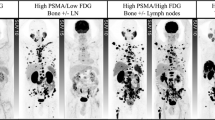
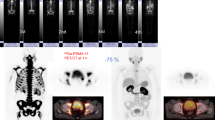
Abstract
Radioligand therapy is a targeted cancer treatment modality in which radioisotopes are utilized in the delivery of radiation at targeted cancer cells, with the goal of sparing normal cells. Prostate cancer is a well-known radiosensitive disease, historically treated with radioisotopes such as Strontium-89, Samarium-153, and Radium-223 for palliation of bone metastases. Recently, prostate specific membrane antigen (PSMA) has recently been employed as a radioligand target due to its unique properties of high expression on the surface of prostate cancer cells, limited expression in normal tissue, function as an internalizing cell surface receptor, and increased expression with androgen deprivation therapy. In 2015, 177Lu-PSMA-617 was first introduced as a promising treatment option for castration-resistant prostate cancer, and 7 years later the results of the phase III VISION trial led to 177Lu-PSMA-617 gaining FDA approval for the treatment of progressive castration-resistant prostate cancer. These results in combination with the inherent properties of 177Lu-PSMA-617 have led to its current exploration as a promising treatment modality beyond progressive metastatic castration-resistant prostate cancer, and into the earlier phases of prostate cancer. This review paper aims to highlight the key phase III randomized controlled trials related to 177Lu-PSMA-617 in all stages of prostate cancer, as well as bring attention to ongoing, earlier phase I/II trials incorporating 177Lu-PSMA-617.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Theranostics radioligand therapy (RLT) refers to the use of a pair of radiopharmaceuticals containing radionuclides for imaging and therapy [1]. It allows for a targeted cancer treatment modality in which radioisotopes are utilized in the delivery of radiation at targeted cancer cells, with the goal of sparing normal cells [2]. The concept was first used by Dr. Saul Hertz in 1941 for the treatment of thyroid disease with radioactive iodine [3]. Since then, RLT has emerged as a promising treatment modality in a variety of radiosensitive diseases. One such radiosensitive disease is prostate cancer, in which RLT has been studied in both localized and metastatic disease states. The beta-emitting radioisotopes, Strontium-89 and Samarium-153, were among the first radioisotopes approved for palliation of painful bony metastases. Strontium-89 was first approved in 1993, while Samarium-153 was approved in 1997 [4, 5]. The alpha-emitting radioisotope, Radium-223, was more recently approved for treatment of symptomatic bone metastases in castration-resistant prostate cancer (CRPC) in 2013 [6]. However, these radioisotopes lacked selectivity for target tissue and have been found to non-selectively bind to areas of increased bone metabolism. The first FDA-approved RLT used peptide receptor radionuclide therapy for progressive midgut neuroendocrine tumors in 2018. This combined the radionuclide 177Lu with the somatostatin analogue DOTATATE to deliver ionizing radiation to tumor cells expressing somatostatin receptors [7]. Now, prostate specific membrane antigen (PSMA) has become an area of interest for targeted radionuclide therapy that is highly-specific for the target tumor tissue.
PSMA, or folate hydrolase I and glutamate carboxypeptidase II, is a cell membrane protein that is expressed on the surface of prostate cancer cells [8]. It has emerged as a beneficial radioligand target in prostate cancer treatment due to its limited expression in normal tissue, high expression levels on the plasma membrane of the tumor cell, function as an internalizing cell surface receptor [9], and increased expression with androgen deprivation therapy (ADT) [10]. In 2015, 177Lu-PSMA-617 was first introduced as a promising modality for CRPC [11], and 7 years later the results of the phase III VISION trial led to 177Lu-PSMA-617 emerging as a potentially favorable treatment modality in earlier stages of prostatic disease [12].
Despite these advancements, prostate cancer remains a highly prevalent disease, and one that will likely see a continued growth in incidence due to an increasingly aging global population. This calls attention to the importance of highlighting the literature supporting the advancements in modern treatments. The purpose of this narrative review is to describe the current body of evidence supporting the use of 177Lu-PSMA-617 in prostate cancer. The results of recent large cohort trials in combination with the inherent properties of 177Lu-PSMA-617 have led to its current exploration as a promising treatment modality beyond progressive metastatic CRPC (mCRPC), and into the earlier phases of prostate cancer treatment.
1.1 Prostate cancer and standard of care treatment
Prostate cancer is the second leading cause of male cancer-related mortality in the United States, with an estimated 288,300 new cases and 34,700 deaths in 2023 [13]. Early detection and management of low to intermediate risk prostate cancer is associated with favorable outcomes including prostate cancer-specific survival at 5 and 10-years approaching 100% [14]. However, the disease becomes more difficult to manage in patients with high-risk disease or evidence of metastasis [15], with approximately one third of all prostate cancer patients developing biochemically recurrent disease after primary definitive therapy [16].
In addition to being a radioligand target, PSMA is also an effective imaging marker. PSMA-directed PET is a non-invasive diagnostic technique to image PSMA positive lesions in individuals with prostate cancer. The routine clinical uses of PSMA PET allows for initial staging of prostate cancer as well as evaluation of metastasis and disease recurrence. Patients who are found to have disease recurrence confirmed on PSMA PET or biochemical recurrence on PSA surveillance, are subsequently offered a variety of treatment options which include hormonal therapy, radiation therapy, or immunotherapy.
Since the 1940s, ADT has been the backbone of prostate cancer treatment for patients with biochemical recurrent disease [17]. The initiation of ADT marks the beginning of treatment for castration-naive prostate cancer (CNPC) [18], with the addition of an androgen receptor pathway inhibitor (ARPI) for patients with metastatic castration naive prostate cancer (mCNPC). Examples of ADT include GnRH agonists such as leuprolide, goserelin, triptorelin, and buserelin. Commonly prescribed treatments for mCNPC are the combination of abiraterone, a first-generation ARPI, and prednisone to ADT [19,20,21], and the second-generation ARPIs, enzalutamide, apalutamide, and darolutamide [22,23,24]. However, despite these regimented therapies, a subset of these patients will continue to exhibit disease progression at which point their disease state becomes known as CRPC.
The treatment of CRPC is vast and varies depending on the presence of metastasis. Nonmetastatic disease is treated with continued ADT and an ARPI such as enzalutamide, apalutamide, and darolutamide [25,26,27]. For metastatic disease without prior ARPI exposure, treatment is augmented by the addition of abiraterone plus prednisone [28, 29] or enzalutamide [25, 30]. Docetaxel plus prednisone is the standard of care for initial chemotherapy in males with relatively rapidly progressing symptomatic mCRPC [31], with cabazitaxel plus prednisone serving as the preferred next-line agent for patients who progressed on treatment with docetaxel and required additional chemotherapy [32]. While cabazitaxel offered a survival benefit in men with taxane-refractory mCRPC relative to current treatment regimens, that survival benefit was only 2.4 months [33, 34].
1.2 Key studies: phase III randomized controlled trials
1.2.1 Progressive castration-resistant prostate cancer
In 2015, 177Lu-PSMA-617 RLT was first introduced as a promising treatment modality for mCRPC [11]. This therapy gained popularity due to its favorable properties, including a long half-life and optimal medium energy. A long half-life is favorable due to a sustained treatment effect, dosing flexibility, and waste minimization. Medium energy is optimal because of targeted cell destruction with minimal damage to the surrounding tissue, and an effective range to balance systemic side effects with effective treatment of the tumor cells. Several subsequent trials reported positive outcomes with treatment including reduced pain, low toxicity, and favorable response rates compared to standard therapy alone [34,35,36,37,38].
In 2021, the results of the TheraP trial, the first randomized study of 177Lu-PSMA-617 RLT, was published [34]. TheraP was a multicenter, unblinded, randomized phase 2 trial in which 200 patients with mCRPC and prior docetaxel treatment were 1:1 randomized to receive either 177Lu-PSMA-617 (6·0–8·5 GBq intravenously every 6 weeks for up to six cycles) or cabazitaxel (20 mg/m2 intravenously every 3 weeks for up to ten cycles). The primary endpoint of this trial was PSA response, defined by a reduction of over 50% from baseline. Secondary endpoints included radiographic progression-free survival (rPFS), adverse events, and death.
TheraP reported compelling evidence in favor of 177Lu-PSMA-617 as an active treatment for men with mCRPC who have progressed after docetaxel chemotherapy. The trial reported a more frequent PSA responses among men in the 177Lu-PSMA-617 group than in the cabazitaxel group (66% vs 37% by intention to treat; 95% CI 16–42; p < 0·0001; and 66% vs 44% by treatment received; [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]; p = 0·0016). Key secondary endpoints also favored the study arm. Progression-free survival (PFS) was significantly improved in the 177Lu-PSMA-617 group (HR, 0.63; 95% CI 0.46–0.86; p = 0.0028). Additionally, grade 3–4 adverse events were less frequent in the 177Lu-PSMA-617 group (33% vs 53%). Overall survival (OS) was similar among both groups (19.1 vs 19.6 months; HR, 0.97; 95% CI 0.70–1.4; p = 0.99), and no deaths were attributed to 177Lu-PSMA-617 therapy [34].
In 2022, the efficacy of 177Lu-PSMA-617 RLT in mCRPC was further expanded upon with the publishing of the phase 3 VISION trial which described the largest assessment of 177Lu-PSMA-617 RLT among mCRPC patients to date [12]. A total of 831 patients with mCRPC previously treated with at least one ARPI and one or two taxane regimens were randomly assigned, in a 2:1 ratio, to receive either 177Lu-PSMA-617 (therapeutic dose of 7.4 GBq for 6 cycles every 6 weeks) in addition to standard care (study group) versus receiving standard care alone (control group). The primary end points of this trial included rPFS and OS. Key secondary end points were objective response, disease control, and time to symptomatic skeletal events (SSE).
VISION reported significant improvement in patient outcomes among the study group compared with the control group. Compared with the control group, patients assigned to the study group demonstrated significantly prolonged rPFS (median, 8.7 vs. 3.4 months; hazard ratio for progression or death, 0.40; 99.2% CI 0.29 to 0.57; P < 0.001) and OS (median, 15.3 vs. 11.3 months; hazard ratio for death, 0.62; 95% CI 0.52 to 0.74; P < 0.001). Key secondary endpoints also favored the study group. The median time to the first SSE or death was 11.5 months in the study group, as compared with 6.8 months in the control group (hazard ratio, 0.50; 95% CI, 0.40 to 0.62; P < 0.001). Among patients who had measurable target lesions, a complete response was noted in 9.2% in the study group and in none of the patients in the control group. A partial response was noted in 41.8% in the study group versus 3% in the control group. Additionally, the proportion of patients with confirmed decreases in the PSA level of at least 50% was 46.0% in the study group compared to 7.1% in the control group (OR 11.19 [95% CI 6.25, 20.04]), while the decrease in PSA level of at least 80% was 33.0% in the study group compared to 2.0% in the control group (OR 23.62 [95% CI 8.57, 65.11]) [12].
The results of the VISION trial, as well as the culmination of efforts of previously reported literature [34,35,36,37], ultimately led to the FDA approval of 177Lu-PSMA-617 RLT for the treatment of patients with mCRPC in 2022 [39]. The results of these trials demonstrate a significant improvement in patient outcomes and suggests a positive step towards disease control in patients with mCRPC.
1.2.2 Taxane-naive metastatic castration-resistant disease
The phase 3 VISION trial showed 177Lu-PSMA-617 significantly prolonged rPFS and OS in patients with mCRPC previously treated with one or more ARPIs and 1–2 taxanes. However, the utilization of 177Lu-PSMA-617 in patients who are taxane-naive had not been previously described. This is being explored by the PSMAfore trial, which has been investigating the outcomes in taxane-naive patients with mCRPC treated with either 177Lu-PSMA-617 or a change in ARPI.
PSMAfore is a multicenter, open-label, randomized phase 3 trial in adults with progressive mCRPC and confirmed PSMA expression by 68 Ga-PSMA-11 PET/CT. In order to be eligible for this trial, patients had to have been taxane-naive in the metastatic setting with a history of receiving one prior ARPI, and who are candidates for a change in ARPI. The trial enrolled 469 patients who were randomized 1:1 to receive 177Lu-PSMA-617 (7.4 GBq IV every 6 weeks for 6 cycles) or a change in ARPI to either abiraterone or enzalutamide. The primary endpoint is rPFS. The key secondary endpoint is OS, as well as safety and tolerability of 177Lu-PSMA-617 and health-related quality of life [40].
Preliminary results were presented in October 2023 and showed the trial met its primary endpoint with a statistically significant benefit in rPFS, with a 59% reduction in the risk of radiographic progression in patients treated with 177Lu-PSMA-617 versus those who received a change in ARPI. The study was also statistically significant for several secondary endpoints, with patients in the experimental arm showing improved quality of life and delay in worsening pain. Patients in the experimental arm were also more than 2.5 times more likely to demonstrate a PSA decline of at least 50% compared with those treated with a change in ARPI alone [41]. Eighty-four percent of patients with progressive disease with ARPI alone chose to cross-over to the experimental arm.
Additional data was presented in September 2024, which further illustrated 177Lu-PSMA-617 prolonged rPFS relative to ARPI change, with a favorable safety profile. The median rPFS was 11.60 months (95% CI 9.30–14.19) in the 177Lu-PSMA-617 group versus 5.59 months (95% CI 4.21–5.95) in the ARPI change group (HR 0.49 [95% CI; 0.39–0.61]). The incidence of grade 3–5 adverse events was also lower in the 177Lu-PSMA-617 group compared to the ARPI change group [42].
The study is estimated to be completed in September 2025. The preliminary results of PSMAfore are encouraging and show the potential of 177Lu-PSMA-617 in improving patient outcomes in taxane-naïve patients with progressive mCRPC.
1.2.3 Castration-naive prostate cancer
The results of the VISION trial provided encouragement for studying the use of 177Lu-PSMA-617 beyond CRPC and into castration-naive disease. It has previously been described that PSMA expression occurs universally both prior to hormone deprivation and in the recurrent disease setting [43,44,45]. Additionally, studies have shown that radio-sensitization occurs with ADT and confers improved OS, local control, disease progression, and distant metastases free survival in comparison to treatments with radiotherapy alone [46, 47]. Following radiation therapy, the androgen receptor signaling pathway repairs the DNA damage caused by radiation. Blocking this pathway with ADT inhibits this repair mechanism, further exposing the cancer cells to radiation therapy [48, 49]. It is hypothesized that with the inhibition of these DNA repair mechanisms through ADT, there will be fewer genomic alterations that could lead to radiation-resistance in mCNPC. These results are being studied in the ongoing PSMAddition trial.
PSMAddition is an international, prospective, open-label, randomized, phase III trial comparing 177Lu-PSMA-617 in combination with standard of care, versus standard of care alone, in adult male patients with mCNPC. Approximately 1126 patients will be randomized 1:1 to receive 177Lu-PSMA-617 (7.4 GBq every 6 weeks for a maximum of 6 cycles) plus standard of care or standard of care alone. Standard of care is defined as a combination of ARPI plus ADT. The primary endpoint is rPFS. Upon confirmed radiographic progression, participants in the standard of care cohort can cross over to the 177Lu-PSMA-617 cohort. The key secondary endpoints include OS, proportion of patients with a PSA decline of ≥ 90% from baseline, time to development of mCRPC, composite PFS, safety, tolerability, and health-related quality of life [50]. As of May 2023, 176 centers are recruiting patients to the study, with an estimated study completion date in February 2026.
1.2.4 Oligometastatic prostate cancer
Oligometastatic prostate cancer (OMPC) refers to an intermediate disease state, between localized disease and widespread metastasis, in which patients are observed to have a limited number of sites of distant metastasis while still being a potentially curable disease [16, 51]. There is not an established number of quantifiable distant sites that define OMPC. Ongoing prospective studies define oligometastases as ranging from 1–5 sites of metastases [52, 53]. OMPC has classically been defined through conventional imaging (CT/MRI and bone scans). However, with the advent of PSMA-based imaging, we are now able to detect previously undetectable cases of OMPC, with PSMA PET/CT now being considered the most accurate diagnostic imaging modality for detecting oligometastasis in prostate cancer patients [54, 55]. Currently stereotactic body radiation therapy (SBRT) is considered the standard of care for OMPC [56], with the goal of delaying initiation of ADT and prolonging PFS [57, 58].
In March 2024, the PSMA-DC study was launched with the goal of evaluating the efficacy of 177Lu-PSMA-617 versus observation after SBRT in delaying castration or disease recurrence in adult patients with PSMA positive OMPC. PSMA-DC is an international, prospective, open-label, multi-center, randomized phase III study. Approximately 450 patients will be enrolled. Following SBRT, participants will be randomized in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq (200 mCi) ± 10% every 6 weeks for a planned 4 cycles) or observation only (control arm). The primary endpoint of PSMA-DC is to evaluate metastasis free survival (MFS). Key secondary endpoints include time to hormonal therapy, time to PSA progression, time to symptomatic progression, rPFS, OS, as well as safety, tolerability, and health-related quality of life. The estimated study completion date is July 2030 [59].
A summary of the key phase III randomized controlled trials (RCTs) related to 177Lu-PSMA-617 use in the various phases of prostate cancer can be found in Table 1.
1.3 Phase I/II 177Lu-PSMA-617 trials
In addition to the large phase III RCTs, there are several earlier phase clinical trials investigating the use of 177Lu-PSMA-617 in the various stages of prostate cancer disease states. We performed a review of clinicaltrial.gov using the keywords “Lu-177 PSMA-617” and “Prostate Cancer” and summarized the ongoing clinical trials herein. A summary of the following studies can be found in Table 2.
1.3.1 Castration-resistant prostate cancer
Immunotherapy with checkpoint inhibitors is actively being investigated in conjunction with 177Lu-PSMA-617 in patients with mCRPC. A phase Ib trial (NCT03805594) began studying the combination of the PD-1 inhibitor, pembrolizumab, with 177Lu-PSMA-617 to determine the recommended phase 2 dose (RP2D), schedule, and objective response rate (ORR) of this combination in approximately 43 patients with mCRPC [60, 61]. Similarly, a phase II trial (NCT05766371) is aiming to study whether a single dose of 177Lu-PSMA-617 followed by pembrolizumab was safe and provides clinical benefit in approximately 45 patients with mCRPC who have previously progressed on at least one prior ARPI (abiraterone, enzalutamide, apalutamide) [61, 63]. Another study that is looking at combining immunotherapy with 177Lu-PSMA-617 is the EVOLUTION study (NCT05150236). In this study, the activity and safety of 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with the CTLA-4 inhibitors, ipilimumab and nivolumab, is also being investigated in a randomized, phase II trial looking at approximately 110 patients with mCRPC. Patients will be randomized in a 2:1 ratio stratified by prior exposure to docetaxel in order to determine the PSA progression free survival (PSA-PFS) at one year [64].
Additionally, the UPLIFT study (NCT05113537) is a phase I/II trial that is uniquely investigating the use of the cyclin-dependent kinase-4 and -6 inhibitor, abemaciclib, prior to administration of 177Lu-PSMA-617 in order to determine the drug limiting toxicities (DLTs) and RP2D for abemaciclib given prior to 177Lu-PSMA-617 at each treatment cycle in approximately 30 mCRPC patients [65].
Several other drugs are also being tested in combination with 177Lu-PSMA-617 in the treatment of mCRPC, including cabozantinib (NCT05613894, CaboLu trial), olaparib (NCT03874884, LuPARP trial), and enzalutamide (NCT04419402, ENZA-p study). The CaboLu trial is a phase 1b dose-escalation study in which the combination of cabozantinib, a multityrosine kinase inhibitor, and 177Lu-PSMA-617 is being investigated to assess the rate of DLTs in the dose-escalation phase and then identifying the maximum tolerated dose (MTD) and/or recommended dose and schedule for the subsequent expansion phase. This study will look at 24 patients with mCRPC [66]. Similarly, the LuPARP study is a phase 1 trial investigating the combination of olaparib, a potent poly ADP-ribose polymerase inhibitor, and 177Lu-PSMA-617 to determine safety and tolerability of this combination in approximately 52 patients with mCRPC [67]. Additionally, the ENZA-p study is a randomized, stratified, 2-arm phase 2 trial that recruited 162 participants with mCRPC not previously treated with chemotherapy in order to determine the PFS of the combination of enzalutamide and 177Lu-PSMA-617. These patients were randomized to either enzalutamide or enzalutamide plus 177Lu-PSMA-617 in a 1:1 ratio [68].
Data from the ENZA-p trial showed a median PSA-PFS of 13.0 months (95% CI 11.0–17.0) in the enzalutamide plus 177Lu-PSMA-617 group and 7.8 months (95% CI 4.3–11.0) in the enzalutamide group (hazard ratio 0.43; 95% CI 0.29–0.63; p < 0.0001). The addition of 177Lu-PSMA-617 to enzalutamide improved PSA-PFS providing evidence of enhanced anticancer activity in patients with mCRPC with risk factors for early progression on enzalutamide and warrants further evaluation of the combination more broadly in metastatic prostate cancer [69].
One study is also evaluating the potential clinical benefit of combining 177Lu-PSMA-617 with chemotherapeutic agents. The LuCaB study (NCT05340374) is evaluating the combination of cabazitaxel and 177Lu-PSMA-617 in a phase I/II study to determine the MTD, DLTs, and RP2D of this combination in approximately 44 patients with mCRPC who have progressed on prior docetaxel and a second-generation androgen receptor antagonist [70].
The safety and tolerability of 177Lu-PSMA-617 in mCRPC patients with moderate and severe renal impairment is being examined by Novartis in a phase II study (NCT06004661). Twenty patients are expected who will be stratified to one of three cohorts based on eGFR at screen (normal, moderately reduced, or severely reduced). The effects of radiation absorption in the kidney and other at-risk organs will be determined, as well as the concentration in blood and radioactivity in the urine [71].
The purpose of the REALITY Study is to analyze the safety and efficacy of RLT targeting PSMA in men with mCRPC in everyday practice. The trial analyzed prospectively collected registry data in 254 patients with mCRPC. Primary endpoints were response to RLT, defined by changes from baseline PSA concentration, PSA-PFS, and OS. Data published in September 2021 demonstrated a greater than 50% PSA reduction in 52.0% of patients (132/254). PSA-PFS was 5.5 (95% CI 4.4–6.6) months and OS, 14.5 (95% CI 11.5–17.5) months. This large, prospectively observed “real-world” cohort of patients with late-stage mCRPC showed 177Lu-PSMA-617 was effective, safe, and well-tolerated [72].
1.3.2 Localized prostate cancer
The LuTectomy trial (NCT04430192) is a phase I/II trial that is seeking to evaluate the utility of 177Lu-PSMA-617 in an earlier disease setting. LuTectomy enrolled 20 men with high PSMA-expressing, high-risk localized or locoregional advanced prostate cancer and evaluated the dosimetry, safety, and efficacy of upfront 177Lu-PSMA-617 prior to radical prostatectomy with pelvic node lymph node dissection. Participants received one to two cycles of 177Lu-PSMA-617 followed by surgery to deduce the radiation absorbed dose in both the prostate and involved lymph nodes. The absorbed radiation doses were a median of 19.6 Gy (IQR 11.3–48.4) for the prostate, and a median of 37.9 Gy (IQR 33.1–50.1) for involved pelvic lymph nodes. The study found that up to two cycles of 177Lu-PSMA-617 prior to radical prostatectomy was safe, well tolerated, and did not compromise surgical safety [73].
1.3.3 Castration-naive prostate cancer
The UpFrontPSMA study (NCT04343885) is investigating the clinical benefit of the combination of 177Lu-PSMA-617 and docetaxel in mCNPC. UpFrontPSMA is a randomized phase 2 study enrolling approximately 140 patients with newly diagnosed mCNPC who will be randomized to either 177Lu-PSMA-617 followed by docetaxel or docetaxel in a 1:1 ratio. The undetectable PSA rate at 12 months is the primary outcome, while the safety of 177Lu-PSMA-617 followed by docetaxel compared to docetaxel alone, time to development of castration resistance between treatment arms, PSA-PFS, and rPFS will also be measured [74].
1.3.4 Oligometastatic prostate cancer
A phase I pilot study out of Memorial Sloan Kettering Cancer Center (NCT05079698) is planning to look at the administration of 177Lu-PSMA-617 prior to SBRT in the castration naive, oligometastatic treatment phase in an effort to prevent or delay metastasis. This study will evaluate approximately 6 patients and measure the proportion of subjects with DLTs due to 177Lu-PSMA-617 [75].
The Bullseye study is phase 2 prospective randomized trial (NCT04443062) that plans to investigate the efficacy of 177Lu-PSMA-617 in approximately 58 OMPC prior to the castration-resistant state. These patients will be randomized in a 1:1 ratio to receive either 177Lu-PSMA-617 or current standard of care (deferred ADT) to compare the fraction of patients that have disease progression within 6 months, and to compare the two arms for the time to disease progression [76].
The POPSTAR II study (NCT05560659) is a randomized phase II parallel cohort trial that plans to evaluate the biochemical PFS of stereotactic ablative body radiotherapy (SABR) alone and SABR plus 177Lu-PSMA-617 in approximately 92 OMPC patients. These patients will be split into a 1:1 ratio to either SABR alone or SABR plus 2 cycles of 177Lu-PSMA-617 over a period of 24 months [77].
1.4 Phase IV 177Lu-PSMA-617 trials
In a phase IV, post-authorization safety study (NCT05803941), Novartis is currently investigating the long-term outcome of known or potential risks of 177Lu-PSMA-617. The study will seek to further characterize any other serious adverse reactions in the long-term in adults with prostate cancer who received at least one dose of 177Lu-PSMA-617 from interventional, phase I-IV Novartis sponsored clinical trials. The study will observe approximately 700 patients with an estimated study completion of July 21, 2033 [78].
1.5 Final considerations
The vast exploration of 177Lu-PSMA-617 throughout all phases of prostate cancer treatment and promising published and anticipated results lead to optimism about the future landscape of prostate cancer treatment. However, the availability and costs associated with the treatment remain a barrier to its extensive utilization, with an expected cost of $122,489 per patient. For comparison, the estimated total treatment cost for docetaxel is $5,940 per patient, while cabazitaxel is between $18,069 and $36,137 per patient. The Canadian Agency for Drugs and Technologies in Health recommends Pluvicto be reimbursed by public drug plans for the treatment of patients with mCRPC. Future efforts will need to be directed towards improving access to care for these therapies [79].
Although the benefits of 177Lu-PSMA-617 therapy have been highly promoted, this treatment is not benign and adverse effects of treatment should be discussed with patients as part of the shared-decision making process prior to treatment initiation. Adverse effects include fatigue, dry mouth, nausea, anemia, changes in bowel movements (constipation or diarrhea), vomiting, thrombocytopenia, urinary tract infection, weight loss, or abdominal pain (80).
2 Conclusion
RLT with 177Lu-PSMA-617 has demonstrated efficacy in progressive mCRPC post-ARPI and 1–2 taxane chemotherapies in patients with PSMA-avid disease through the results of the VISION trial. Preliminary results from PSMAfore in taxane-naïve mCRPC are promising which leads to anticipation of results of PSMAddition in CNPC and PSMA-DC in OMPC. While these large phase III RCTs are currently underway, numerous additional smaller phase I and II studies incorporating 177Lu-PSMA-617 in combination with other agents are being planned. This review article highlights the many ongoing studies of 177Lu-PSMA-617 which are investigating expansion of use to all phases of prostate cancer, as well as brings attention to the additional proposed adjunct uses of 177Lu-PSMA-617 in prostate cancer.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ADT:
-
Androgen deprivation therapy
- ARPI:
-
Androgen receptor pathway inhibitor
- CNPC:
-
Castration naïve prostate cancer
- CRPC:
-
Castration resistant prostate cancer
- DLT:
-
Drug-limiting toxicity
- MFS:
-
Metastasis-free survival
- mCNPC:
-
Metastatic castration naive prostate cancer
- mCRPC:
-
Metastatic castration resistant prostate cancer
- MTD:
-
Maximum tolerated dose
- OMPC:
-
Oligometastatic prostate cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PSA-PFS:
-
Prostate specific antigen progression free survival
- PSMA:
-
Prostate specific membrane antigen
- RCT:
-
Randomized controlled trial
- RLT:
-
Radioligand therapy
- RP2D:
-
Recommended phase 2 dose
- rPFS:
-
Radiographic progression-free survival
- SABR:
-
Stereotactic ablative body radiotherapy
- SBRT:
-
Stereotactic body radiation therapy
- SSE:
-
Symptomatic skeletal event
References
Bannik K, Madas B, Jarzombek M, Sutter A, Siemeister G, Mumberg D, et al. Radiobiological effects of the alpha emitter Ra-223 on tumor cells. Sci Rep. 2019;9(1):18489.
Guerra Liberal FDC, Tavares AAS, Tavares JMRS. Palliative treatment of metastatic bone pain with radiopharmaceuticals: a perspective beyond Strontium-89 and Samarium-153. Appl Radiat Isot. 2016;110:87–99.
Fahey FH, Grant FD. Celebrating eighty years of radionuclide therapy and the work of Saul Hertz. J Appl Clin Med Phys. 22. United States: © 2021 The Authors. Journal of Applied Clinical Medical Physics published by Wiley Periodicals, Inc. on behalf of American Association of Physicists in Medicine.; 2021. p. 4–10.
Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25(5):805–13.
Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63(5):940–5.
Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738–46.
Hennrich U, Kopka K. Lutathera (®): the first FDA- and EMA-Approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals. 2019;12(3):114.
Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. (177)Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58(8):1196–200.
Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58(18):4055–60.
Bakht MK, Oh SW, Youn H, Cheon GJ, Kwak C, Kang KW. Influence of androgen deprivation therapy on the uptake of PSMA-targeted agents: emerging opportunities and challenges. Nucl Med Mol Imaging. 2017;51(3):202–11.
Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benešová M, Mier W, et al. [1⁷⁷Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(6):987–8.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–103.
Jain MA, Leslie SW, Sapra A. Prostate Cancer Screening. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473–80.
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89.
Fakhrejahani F, Madan RA, Dahut WL. Management options for biochemically recurrent prostate cancer. Curr Treat Options Oncol. 2017;18(5):26.
Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol. 2002;168(1):9–12.
Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85.
Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. 2000;355 (9214):1491-8.
Kumar G. LATITUDE: a landmark trial for high-risk metastatic castration-sensitive prostate cancer: Final overall survival analysis. Indian J Urol. 2020;36(1):71–2.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–51.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–86.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–42.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–46.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–18.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American society of clinical oncology and cancer care ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–48.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
Paller CJ, Antonarakis ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117–24.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33.
Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang SP, Kong G, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of (177)Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61(6):857–65.
Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45(1):19–31.
Drug USFa, Administration., @font-face, Math" f-fC, 4 p-, mso-font-charset:0, et al. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer.
Sartor AO, Morris MJ, Chi KN, Bono JSD, Shore ND, Crosby M, et al. PSMAfore: A phase 3 study to compare 177Lu-PSMA-617 treatment with a change in androgen receptor pathway inhibitor in taxane-naïve patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2022;40(6_suppl):TPS211-TPS.
Novartis Pluvicto™ shows clinically meaningful and highly statistically significant rPFS benefit in patients with PSMA-positive metastatic castration-resistant prostate cancer in the pre-taxane setting 2023 [https://www.novartis.com/news/media-releases/novartis-pluvictotm-shows-clinically-meaningful-and-highly-statistically-significant-rpfs-benefit-patients-psma-positive-metastatic-castration-resistant-prostate-cancer-pre-taxane-setting.
Morris MJ, Castellano D, Herrmann K, de Bono JS, Shore ND, Chi KN, et al. (177)Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. Lancet. 2024;404(10459):1227–39.
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61.
Hope TA, Truillet C, Ehman EC, Afshar-Oromieh A, Aggarwal R, Ryan CJ, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58(1):81–4.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40.
Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–11.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1066–73.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245–53.
Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–71.
Tagawa ST, Sartor AO, Saad F, Reid AH, Sakharova OV, Feng FY, et al. PSMAddition: A phase 3 trial to compare treatment with 177Lu-PSMA-617 plus standard of care (SoC) and SoC alone in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2023;41(16_suppl):TPS5116-TPS.
Mahjoub S, Heidenreich A. Oligometastatic prostate cancer: definition and the role of local and systemic therapy: a narrative review. Transl Androl Urol. 2021;10(7):3167–75.
Foster CC, Weichselbaum RR, Pitroda SP. Oligometastatic prostate cancer: Reality or figment of imagination? Cancer. 2019;125(3):340–52.
Fodor A, Berardi G, Fiorino C, Picchio M, Busnardo E, Kirienko M, et al. Toxicity and efficacy of salvage carbon 11-choline positron emission tomography/computed tomography-guided radiation therapy in patients with lymph node recurrence of prostate cancer. BJU Int. 2017;119(3):406–13.
Aluwini SS, Mehra N, Lolkema MP, Oprea-Lager DE, Yakar D, Stoevelaar H, et al. Oligometastatic prostate cancer: results of a dutch multidisciplinary consensus meeting. Eur Urol Oncol. 2020;3(2):231–8.
McCarthy M, Francis R, Tang C, Watts J, Campbell A. A multicenter prospective clinical trial of (68)Gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution compared with standard imaging. Int J Radiat Oncol Biol Phys. 2019;104(4):801–8.
Guidelines NCCN. Prostate cancer (version 4.2023) https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Rao A, Vapiwala N, Schaeffer EM, Ryan CJ. Oligometastatic prostate cancer: a shrinking subset or an opportunity for cure? Am Soc Clin Oncol Educ Book. 2019;39:309–20.
Juan GR, Laura FH, Javier PV, Natalia VC, Galante RMI, Enrique RG, et al. Where do we stand in the management of oligometastatic prostate cancer? A comprehensive review. Cancers. 2022;14(8):2017.
Novartis. An open-label study comparing lutetium (177Lu) Vipivotide Tetraxetan Versus Observation in PSMA Positive OMPC. (PSMA-DC) [updated April 18, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05939414.
Aggarwal RR, Sam SL, Koshkin VS, Small EJ, Feng FY, Kouchkovsky ID, et al. Immunogenic priming with 177Lu-PSMA-617 plus pembrolizumab in metastatic castration resistant prostate cancer (mCRPC): a phase 1b study. J Clin Oncol. 2021;39(15_suppl):5053.
Immunogenic priming with PSMA-targeted radioligand therapy in advanced prostate cancer: a phase 1b study of 177Lu-PSMA-617 in combination with pembrolizumab. 2019. https://clinicaltrials.gov/study/NCT03805594.
Aggarwal R, Starzinski S, de Kouchkovsky I, Koshkin V, Bose R, Chou J, et al. Single-dose (177)Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023;24(11):1266–76.
A phase 2 study of Pembrolizumab Plus 177Lu-PSMA-617 in patients with metastatic castration resistant prostate cancer. 2023. https://clinicaltrials.gov/study/NCT05766371.
Phase II study of radionuclide 177Lu-PSMA therapy versus 177Lu-PSMA in combination with ipilimumab and nivolumab for Men with metastaticcastration resistant prostate cancer (mCRPC). 2021. https://clinicaltrials.gov/study/NCT05150236.
Phase I/II Study of CDK4/6 inhibition with abemaciclib to upregulate PSMA expression prior to 177Lu-PSMA-617 treatment in patients with metastatic castrate resistant prostate cancer (mCRPC) previously treated with novel hormonal agents and chemotherapy. 2021. https://clinicaltrials.gov/study/NCT05113537.
A phase Ib dose-escalation study of cabozantinib in combination with lutetium-177 (177Lu)-PSMA-617 in patients with metastatic castration-resistant prostate cancer (mCRPC). 2022. https://clinicaltrials.gov/study/NCT05613894.
177Lu-PSMA-617 therapy and olaparib in patients with metastatic castration resistant prostate cancer. 2019. https://clinicaltrials.gov/study/NCT03874884.
ENZA-p: A randomised phase II trial using PSMA as a therapeutic agent and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). 2020. https://clinicaltrials.gov/study/NCT04419402.
Emmett L, Subramaniam S, Crumbaker M, Nguyen A, Joshua AM, Weickhardt A, et al. [<sup>177</sup>Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5):563–71.
Cabazitaxel in Combination With 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer. 2022. https://clinicaltrials.gov/study/NCT05340374.
An Open-label Dosimetry, Biodistribution, Tolerability and Safety Study of Lutetium (177Lu) Vipivotide Tetraxetan in Participants With Progressive PSMA-Positive Metastatic Castration-Resistant Prostate Cancer (mCRPC) With Moderately and Severely Impaired and With Normal Renal Function. 2023. https://clinicaltrials.gov/study/NCT06004661.
Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study). Eur J Nucl Med Mol Imaging. 2022;49(3):1075–85.
Study of the Dosimetry, Safety and Potential Benefit of 177Lu-PSMA-617 Radionuclide therapy prior to radical prostatectomy in men with high-risk localised prostate cancer. 2020. https://clinicaltrials.gov/study/NCT04430192.
UpFrontPSMA : A Randomised Phase 2 Study of Sequential 177Lu-PSMA617 and docetaxel versus docetaxel in metastatic hormone-naive prostate cancer. 2020. https://clinicaltrials.gov/study/NCT04343885.
A Pilot Study of Stereotactic Body Radiotherapy (SBRT) and 177Lu-PSMA-617 for the treatment of hormone sensitive, oligometastatic prostate cancer. 2021. https://clinicaltrials.gov/study/NCT05079698.
Lutetium-177-PSMA-617 Radioligand therapy in oligo-metastatic hormone sensitive prostate cancer. 2020. https://clinicaltrials.gov/study/NCT04443062.
Lu-PSMA for Oligometastatic prostate cancer treated with stereotactic ablative radiotherapy, a randomised phase II parallel cohort trial. 2022. https://clinicaltrials.gov/study/NCT05560659.
A Phase IV, Post-Authorization Safety Study to Investigate the Long-Term Safety of Lutetium (177Lu) Vipivotide Tetraxetan in Adult Participants With Prostate Cancer [Internet]. 2023. https://clinicaltrials.gov/study/NCT05803941.
CADTH Reimbursement Reviews and Recommendations. Lutetium ((177)Lu) Vipivotide Tetraxetan (Pluvicto): CADTH Reimbursement Recommendation: Indication: Treatment of adults with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer who have received at least one androgen receptor pathway inhibitor and taxane-based chemotherapy. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023.
Fallah J, Agrawal S, Gittleman H, Fiero MH, Subramaniam S, John C, et al. FDA approval summary: Lutetium Lu 177 Vipivotide Tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2023;29(9):1651–7.
Acknowledgements
Not applicable
Funding
The authors have no sources of funding to declare.
Author information
Authors and Affiliations
Contributions
AK, MZ, and BJ contributed to the study conception and design. AK, MZ, and MR contributed to manuscript preparation and drafting. Expert review was performed by BJ. All authors approved the submitted version.
Corresponding author
Ethics declarations
Human ethics and consent to participate declarations
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Konopnicki, A., Zaliznyak, M., Roy, M. et al. The therapeutic use of 177 Lu-PSMA-617 radioligand therapy in prostate cancer treatment: a review of literature and ongoing trials. Discov Onc 15, 791 (2024). https://doi.org/10.1007/s12672-024-01680-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01680-z