Abstract
In the last decade, it has been discovered that intestinal flora can affect various organ-specific cancers by altering the body's energy balance, synthesizing genetic toxins and small signaling molecules, and initiating and modulating immune responses. In this review, we will focus on elucidating the role of intestinal flora based on its molecular mechanisms and its possible impact on head and neck cancers in the near future, and explore how it may be a novel approach to treating head and neck cancers in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Gut flora is a complex and diverse microbial ecosystem containing bacteria, fungi, archaea and viruses [1]. With more research, it has been found that the gut flora plays an important role in pro-inflammatory or anti-inflammatory responses [2]. For example, the intestinal bacterium Helicobacter pylori is classified as a class 1 carcinogen [3], and its presence may be closely associated with the development and progression of certain types of tumors [4]. In contrast, members of the intestinal flora such as bifidobacteria and lactobacilli are thought to reduce tumorigenesis through immunomodulatory effects [5]. Significant differences in the microbial composition of fecal samples from individuals with effective and ineffective immunotherapy have been identified [6]. Although certain intestinal bacteria may be associated with the development of specific tumors, the exact microbial composition associated with tumorigenesis requires further investigation [7].
2 History of intestinal flora
Since the first microscopic observation of microorganisms in the gut in 1683, it has been recognized that lactic acid bacteria (LAB) are beneficial to health and can affect health by altering the composition of the gut flora. After more than 300 years, the Human Gut Macrogenome Project has revealed the complexity and diversity of gut microbes and their strong association with a wide range of diseases [7,8,9,10,11,12,13,14,15,16] Future research will further explore the relationship between the gut microbiota and health and develop more effective treatments to improve the gut microecosystem (Fig. 1).
3 Mechanisms of gut flora in disease
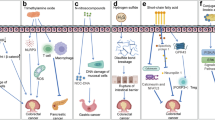
The main areas involved are as follows: gut-brain axis: gut flora interacts with the brain by modulating neurotransmitters, affecting the vagus and enteric nervous systems, and the immune system, and thus is associated with diseases such as stroke, epilepsy, and Alzheimer's disease [17,18,19]. Enterohepatic axis: intestinal flora regulates liver function by affecting metabolites, disrupting the intestinal barrier, and regulating the biological clock, and has been associated with the development of liver disease [20,21,22,23]. Intestinal-lung axis: the intestinal flora influences lung disease through the immune system by stimulating immune cells, secreting antimicrobial substances, and regulating interactions between the gut and the lungs [24,25,26,27]. Intestinal-renal axis: the intestinal flora interacts with the kidneys through metabolic and immune pathways and affects renal function [28,29,30]. Inflammatory bowel disease (IBD): dysbiosis of the intestinal flora is a key factor in IBD, affecting immune response, nutrition, and host defenses to promote or exacerbate disease [31,32,33,34,35]. Overall, gut flora regulates host immune, metabolic, and barrier functions through a variety of mechanisms, and imbalances can lead to the onset or exacerbation of a wide range of diseases, revealing its importance as a key regulator of host health (Fig. 2).
4 Intestinal flora and tumors
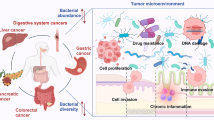
There are approximately 100 trillion microorganisms in the gut [36], and studies have shown that the microbiota plays an important role in carcinogenesis, including influencing host cell proliferation and death, altering immune system activity, and affecting metabolism. An imbalanced microbiota produces metabolites that in turn affect tumor cell proliferation, invasion, and metastasis [37,38,39]. Therefore, the gut flora has an important role in tumorigenesis and progression. Although studies have been conducted to explore the relationship between gut microbiota and head and neck squamous cell carcinoma (HNSCC), this area is still under constant research. Next, we will focus on the role and mechanisms of gut microbiota in several common head and neck squamous cell carcinomas.
4.1 Oral cancer and intestinal flora
Oral cancer (OC) is the sixth most common malignant tumor in the world [40], and it is a common tumor in the head and neck region with a very high recurrence rate. With the deepening understanding of oral cancer, more and more studies have begun to focus on the role of intestinal flora in the pathogenesis of oral cancer, and it has been found that intestinal flora is closely related to oral cancer and is mainly driven by immune mechanisms triggered by a variety of microorganisms [41,42,43]. The oral and intestinal microbiota have emerged as potential cancer biomarkers, and microbiota residing in the oral gastrointestinal tract have been found to be associated with carcinogenesis as well as modulation of response to anticancer therapy in a variety of tumor types [44]. The oral microbiota is interconnected with the lung and gut microbiota in a complex manner and is able to regulate inflammatory signaling through coordinated interactions between different species of resident bacteria, thereby promoting TLR-2-mediated T-cell activation, sustaining the oral microenvironment and promoting tumorigenesis [45]. Recent studies have concluded that oral health is associated with changes in the composition and structure of the gut bacterial flora attached to the colon [46]. Imbalances in the gut microbiota (e.g., a decrease in probiotics and an increase in harmful bacteria) are thought to be associated with a variety of diseases, including cancer. Improving the health of the microbiota, e.g., through dietary modifications, use of probiotics or antibiotics, may help to reduce the occurrence or recurrence of oral cancer. Therefore, studying the role of the gut microbiota in oral cancer could help develop new prevention and intervention strategies to reduce the incidence of oral cancer. The study of gut microbiota in oral cancer not only contributes to a better understanding of the pathogenesis of oral cancer, but also offers new possibilities for early diagnosis, personalized therapy, disease prevention, and the development of microbiomics.
4.2 Nasopharyngeal cancer and intestinal flora
Nasopharyngeal cancer (NPC) is one of the common malignant tumors in Southeast and East Asia, and about 80% of NPC patients are in advanced stages at the time of diagnosis [47]. Existing studies have shown that nasopharyngeal cancer is associated with structural imbalance of the intestinal flora, especially in the nasopharyngeal cancer family group, where Trichoderma spp, Klebsiella spp, Toxococcus spp, and Prevotella spp. were significantly increased, whereas Trichoderma spp. and Puccinia rosea spp. were significantly decreased. In addition, metabolites of the intestinal anaerobic bacterium C. ramosum stimulate cellular secretion of 5-hydroxytryptophan, leading to elevated plasma levels of 5-hydroxytryptophan, which may contribute to nasopharyngeal carcinogenesis [48]. The use of probiotics in combination with radiotherapy for simultaneous radiotherapy treatment of nasopharyngeal cancer patients may significantly enhance host immunity by altering the intestinal flora. The interaction between the host immune system and the microbiota may underlie the role of the microbiota in cancer therapy. Thus, modulation of gut flora may contribute to improving the prognosis and treatment outcome of nasopharyngeal carcinoma patients [49], while the interaction between the host immune system and the microbiota may reveal the role of the microbiota in cancer therapy [50]. The development of nasopharyngeal carcinoma is not only related to genetic factors and viral infections, but environmental factors also play an important role. Gut flora, as mediators of host-environment interactions, may play a bridging role in the influence of environmental factors on cancer development. By studying how gut flora responds to external environmental changes and thus influences nasopharyngeal cancer development, it may provide a broader perspective on cancer prevention and intervention. Studying the relationship between nasopharyngeal cancer and intestinal flora not only helps to reveal the pathogenesis of nasopharyngeal cancer, but also provides new ideas for early diagnosis, optimization of therapeutic strategies, and design of preventive measures. With the development of microbiomics technology, the role of intestinal flora in cancer research will be more and more valued, and it is expected to become an important tool in tumor therapy in the future, promoting the development of personalized cancer treatment and precision medicine.
4.3 Thyroid cancer and intestinal flora
Thyroid cancer (TC) is the most common endocrine malignancy [51]. Recent studies have shed preliminary light on the thyroid-gut axis, suggesting that gut flora and their metabolites may act on the thyroid by influencing intestinal micronutrient uptake, conversion and storage, as well as immune regulation, providing new insights into the pathogenesis of thyroid disorders and clinical management strategies [52]. The thyroid gland requires iodine to synthesize thyroid hormones, and tens of thousands of microorganisms in the gut play a key role in regulating iodine metabolism. Studies have shown that a decrease in gut flora may affect radioactive iodine absorption and thyroid function [53]. The dose of levothyroxine required to maintain a stable TSH level is closely related to the intestinal flora [54]. Serum metabolomics analysis of thyroid cancer patients with distant metastases also showed that the flora of the thyroid cancer group was higher in abundance and diversity than that of healthy controls and correlated with changes in serum lipid metabolites [55]. A high-throughput sequencing study comparing the structural characteristics of microbial communities in 36 patients with thyroid cancer, 72 patients with thyroid nodules, and 73 healthy controls also showed that thyroid cancer and thyroid nodules were strongly associated with changes in the flora [56]. In addition, a decreased abundance of certain short-chain fatty acids (SCFAs) producing bacteria, which are thought to have a regulatory role in the immune microenvironment, has been found in patients with thyroid cancer [57]. Cholesterol increased the aggressiveness of thyroid cancer, while the thyroid cancer group showed significant changes in the abundance and composition of intestinal flora and metabolites, genera that were significantly associated with disturbed lipid metabolism in thyroid cancer patients [58]. Early diagnosis of thyroid cancer still faces several challenges, and current screening methods (e.g., ultrasound, fine-needle aspiration biopsy, etc.) rely heavily on diagnostic imaging and pathology. As an important biomarker reflecting the health status of the human body, it has been found that the composition of intestinal flora may be closely related to the development of thyroid cancer. By analyzing the differences in intestinal flora between thyroid cancer patients and healthy individuals, specific flora patterns associated with thyroid cancer may be identified, thus providing new diagnostic markers for early screening. For example, overgrowth of certain flora or imbalance of flora may indicate risk of thyroid cancer. Thyroid cancer is closely linked to thyroid hormone levels. The interaction of gut flora with the endocrine system has become an increasingly important area of research. Studies have shown that gut flora can influence the metabolism and regulation of hormones in the body, possibly by altering thyroid hormone levels, which in turn affects the development of thyroid cancer. Understanding how gut flora interacts with thyroid hormones will help to shed light on the biology of thyroid cancer and provide new strategies for treatment.
4.4 Neurogliomas and gut flora
The maturation and development of the human central nervous system (CNS) is regulated by intrinsic and extrinsic factors. Studies have shown that specific microbiota can influence CNS physiology and neurochemistry [59], the gut-brain axis has been used to define the relationship between the microbiota and the brain and its interactions, and ecological dysregulation of microorganisms may contribute to the progression of CNS diseases [60]. Recent studies have shown that the microbiota has an impact on the nature and function of microglia [61]. Glioblastoma is a malignant tumor with a particularly high mortality rate [62], and new therapeutic agents and approaches are needed to combat this deadly disease. Recent studies have demonstrated the potential role of the microbiota in immuno-oncology [63], for example, analysis of fecal samples from patients with metastatic melanoma showed that in patients with PD-1 inhibition using therapeutic antibodies, Bifidobacterium longum, Pseudomonas aeruginosa and Enterococcus faecalis (Enterococcus faecalis) were increased in abundance, suggesting that certain microorganisms in the gut may play a supportive role in enhancing the effects of PD-1 inhibition [64]. Glioma development leads to dysregulation of gut ecology in a mouse model, with increased abundance of Micrococcus verrucosus and Ackermansia spp. following glioma growth and a significant increase in fecal Fusarium thick-walled/ Fusarium anisum) ratios changed significantly, with increased abundance of Fusarium verrucosum and Ackermansia spp. in the feces of mice [65]. A meta-analysis showed an association between human cytomegalovirus (HCMV) and gliomas, and polyomavirus and adenovirus infections have also been associated with glioma development [66]. Enterobacteriaceae, which are over-represented in meningiomas, also inhibit short-chain fatty acid (SCFA)-producing bacteria, leading to ecological dysregulation of the immune and intestinal environments, whereas Akkermansia spp. may induce inflammatory responses, neurotoxicity, and disruption of the blood–brain barrier in the microenvironment of gliomas, through their ability to degrade the intestinal mucosal layer. Thus, the ecological dysregulation of the gut microbiota is more severe in glioma patients, and Lactobacillus spp. and Agrobacterium spp. may exert antitumor activity through the production of butyrate, which prevents pathogen invasion, slows tumor progression, and modulates the immune response of the central nervous system. In addition, Bifidobacterium spp. contribute to the dynamic balance of immunity, neurohormones and metabolism [67]. The immune microenvironment of gliomas is an important factor in tumor therapy. It has been found that intestinal flora, by regulating the immune system, may have an impact on the immune escape mechanisms of glioma. Intestinal flora are able to influence the function of peripheral immune cells through activation of the gut-immune axis, which in turn affects the immune surveillance function of tumors. Some beneficial flora (e.g., lactobacilli, bifidobacteria, etc.) may enhance the recognition and clearance of glioma cells by the immune system by promoting anti-tumor immune responses. Imbalance of intestinal flora may lead to immune tolerance or immune escape, weakening tumor immune surveillance, which in turn promotes the growth and metastasis of glioma. Gut flora may play a crucial role in glioma development, immune response, and treatment response. An in-depth study of the relationship between gut flora and gliomas will not only help to unravel the mechanisms of glioma genesis, but may also provide new strategies and tools for early screening, personalized treatment, and improvement of patients' quality of life. With the continuous development of intestinal microbiology and tumor immunology, the application of intestinal flora in glioma research is very promising and may become an important part of glioma treatment in the future (Fig. 3).
5 Gut flora in the treatment of head and neck tumors
Cancer radiotherapy may cause a decrease in immune system function and exacerbate mucosal toxicity responses in patients, and the intestinal flora may play an important role in regulating anticancer immune responses and attenuating the side effects of radiotherapy [68]. Macrogenomic sequencing analysis showed that radiotherapy leads to a reduction in the number and diversity of intestinal flora and alters the structure of intestinal flora in nude mice. Meanwhile, raw wheat and turmeric dispersions could effectively protect the diversity of intestinal flora and partially restore the flora imbalance caused by radiotherapy [69]. By modulating the intestinal flora, the probiotic combination significantly enhanced the immune response and attenuated the severity of oral mucositis (OM) in nasopharyngeal cancer patients undergoing radiotherapy [70]. In a rat model, the probiotic mixture similarly ameliorated the severity of OM, attenuated the inflammatory response, and promoted apoptosis and altered intestinal permeability, while restoring the structure of the intestinal flora. A modified probiotic combination significantly reduced the severity of OM in patients with nasopharyngeal carcinoma (NPC), an effect that may have been achieved by enhancing the immune response and modulating the structure of the intestinal flora [71]. Recent studies have shown that Bacteroides thickeniensis was significantly reduced and Bifidobacterium bifidum was increased in postoperative thyroid cancer (TC) patients treated with 131I, leading to a significant decrease in the ratio of the two after treatment [72]. In contrast, probiotics significantly restored gut and oral microbial diversity and reduced the incidence of complications in patients after thyroid cancer resection [73]. A growing body of research suggests that microorganisms and their derivatives can be used as part of cancer therapy, including three main types: 1 microorganisms produce active substances, such as bacteriocins and antimicrobial peptides, that can be used for the treatment of gliomas through different mechanisms [74]; 2 phages can target gliomas and deliver drugs, and there is a strong affinity between phages and peptides of glioma-initiating cells, which allows for precise drug delivery to gliomas [75]; 3 microbial targeted therapies, where many bacteria can cross the blood–brain barrier into the central nervous system through a unique mechanism, offering the possibility of bacterial targeting of gliomas, as well as viruses, which can cross the blood–brain barrier into gliomas through systemic drug delivery using immune cells as carriers [76]. Metabolites of gut flora may reduce the migration and invasive capacity of glioma cells in vitro by inhibiting the downstream SDF-1 and CXCR4 signaling pathways [77]; in addition, gut flora may be involved in the regulation of glioma progression and individualized efficacy of treatment through immunomodulatory effects, and gut bacteria have been identified as potential biomarkers and clinical therapeutic targets [78]. Although gut microbes may play an important role in cancer development and treatment, more studies are needed to explore their specific functions in depth.
6 Summary and prospects
Currently, the treatment of head and neck tumors is relatively homogeneous, and a common protocol is to combine radiotherapy with immunotherapy or targeted therapy, but these treatments are usually accompanied by greater toxicity and side effects. Gut flora not only regulates tumor immune responses, but may also be involved in tumor progression, and thus has a potentially important role in tumor desensitization and therapy. The role of microbiota in the tumor microenvironment is gradually gaining attention, and if stronger immune functions can be activated by regulating gut flora, this could be a key breakthrough in tumor desensitization and therapy. With our deeper understanding of the relationship between gut bacteria and treatments such as immunotherapy and radiotherapy, studies have shown that certain genera of gut bacteria are able to alleviate serious complications caused by radiotherapy. Therefore, how to regulate intestinal flora has become an emerging topic and research direction in the field of anti-tumor therapy in the future. Studying the effects of intestinal flora on head and neck squamous cell carcinoma is scientifically important and may provide new perspectives and strategies for future cancer treatment and prevention. The following are several potential implications and future directions:
6.1 The role of intestinal flora in the tumor immune microenvironment
Recent studies have shown that intestinal flora is not only critical for intestinal health, but also has a profound impact on the systemic immune system. For head and neck squamous cell carcinoma (HNSC), intestinal flora may influence tumorigenesis and progression by modulating immune responses, promoting immune tolerance or immune escape. Studying the relationship between intestinal flora and the immune microenvironment of HNSCC may help to understand tumor immune escape mechanisms and provide new targets for immunotherapy.
6.2 Influence of intestinal flora on tumor therapeutic response
Gut flora may play an important role in the therapeutic response of tumors, especially in the application of immunotherapy such as immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors). Studies have shown that the composition of the gut flora may influence the efficacy of immunotherapy. For example, some probiotics may enhance the efficacy of immunotherapy by altering the diversity of the gut flora. Therefore, investigating the relationship between gut flora and the response of HNSCC patients to different treatments, including immunotherapy, radiotherapy, and chemotherapy, will help develop more effective and personalized treatment strategies.
6.3 Relationship between microorganisms and tumor metabolism
Intestinal flora not only has an impact on the immune system, but also regulates systemic metabolism through its metabolites (e.g., short-chain fatty acids). Imbalance of intestinal flora may affect the metabolic environment of tumors and further promote tumor growth and metastasis. Studying the interactions between gut flora and HNSCC metabolism could reveal the metabolic reprogramming mechanism of tumors and thus provide new metabolic targets for cancer therapy.
6.4 Potential for individualized therapy
The composition of gut flora varies significantly across individuals, which means that the gut flora characteristics of each HNSCC patient may affect their response to treatment. By analyzing a patient's gut flora, physicians can tailor a more personalized treatment plan, including dietary modifications and the use of probiotics or prebiotics, based on specific flora characteristics. Further research will help develop precision medicine strategies based on gut flora analysis to improve treatment efficacy and reduce side effects.
6.5 Prevention and early diagnosis
If the characteristics of gut flora are closely related to the occurrence, progression or prognosis of HNSCC, then early warning of cancer can be achieved to some extent by monitoring the gut flora. In addition, studies have shown that imbalance of certain microbial groups may be associated with cancer susceptibility, and therefore, maintaining the balance of gut flora by adjusting it may become a new cancer prevention strategy.
6.6 Exploring gut-tumor interactions
Gut flora not only affects tumor growth through immune and metabolic mechanisms, but may also interact with tumor cells through direct bacterial metabolites (e.g., fatty acids, peptides, etc.). Understanding the molecular mechanisms of these interactions can help develop novel targeted therapeutic approaches, such as using metabolites of gut microorganisms to modulate the tumor microenvironment or to act directly on tumor cells.
6.7 Advancement of interdisciplinary research
Studying the impact of gut flora on HNSCC requires not only the deep integration of multiple disciplines, such as gut microbiology, oncology, immunology and metabolism, but also the support of big data analysis and precision medicine technology. Through interdisciplinary cooperation, the multidimensional role of intestinal flora in cancer development can be more comprehensively understood and new ideas for cancer prevention, diagnosis and treatment can be provided.
6.8 Public health and nutritional interventions
Modification of gut flora through diet, lifestyle and probiotics may be a potential means of adjuvant therapy for HNSCC. In the future, dietary intervention strategies based on gut flora may become an effective public health tool to help reduce the incidence of head and neck squamous cell carcinoma.
In conclusion, studying the effects of intestinal flora on head and neck squamous cell carcinoma may provide new breakthroughs in immunotherapy, metabolic regulation, personalized therapy, and early diagnosis of cancer. With further understanding of the relationship between intestinal microecology and tumor biology, new therapeutic targets, therapies, and preventive strategies may emerge in the future, thus improving the effectiveness of cancer treatment and the quality of patient survival. However, although the effect of intestinal flora on HNSCC is an area of research with great potential, there are many limitations in the existing findings due to individual differences in intestinal flora, complex tumor microenvironment, treatment interference with flora, and technical limitations. In the future, larger and higher-quality clinical studies with a multidisciplinary and collaborative approach are needed to explore in depth the relationship between intestinal flora and HNSCC, as well as to overcome existing technical and ethical barriers, with the aim of providing more effective strategies for cancer prevention, diagnosis, and treatment.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- LAB:
-
Lactic acid bacteria
- GABA:
-
Y-aminobutyric acid
- 5-HT:
-
Pentraxin
- MIS:
-
Mucosal immune system
- IBD:
-
Inflammatory bowel disease
- IAP:
-
Inhibitor of apoptosis
- DAB:
-
Peroxidase
- HNSCC:
-
Head and neck squamous cell carcinoma
- OC:
-
Oral cancer
- NPC:
-
Nasopharyngeal Carcinoma
- TC:
-
Thyroid cancer
- CNS:
-
Central nervous system
- HCMV:
-
Human cytomegalovirus
References
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71.
Brown RL, Larkinson M, Clarke TB. Immunological design of commensal communities to treat intestinal infection and inflammation. PLoS Pathog. 2021;17(1): e1009191.
Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108.
Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141(5):1696-708.e1-2.
Kuugbee ED, Shang X, Gamallat Y, et al. Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig Dis Sci. 2016;61(10):2908–20.
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Porter JR. Antony van Leeuwenhoek: tercentenary of his discovery of bacteria. Bacteriol Rev. 1976;40(2):260–9.
Majem LS, Marcos AMR, Guardia JAM, Batrina JA, Marcos A, Anta RMO. Alimentos Funcionales. Probióticos. 2002.
Bourdelles F, Avril JL, Ghnassia JC. Quantitative study of the faecal flora of breast- or bottle-fed neonates (author’s transl). Archives Franaises de Pédiatrie. 1981;38(1):35–9.
Ursell LK, Haiser HJ, Van Treuren W, et al. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology. 2014;146(6):1470–6.
Wilson KH, Blitchington RB. Human colonic biota studied by ribosomal DNA sequence analysis. Appl Environ Microbiol. 1996;62(7):2273–8.
Lederberg J. Infectious History. Science. 2000;288(5464):287–93.
Breitbart M, Hewson I, Felts B, et al. Bacteriophages, transposons, and plasmids: metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185(20):6220–3.
Klaassens ES, De Vos WM, Vaughan EE. Metaproteomics approach to study the functionality of the microbiota in the human infant gastrointestinal tract. Appl Environ Microbiol. 2007;73(4):1388–92.
Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci. 2011;108(7):3047–52.
Sebastián Domingo JJ, Sánchez SC. From the intestinal flora to the microbiome. Rev Esp Enferm Dig. 2018;110(1):51–6.
Cryan JF, O’Riordan KJ, Cowan C, Sandhu KV, Dinan TG. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013.
Morais LH, Schreiber HL IV, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–55.
Farhangi MA, Vajdi M. Gut microbiota–associated trimethylamine N, xide and increased cardiometabolic risk in adults: a systematic review and dose-response meta-analysis. Nutr Rev. 2020. https://doi.org/10.1186/s12937-020-00592-2.
Wang T, Rong X, Zhao C. Circadian rhythms coordinated with gut microbiota partially account for individual differences in hepatitis B-related cirrhosis. Front Cell Infect Microbiol. 2022;12: 936815.
Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic fatty liver disease and the gut-liver axis: exploring an undernutrition perspective. Gastroenterology. 2022;162(7):1858-1875.e2.
Lifeng L, Yunhai Y, Yunyun L, Junwei Q, Xuejing L, Gengyun Z. A comprehensive genome survey provides novel insights into bile salt hydrolase (BSH) in lactobacillaceae. Molecules. 2018;23(5):1157.
John II, Behrendt CL, Ruhn KA, et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell. 2021. https://doi.org/10.1016/j.cell.2021.07.001.
Mjösberg J, Rao A. Lung inflammation originating in the gut. Science. 2018;359(6371):36–7.
Yun KY, Zuo T, Lui CY, Zhang F, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706.
Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010;11(7):558–60.
Perrone EE, Jung E, Breed E, et al. Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock. 2012;38(1):68–75.
Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–56.
Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. 2014. https://doi.org/10.2215/CJN.00490114.
Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24(1):88–99.
Marchesi JR, Adams DH, Fava F, Hermes G, Hart A. The gut microbiota and host health. 2015.
LeBlanc JG, Laiño JE, del Valle MJ, et al. B-group vitamin production by lactic acid bacteria–current knowledge and potential applications. J Appl Microbiol. 2011;111(6):1297–309.
Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113(12):2019–40.
Sekirov I, Russell SL, Antunes L, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
Kamada N, Kim YG, Sham HP, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(6086):1325–9.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Chen Y, Zhou J, Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 2021;11: 625913.
Bai X, Wei H, Liu W, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71(12):2439–50.
Chen W, Wen L, Bao Y, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci U S A. 2022;119(52): e2203894119.
Rodríguez-Molinero J, Migueláñez-Medrán B, Puente-Gutiérrez C, et al. Association between oral cancer and diet: an update. Nutrients. 2021. https://doi.org/10.3390/nu13041299.
Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome. J Oral Maxillofac Surg. 2010;68(6):1270–5.
Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol. 2016;8:32762.
Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–40.
Oliva M, Schneeberger P, Rey V, et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br J Cancer. 2021;124(9):1543–51.
Nocini R, Muzio LL, Gibellini D, et al. Oral microbiota in oropharyngeal cancers: Friend or foe. Front Oncol. 2022;12: 948068.
Xu AA, Hoffman K, Gurwara S, et al. Oral Health and the altered colonic mucosa-associated gut microbiota. Dig Dis Sci. 2021;66(9):2981–91.
He YQ, Wang TM, Ji M, et al. A polygenic risk score for nasopharyngeal carcinoma shows potential for risk stratification and personalized screening. Nat Commun. 2022;13(1):1966.
Peters M, Meijer C, Fehrmann R, et al. Serotonin and dopamine receptor expression in solid tumours including rare cancers. Pathol Oncol Res. 2020;26(3):1539–47.
Guo H, Chou WC, Lai Y, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020. https://doi.org/10.1126/science.aay9097.
Yu ZK, Xie RL, You R, et al. The role of the bacterial microbiome in the treatment of cancer. BMC Cancer. 2021;21(1):934.
Gu Y, Yu Y, Ai L, et al. Association of the ATM gene polymorphisms with papillary thyroid cancer. Endocrine. 2014;45(3):454–61.
Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6(4):R52–8.
Vought RL, Brown FA, Sibinovic KH, McDaniel EG. Effect of changing intestinal bacterial flora on thyroid function in the rat. Horm Metab Res. 1972;4(1):43–7.
Yao Z, Zhao M, Gong Y, et al. Relation of gut microbes and L-thyroxine through altered thyroxine metabolism in subclinical hypothyroidism subjects. Front Cell Infect Microbiol. 2020;10:495.
Shen CT, Zhang Y, Liu YM, et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2017;87(6):844–52.
Yano Y, Matsui T, Uno H, Hirai F, Futami K, Iwashita A. Risks and clinical features of colorectal cancer complicating Crohn’s disease in Japanese patients. J Gastroenterol Hepatol. 2008;23(11):1683–8.
Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8.
Asghari A, Umetani M. Obesity and cancer: 27-hydroxycholesterol, the missing link. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21144822.
Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526(7573):312–4.
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–32.
Erny D, de Angelis Hrabě AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77.
Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5 Suppl):S2-8.
Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382–96.
Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8.
Patrizz A, Dono A, Zorofchian S, et al. Glioma and temozolomide induced alterations in gut microbiome. Sci Rep. 2020;10(1):21002.
Farias K, Moreli ML, Floriano VG, da Costa VG. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch Virol. 2019;164(5):1249–57.
Jiang H, Zeng W, Zhang X, Pei Y, Zhang H, Li Y. The role of gut microbiota in patients with benign and malignant brain tumors: a pilot study. Bioengineered. 2022;13(3):7847–59.
Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85.
Yang JB, Zhu DQ, Shao M, et al. Effects of Shengmai Jianghuang San on intestinal flora in nude mice with radio resistant cells of nasopharyngeal carcinoma. Zhongguo Zhong Yao Za Zhi. 2019;44(3):553–8.
Jiang C, Wang H, Xia C, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081–90.
Xia C, Jiang C, Li W, et al. A Phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front Immunol. 2021;12: 618150.
Zheng L, Zhang L, Tang L, et al. Gut microbiota is associated with response to (131)I therapy in patients with papillary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2023;50(5):1453–65.
Lin B, Zhao F, Liu Y, et al. Randomized clinical trial: probiotics alleviated oral-gut microbiota dysbiosis and thyroid hormone withdrawal-related complications in thyroid cancer patients before radioiodine therapy following thyroidectomy. Front Endocrinol (Lausanne). 2022;13: 834674.
Cheng SY, Chen NF, Kuo HM, et al. Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis. 2018;23(5–6):314–28.
Zhang M, Lu W. Enhanced glioma-targeting and stability of (L)GICP peptide coupled with stabilized peptide (D)A7R. Acta Pharm Sin B. 2018;8(1):106–15.
Al-Obaidi M, Desa M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38(7):1349–68.
Kim H, Roh HS, Kim JE, Park SD, Park WH, Moon JY. Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells. Nutr Res Pract. 2016;10(3):259–64.
Hou X, Du H, Deng Y, et al. Gut microbiota mediated the individualized efficacy of Temozolomide via immunomodulation in glioma. J Transl Med. 2023;21(1):198.
Acknowledgements
Not applicable.
Funding
Science and Technology Foundation Project of Guizhou Provincial Health Commission [gzwkj2023-118]; Qiandongnan Science and Technology Plan (Self-funded) Project, Document No. J [2023] 93.
Author information
Authors and Affiliations
Contributions
Su Xiaolu completed an extensive literature review and drafted the manuscript, which was reviewed, revised and guided by Ma Faqiang.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Registration form and registration number of the study/trial
Not applicable.
Animal studies
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xialu, S., Faqiang, M. Mechanisms of action of intestinal microorganisms and advances in head and neck tumors. Discov Onc 16, 303 (2025). https://doi.org/10.1007/s12672-025-02035-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-025-02035-y