clinical trial data [2,3,4]. An important implication of our findings is that other intrinsic and extrinsic factors at baseline may allow the identification of patient groups most likely to benefit from early dual ICS/LABA therapy, in order to prevent further airway damage and remodelling [50]. Personalised management of patients with asthma should consider those factors, particularly high BMI, low FEV1, exacerbation history and female sex, which have been identified as independent predictors of future asthma exacerbation [51].
From a clinical perspective, it may thus be helpful to consider the heterogeneity of disease status and treatment effects longitudinally. For example, in a patient at increased risk of exacerbation, opting to continue with ICS monotherapy in the disease course may expose the patient to more exacerbations (potentially inducing further airway damage and remodelling) than with earlier treatment step-up to o.d. ICS/LABA therapy. However, if the patient was a smoker, the potential benefit of step-up would be less impactful if they chose to continue smoking. It is likely that smoking has a dual deleterious effect on asthma, changing the inflammatory subtype (and thereby reducing ICS sensitivity, requiring higher doses), while also increasing damage to airways with subsequent remodelling. The novelty of our modelling findings lies in the quantification of these traits and their impact numerically.
Strengths of this study include the individual patient level data from a large pool of patients following different interventions. While most trials monitor immediate effects of treatment or longer-term exacerbation risk, these effects are usually not assessed in the same patient over time at an individual level. Our modelling strategy enables the analysis of how both interact longitudinally at an individual patient level. We specifically selected studies for inclusion that were ≥ 24 weeks’ duration to utilise the most accurate extrapolated annualised exacerbation rates. Moreover, the availability of model parameter estimates, including covariates describing the effect of clinical and demographic baseline characteristics along with the effect of different pharmacological interventions allowed the evaluation of the effect of interindividual differences, i.e. patient- and disease-related factors, on symptom control, reliever use and exacerbation risk, independently from that of the underlying maintenance therapy with ICS/LABA.
Limitations of this study include potential selection bias: clinical trials often exclude comorbidities, and are more frequently monitored, which may not reflect clinical practice. However, our use of high-quality randomised clinical trial data is likely to counteract some bias that might be seen in observational studies, allowing for extrapolation of the effect of covariates across a clinically relevant range with sufficient precision to describe their implication for patients with moderate–severe asthma in real-life settings [52]. Limitations of the individual models are described in the Supplementary Materials (Supplements 1–3).
Conclusions
Differences between individuals’ clinical and demographic characteristics are associated with heterogeneity in terms of asthma symptoms, reliever use, long-term exacerbation rates and treatment responses. Our modelling and simulation approach in moderate–severe asthma identified the relative contributions of baseline ACQ-5 score, BMI, asthma exacerbation history and smoking to clinical outcomes with different ICS and ICS/LABA therapies o.d. or b.i.d. These findings may provide clinicians with a more structured way to identify patients with treatable traits who warrant personalised asthma management.
Data Availability
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
2023 GINA Report, Global Strategy for Asthma Management and Prevention. Available at: https://ginasthma.org/2023-gina-main-report/. Accessed 16/10/2023.
Oosterholt S, Pavord ID, Brusselle G, et al. Modelling Asthma TrEatment Responses (MASTER): effect of individual patient characteristics on the risk of exacerbation in moderate or severe asthma: a time-to-event analysis of randomized clinical trials. Br J Clin Pharmacol. 2023. https://doi.org/10.1111/bcp.15801.
van Dijkman SC, Yorgancıoğlu A, Pavord I, et al. Effect of individual patient characteristics and treatment choices on reliever medication use in moderate-severe asthma: a Poisson analysis of randomised clinical trials. Adv Ther. 2024;41:1201–25.
Singh D, Oosterholt S, Pavord I, et al. Understanding the clinical implications of individual patient characteristics and treatment choice on the risk of exacerbation in asthma patients with moderate–severe symptoms. Adv Ther. 2023;40:4606–25.
Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7.
Bateman ED, Reddel HK, Eriksson G, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010;125:600–8.
Latorre M, Pistelli R, Carpagnano GE, et al. Symptom versus exacerbation control: An evolution in GINA guidelines? Ther Adv Respir Dis. 2023;17:17534666231159261.
von Bülow A, Hansen S, Sandin P, et al. Severe asthma trajectories in adults: findings from the NORDSTAR cohort. Eur Respir J. 2023;62:2202474.
Chen W, FitzGerald JM, Lynd LD, et al. Long-term trajectories of mild asthma in adulthood and risk factors of progression. J Allergy Clin Immunol Pract. 2018;6:2024–32.
Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13.
Yii ACA, Tan JHY, Lapperre TS, et al. Long-term future risk of severe exacerbations: distinct 5-year trajectories of problematic asthma. Allergy. 2017;72:1398–405.
Haselkorn T, Szefler SJ, Chipps BE, et al. Disease burden and long-term risk of persistent very poorly controlled asthma: TENOR II. J Allergy Clin Immunol Pract. 2020;8:2243–53.
De Prado GL, Pavord I, Busse W, et al. Effect of dupilumab on prevention of lung function decline in patients with uncontrolled moderate-to-severe asthma: ATLAS trial design. ERJ Open Res. 2023;9:00417–2022.
Bai TR, Vonk JM, Postma DS, et al. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–6.
Sears MR. Lung function decline in asthma. Eur Respir J. 2007;30:411–3.
Soremekun S, Heaney LG, Skinner D, et al. Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study. Thorax. 2023;78:643–52.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80:372–80.
Daley-Yates PT, Aggarwal A, Plank M. Pharmacological basis of differences in dose response, dose equivalence, and duration of action of inhaled corticosteroids. Adv Ther. 2024;41(5):1995–2009.
Wojciechowski J, Mukherjee A, Banfield C, Nicholas T. Model-informed assessment of probability of Phase 3 success for ritlecitinib in patients with moderate-to-severe ulcerative colitis. Clin Pharmacol Ther. 2024. https://doi.org/10.1002/cpt.3251.
Healy P, Verrest L, Felisi M, Ceci A, Della PO. Dose rationale for gabapentin and tramadol in pediatric patients with chronic pain. Pharmacol Res Perspect. 2023;11(5): e01138.
Polhamus DG, Dolton MJ, Rogers JA, et al. Longitudinal exposure-response modeling of multiple indicators of Alzheimer’s disease progression. J Prev Alzheimers Dis. 2023;10(2):212–22.
D’Agate S, Musuamba FT, Jacqz-Aigrain E, Della PO. Simplified dosing regimens for gentamicin in neonatal sepsis. Front Pharmacol. 2021;12: 624662.
Saeed MA, Vlasakakis G, Della PO. Rational use of medicines in older adults: Can we do better during clinical development? Clin Pharmacol Ther. 2015;97(5):440–3.
Manolis E, Musuamba FT, Karlsson KE. The European Medicines Agency experience with pediatric dose selection. J Clin Pharmacol. 2021;61(Suppl 1):S22–7.
O’Byrne PM, Bleecker ER, Bateman ED, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J. 2014;43:773–82.
Bateman ED, O’Byrne PM, Busse WW, et al. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014;69:312–9.
Busse WW, O’Byrne PM, Bleecker ER, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients ≥12 years old with asthma: a randomised trial. Thorax. 2013;68:513–20.
Woodcock A, Bleecker ER, Lotvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest. 2013;144:1222–9.
Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting beta2-agonists (ICS/LABA) in patients with uncontrolled asthma: An open-label, randomized, controlled trial. Respir Med. 2018;141:111–20.
Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: An open-label, parallel group, randomised controlled trial. Lancet. 2017;390:2247–55.
Dahl R, Chuchalin A, Gor D. EXCEL: a randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respir Med. 2006;100:1152–62.
FitzGerald JM, Boulet LP, Follows RM. The CONCEPT trial: a 1-year, multicenter, randomized, double-blind, double-dummy comparison of a stable dosing regimen of salmeterol/fluticasone propionate with an adjustable maintenance dosing regimen of formoterol/budesonide in adults with persistent asthma. Clin Ther. 2005;27:393–406.
Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol(100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52(10):1073–83.
Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84.
Chain AS, Dieleman JP, van Noord C, et al. Not-in-trial simulation I: bridging cardiovascular risk from clinical trials to real-life conditions. Br J Clin Pharmacol. 2013;76(6):964–72.
Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;16(13):92.
Egleston BL, Cropsey KL, Lazev AB, Heckman CJ. A tutorial on principal stratification-based sensitivity analysis: application to smoking cessation studies. Clin Trials. 2010;7(3):286–98.
Garcia G, van Dijkman S, Pavord I, et al. A simulation study of the effect of clinical characteristics and treatment choice on reliever medication use, symptom control and exacerbation risk in moderate-severe asthma. [Placeholder for Master 6 citation when published].
Berger WE, Noonan MJ. Treatment of persistent asthma with Symbicort (budesonide/formoterol inhalation aerosol): an inhaled corticosteroid and long-acting beta2-adrenergic agonist in one pressurized metered-dose inhaler. J Asthma. 2010;47(4):447–59.
Balki A, Balamurugan S, Bardapurkar S, et al. Comparison of fluticasone/formoterol with budesonide/formoterol pMDI in adults with moderate to severe persistent asthma: results from a 12-week randomized controlled trial. Pulm Pharmacol Ther. 2018;48:28–36.
Singh D, Garcia G, Maneechotesuwan K, et al. New versus old: The impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther. 2022;39(5):1895–914.
Daley-Yates P, Singh D, Igea JM, et al. Assessing the effects of changing patterns of inhaled corticosteroid dosing and adherence with fluticasone furoate and budesonide on asthma management. Adv Ther. 2023;40(9):4042–59.
Furuhashi K, Fujisawa T, Hashimoto D, et al. Once-daily fluticasone furoate/vilanterol combination versus twice-daily budesonide/formoterol combination in the treatment of controlled stable asthma: a randomized crossover trial. J Asthma Allergy. 2019;12:253–61.
Shimizu Y, Shiobara T, Arai R, Chibana K, Takemasa A. Real-life effectiveness of fluticasone furoate/vilanterol after switching from fluticasone/salmeterol or budesonide/formoterol therapy in patients with symptomatic asthma: Relvar Ellipta for Real Asthma Control Study (RERACS study). J Thorac Dis. 2020;12(5):1877–83.
Macabeo B, Quenéchdu A, Aballéa S, et al. Methods for indirect treatment comparison: results from a systematic literature review. J Mark Access Health Policy. 2024;12(2):58–80.
Goteti K, Garcia R, Gillespie WR, et al. Model-based meta-analysis using latent variable modeling to set benchmarks for new treatments of systemic lupus erythematosus. CPT Pharmacometrics SystPharmacol. 2024;13(2):281–95.
Armstrong AW, Warren RB, Zhong Y, et al. Short-, mid-, and long-term efficacy of deucravacitinib versus biologics and nonbiologics for plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2023;13(11):2839–57.
Sui Z, Zhu H, Luo J, et al. Quantitative comparison of the efficacy of clinical drug treatments for primary progressive multiple sclerosis. J Clin Neurosci. 2023;113:45–53.
Svedsater H, Stynes G, Wex J, et al. Once-daily fluticasone furoate/vilanterol versus twice daily combination therapies in asthma-mixed treatment comparisons of clinical efficacy. Asthma Res Pract. 2016;8(2):4.
Zhang J, Dong L. Status and prospects: personalized treatment and biomarker for airway remodeling in asthma. J Thorac Dis. 2020;12:6090–101.
Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: A respiratory effectiveness group initiative. J Allergy Clin Immunol Pract. 2017;5:1015–24.
European Medicines Agency. Concept paper on the extrapolation of efficacy and safety in medicine development, EMA/129698/2012, 22/06/2012 (Accessible at: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-concept-paper-extrapolation-efficacy-and-safety-medicine-development_en.pdf).
Medical Writing and/or Editorial Assistance
Medical Writing and Editorial assistance. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Jenni Lawton, PhD, of Ashfield MedComms (Macclesfield, UK), an Inizio company, and was funded by GSK.
Funding
This investigation is part of the MASTER (Modelling Asthma TrEatment Responses) study, which has been funded by GlaxoSmithKline (study no. 218915). GSK has also funded the Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Sean Oosterholt and Janna Duong were involved in the analysis and interpretation of study data, drafting and critical revision of the manuscript; Pierluigi Paggiaro, Gabriel Garcia, Nicolas Roche, Manish Verma, Maximilian Plank and Anurita Majumdar were involved in the interpretation of study data, drafting and critical revision of the manuscript; Oscar Della Pasqua was involved in the conception/design and interpretation of study data, drafting and critical revision of the manuscript. All authors had access to the study data, take responsibility for the accuracy of the analysis and had authority in the decision to submit the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Pierluigi Paggiaro received honoraria for education activities, received grants for advisory boards and education activities from AstraZeneca, Chiesi, GSK, Guidotti and Sanofi. Gabriel Garcia has received research grants from AstraZeneca, Boehringer Ingelheim, GSK, Novartis and Sanofi, and for acting as a consultant/advisor/speaker for AstraZeneca, GSK, Novartis, and Sanofi. Nicolas Roche is in receipt of grants from Boehringer Ingelheim, GSK, Novartis, Pfizer; personal consulting fees from AstraZeneca, Austral, Bayer, Biosency, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer, Sanofi, Teva; personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, MSD, Novartis, Pfizer, Sanofi, Teva, Zambon; and support for attending meetings and/or travel from AstraZeneca, Chiesi, and GSK. Manish Verma, Maximilian Plank, Sean Oosterholt, Janna K. Duong, Anurita Majumdar and Oscar Della Pasqua are GSK employees and hold stocks/shares in GSK.
Ethical Approval
This article is based on in silico modelling and simulation and does not contain any new studies with human participants or animals performed by any of the authors. All clinical data used for the development and validation of the different models, as well as those required for re-sampling of the baseline characteristics of the virtual patient cohorts which were generated for the evaluation of the different simulation scenarios described in this study were derived from clinical trials that have been performed according to the Declaration of Helsinki and were approved by the required ethics committee(s) and/or ethics review board(s). Re-use of the data for the purpose of the current investigation is in alignment with the terms of informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Paggiaro, P., Garcia, G., Roche, N. et al. Baseline Characteristics and Maintenance Therapy Choice on Symptom Control, Reliever Use, Exacerbation Risk in Moderate–Severe Asthma: A Clinical Modelling and Simulation Study. Adv Ther 41, 4065–4088 (2024). https://doi.org/10.1007/s12325-024-02962-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02962-2