Abstract
Objective
This study investigated resveratrol impact on oral squamous cell carcinoma (OSCC) via the NORAD/IGF2BP2/PDK1 pathway.
Methods
CAL-27, SCC-25, and KB cell lines were used to evaluate cell proliferation, cell cycle arrest, and protein expression. Key molecular markers were assessed using Western blot, RNA interference, and functional assays.
Results
Resveratrol inhibited the growth of CAL-27, KB, and SCC-25 cancer cell lines in a dose-dependent manner, with IC50 values of 70, 145, and 125 μg/mL, respectively (P < 0.01). In CAL-27 cells, 50 μg/mL resveratrol induced G2/M arrest (P < 0.05); 100 μg/mL caused S and G2/M phase arrest (P < 0.01). Thirteen proteins changed significantly: cPKCα and Notch4 were upregulated, while p-ERK, p-PDK1, p-Cdc2, p-RB, NORAD, IGF2BP2, CDK2, Cdc2P34, Cyclin E, 14–3-3beta, and XIAP were downregulated. si-NORAD groups showed lower CAL-27 proliferation than control. IGF2BP2 silencing reduced proliferation to 69.13% in HSC3 and 74.01% in CAL-27 (P < 0.001) and decreased invasion to 72.85% and 52.44% (P < 0.001). PDK1 overexpression enhanced the proliferation and migration of hypopharyngeal cancer cells.
Conclusion
Resveratrol inhibits OSCC proliferation, particularly in CAL-27 cells. It affects NORAD, IGF2BP2, and PDK1 pathways, altering cell cycle protein expression and causing S and G2/M phase arrest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
The rapid growth, diffusion, and metastasis of tumor cells are related to too little cell death or too much proliferation [1]. The weakening or loss of apoptotic capacity causes this disease. Apoptosis is a normal physiological phenomenon in which the body actively removes excess or defective cells under strict gene regulation and is also the main mechanism for maintaining normal metabolism [2, 3]. The body maintains normal physiological function by removing aging, damaged, or potentially toxic cells to maintain ontogeny or balance the proliferation and death of cells in the adult body under normal circumstances to maintain a dynamic balance between them. Tumor genesis correlates with the abnormal proliferation and differentiation of tumor cells and the changes in apoptosis genes [4]. The disorder of its regulatory mechanism will lead to tumorigenesis. Therefore, the strategy of inducing tumor cell apoptosis has become the focus of tumor therapy in recent years. Lately, the emphasis on using Chinese herbs in the oncology therapeutic field has been increasing [5]. Epidemiology and many animal experiments have confirmed that many natural ingredients in human food can block tumorigenesis and positively impact prevention and treatment. Resveratrol is a natural plant active ingredient and one of the effective tumor chemoprevention drugs [5, 6]. Resveratrol is a non-flavonoid polyphenolic compound isolated from the root of Rhizoma Rehmanniae for the first time in 1940 [7]. Its tumor consequences, particularly on Oral Squamous Cell Carcinoma (OSCC), have still not been clarified.
2 Materials and methods
2.1 Cell line
Human oral squamous cell carcinoma cell lines CAL-27, SCC-25, and KB were donated by the David Y. Zhang Laboratory, Mount Sinai Medical College (New York, NY, USA). HSC3, a human tongue squamous cell carcinoma cell line, was obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank (Tokyo, Japan). Hypopharyngeal cancer cells (FaDu) were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in a 5% CO2 incubator.
2.2 Experimental reagent
2.2.1 Resveratrol
Resveratrol (Catalog #R5010, purity ≥ 99%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dissolve and prepare the original solution of 100 mmol/L with DMSO, sub-pack, keep away from light at − 20 ℃, and set aside. Before the experiment, the RPMI1640 culture medium was diluted into different solutions, and the final concentration of DMSO was less than 0.5%.
2.2.2 Opti-MEM
Opti-MEM (Catalog #31985062) is obtained from American GIBCO company, Powder agarose (Catalog #445593) is obtained from French Biotest company, Fast Digest Bam HI (Catalog #FD0054) and Fast Digest Hind III (Catalog #FD0504) are obtained from Shanghai Thermo Scientific Company. Lipofectamine2000 (Catalog #11668019) is obtained from American Invitrogen company, plasmid mass (Catalog #D6900-0) preparation kit, TRI goat pure, Super M-MLV reverse transcriptase, Ribonuclease (RNase) inhibitor, and 2 × Power Taq PCR Master Mix are obtained from Beijing Bio Teke biotechnology limited company, SYBR Green (Catalog #S9430) is obtained from German Sigma company. Western and IP cell lysate, BCA protein concentration determination kit (Catalog #23227), and other Western blotting reagents were obtained from Shanghai Biyuntian Biotechnology Co., Ltd.
2.3 Methods
2.3.1 Cell culture
The CAL-27 cell line is a human tongue epithelial cell line. The cells were maintained in a DMEM medium containing 10% fetal bovine serum and 100 U/mL penicillin/streptomycin. They were routinely passaged at 37 ℃ and 5% CO2. KB cell line was derived from human oral epithelial carcinoma, and the SCC-25 cell line was derived from tongue squamous cell carcinoma. The above two kinds of cells were maintained in DMEM: Ham'sF12 = 1:1 medium containing 10% fetal bovine serum and 100U/mL penicillin/streptomycin and were routinely passaged at 37 ℃ and 5%CO2. The cells were passaged every 2–3 days, and the cells in the logarithmic growth phase were adopted in the experiment.
2.3.2 MTT assay was adopted to detect the inhibitory effect of different concentrations of resveratrol on tumor cells
The assay was performed using an MTT Kit (Catalog #TOX1, Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. The inhibitory effect of GTE on the growth of three kinds of squamous cell carcinoma was determined by the MTT method. The cells in the logarithmic growth phase were inoculated in 96-well culture dishes with a density of 5 × 103/well and maintained at 5% CO2 for 24 h, and then were given different concentrations of GTE (0, 10, 25, 50, 100, 200, 400 μg/mL). Each group contained 3 multiple holes and were maintained for 72 h. After 72 h, 8 μL MTT with a 5 mg/mL concentration was added to each well. After 3 h of incubation, the supernatant was discarded, and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve the crystals. The absorbance (OD) was immediately determined by enzyme labeling at 570 nm. The above experiments were repeated at least 3 times. Cell growth inhibition rate (%) = (control group OD value-administration group OD value)/control group OD value × 100%.
2.3.3 Analysis of cell cycle by flow cytometry (FCM)
CAL-27 cells in the logarithmic growth phase were maintained in a petri dish with a density of 5 × 105/well. After 24 h, different concentrations of GTE (0, 25, 50, 100 μg/mL) were added and co-maintained for 72 h. Rinse with PBS and centrifuge at 1000×g for 5 min, fix with cold 70% ethanol for 30 min, and remove by centrifugation. Before staining, the fixed solution was removed by PBS centrifugation at 1000×g for 5 min, 200 μL ribonuclease (RNase) was added, and 800 μL propidium iodide (PI) staining solution was mixed in a water bath 30 min at 37 ℃. The results were measured by flow cytometry and assessed by software.
2.3.4 Detection of protein expression by protein chip technique
CAL-27 cells were maintained in a petri dish with a diameter of 10 cm to 1 × 106/L, and 100 μg GTE was added after 24 h culture and then incubated for 48 h. After 48 h, the cells were harvested and centrifuged at 1000 r/min for 10 min at the temperature of 4 ℃. The supernatant was discarded, and the cells were rinsed with PBS twice to collect the lower sediment. 300 μL of cell lysate containing protease inhibitor was added to each precipitated cell, and the 3–5 min of the cell was lysed. The lysate was treated with ultrasound for 15 s 2 times, then 14,000 r/min centrifugation 15 min at the temperature of 4 ℃. The BCA protein assay kit determined the protein concentration. The cell extract containing 300 μg protein was added to a slot across the full gel width, and the protein was separated by Sodium Dodecyl Sulfate (SDS) polyacrylamide gel electrophoresis. Transfer the protein to the nitrocellulose membrane, incubated in 5% milk or 3%BSA at room temperature for 1 h, and entrap the membrane with a device with 20 swimming lanes (Bio-Rad, Hercules, CA). Two or three kinds of primary antibodies were added to each swimming lane and spent the night at the temperature of 4 ℃. After washing the membrane, the second antibody was added, and 45 min was incubated at room temperature. Using Chemi DocXRS system imaging and scanning optical density, the optical density of different corresponding protein expression levels differs. The above experiments were repeated at least 3 times.
2.3.5 Detection of protein expression by Western blot
CAL-27 cell line was selected as the representative to verify the expression of Noncoding RNA Activated by DNA Damage (NORAD), Insulin-Like Growth Factor 2 mRNA-Binding Protein 2 (IGF2BP2), and Phosphoinositide-Dependent Protein Kinase-1 (PDK1) proteins. The cells were maintained in a petri dish with a diameter of 10 cm, and the concentration was 1 × 106 × 10 ~ 6 × 10 ~ 6 ppm L. After 24 h of culture, GTE (0, 10, 25, 50, 100 μg/mL) was added and incubated for 48 h. After 48 h, the cells were harvested, and 10 min was centrifuged by 1000 r/min at the temperature of 4 ℃. Remove the supernatant, rinse the cells with PBS twice, and collect the lower precipitates. The protein was extracted using the above method, and 20 μg protein was transferred to a nitrocellulose membrane by 10%SDS polyacrylamide gel electrophoresis. The first antibody of CDK4, CDK6, and p-PDK1 was added to the nitrocellulose membrane (diluted by 1:1000) and overnight at the temperature of 4 ℃. After rinsing the membrane, the second antibody was added and incubated at room temperature for 1 h. The protein emission signal was measured by autoradiography, with β-actin (diluted by 10,000) as the internal control. The above experiments were repeated at least 3 times.
2.3.6 Liposome transfection
RNA interference technique knocks down the expression level of NORAD in cells CAL-27 cells were inoculated into 6-well plates and divided into si-NORAD- 1 group, si-NORAD-2 group, and si-NORAD-3 group according to the transfection of the si-NORAD plasmid. When the fusion degree of cells reached 30–45%, 500 μL medium without bovine serum was mixed with 5 μL Lipofectamine 2000, and 500 μL medium without bovine serum was mixed with 5 μL si RNA at room temperature after 5 min. The above two samples were mixed together and placed for 20 min at room temperature, then inoculated in a 6-well plate, maintained in an incubator for 4–6 h, and then replaced with fresh 1640 complete medium. QRT-PCR measured transfection efficiency after 24–48 h, and Western blotting measured protein expression after 48–72 h. The above experiments were repeated at least 3 times.
2.3.7 Cell scratch healing test to detect the migration ability of cancer cells
(1) Inoculation cells: the cells in the logarithmic growth phase were inoculated in a 6-well plate, about 3 × 105 cells per well and evenly distributed, maintained in 1640 complete medium, and marked with 5 parallel lines on the back of the 6-well plate. (2) artificial scratches t 3 × 105 cells per well and evenly distributed, maintained in 1640 complete medium, and marked with 5 parallel lines on the back of the 6-well plate. (3) Artificial scratches: 24 h after transfection, 200 μL gun head was adopted to draw two straight lines perpendicular to the parallel line on the back of the cell, PBS was rinsed twice, and then 1640 medium containing 1% fetal bovine serum was added to 2 mL. The healing ability of the cell was adopted to detect the migration ability of the cell. (4) The migration distance of the cells to the scratch area was carefully observed and photographed under an inverted microscope at 0 h, 24 h after that. The scratch healing area was calculated using ImageJ software. The above experiments were repeated at least 3 times.
2.3.8 Trans well invasion experiment
(1) The Trans well chamber was adopted with an 8 μ m pore diameter and 6.5 mm diameter. The upper chamber of the trans well chamber was coated with Matrigel matrix glue, dried at the temperature of 4 ℃, and rinsed gently with 1640 serum-free medium. (2) The cells were digested and counted after being maintained for 24 h. The cells in each group were made into serum-free 1640 single-cell suspension containing Bovine Serum Albumin (BSA) and transferred to the upper chamber of the Trans well chamber according to 2 × 10 5 cells. The lower chamber was 1640 medium containing 10% Fetal Bovine Serum (FBS). (3) after routine culture for 12 h, take out the chamber, discard the liquid in the upper chamber, rinse with Phosphate-Buffered Saline (PBS), gently wipe off the invasive cells in the upper chamber with cotton swabs, fix the membrane with 4% paraformaldehyde, then dye 15 min with crystal violet, rinse with PBS, and then take pictures under a high-power microscope and randomly select 10 visual fields to count and take the mean. The above experiments were repeated at least 3 times.
2.3.9 CCK-8 experimental detection of cell proliferation
The cells in each group were counted and inoculated in a 96-well plate with a density of 5 × 103 cells per well and 100 μL medium. There were 5 multiple holes in each group. 10% CCK-8 reagent (Dojindo) was added at 24, 48, 72, and 96 h, incubated at 37 ℃ for 1.5 h, and the value of 450 nma was measured by enzyme labeling instrument. The cell proliferation rate was calculated.
2.4 Statistical analysis
Methylthiazolyldiphenyl-Tetrazolium Bromide (MTT), qRT-PCR, WB, invasion, and scratch healing were repeated 3 times. The data were assessed by SPSS21.0 statistical software, and the cell growth curve was drawn by GraphPadPrism8.0 software. The exploratory values are expressed as mean, standard error, and the normally distributed measured data were especially compared to the two groups using the t-test. The distinctions were statistically important (P < 0.05 or P < 0.01). software, and the cell growth curve was drawn by GraphPadPrism8.0 software. The exploratory values are expressed as mean, standard error, and the normally distributed measured data were especially compared to the two groups using the t-test. The distinctions were statistically important (P < 0.05 or P < 0.01).
3 Results
3.1 Comparison of inhibitory effects of resveratrol on OSCC cell lines with various concentrations
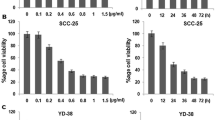
The inhibitory rates of resveratrol at 50, 100, 200, 400 μg/mL on CAL-27, 100, 200, 400 μg/mL resveratrol on KB and 25, 50, 100, 200, 400 μg/mL resveratrol SCC-25 cells were increased. Among the three cell lines tested, CAL-27 demonstrated the highest sensitivity to resveratrol, as evidenced by its lowest IC50 value (70 μg/mL). This sensitivity made it a suitable model for evaluating the mechanistic effects of resveratrol. The statistical difference was discovered in the amount of the drug fashion (P < 0.05). The 50% inhibitory concentration (IC50) of resveratrol on CAL-27, KB, and SCC-25 cells was 70, 145, and 125 μg/mL, respectively. All the results are indicated in Table 1.
3.2 Cellular cycle of CAL-27 cells with an increased concentration gradient of resveratrol
Secondly, we found 50 μg/mL resveratrol mediated G2/M arrest of CAL-27 cells (P < 0.05). Resveratrol (100 μg/mL) could mediate the S phase and G 2/M phase arrest of CAL-27 cells (P < 0.05). The amounts of cells in the G-0/G-1 phase are downregulated. All the results are indicated in Table 2.
3.3 The levels of various proteins in CAL 27 cells with treatment of resveratrol
We adopted protein chip technology to analyze the effect of GTE on the signal transduction-related proteins in CAL-27 cells. In total, 107 phosphorylated and non-phosphorylated proteins were measured, and 13 proteins changed significantly (P < 0.05). The protein levels of c PKC α and Notch 4 were upregulated, while the expressions of p-ERK, p-PDK1, p-Cdc2, p-RB, NORAD, IGF2BP2, CDK2, Cdc2P34, Cyclin E, 14-3-3beta and XIAP were down-regulated. All the results are indicated in Fig. 1.
3.4 Comparison of detecting protein levels
NORAD, IGF2BP2 and PDK1 levels in CAL-27 cells treated with 25, 50, 100 μg/mL resveratrol decreased. Meanwhile, the higher the concentration of resveratrol, the higher the %age of inhibition. and the variations had statistical distinctions (P < 0.05). All the results are indicated in Fig. 2 and Table 3.
3.5 NORAD effect on the proliferative cells of cancer
Compared with the CAL-27 group, the cell proliferative capability of the si-NORAD—1 and si-NORAD-2 groups was obviously weaker at 48 h, 72 h, and 96 h, but there was no difference between the si-NORAD—1 group and the si-NORAD-2 group. The results indicated that the proliferative capability of CAL-27 cells decreased largely after interfering with NORAD expression. All the results are indicated in Fig. 3.
3.6 NORAD has an influence on the capacity of cancerous cells to migrate
The results suggested that markable differences were observed in the percentage of scratch healing area between si-NORAD-1 and si-NORAD-2 groups compared with the Con group [(0.43% ± 0.06) vs (0.81 ± 0.07), (0.48 ± 0.04) vs (0.83 ± 0.08), P < 0.05]. No great differences existed between si-NORAD-1 and si-NORAD-2 groups. The migration ability of YES-2/CDDP-R cells decreased significantly after knocking down the expression of NORAD (P < 0.05). All the results are indicated in Fig. 4.
3.7 NORAD effect on the invasive ability of cancer cells
We assessed the effect of NORAD on the invasive ability of CAL-27 cells. After 24 h, the cells of the Con group, si-NORAD-1 group and si-NORAD-2 group could pass through the Matrigel filter membrane. The number of transmembrane cells in the Con group, si-NORAD-1 group and si-NORAD-2 group was 66.03 ± 14.01, 12.35 ± 5.03, and 18.01 ± 5.04 respectively. The amounts of trans-membrane units in si-NORAD-1 and si-NORAD-2 groups were largely greater than Con group (12.34 ± 5.01) vs (66.04 ± 14.02), (18.01 ± 5.02) vs (66.05 ± 14.02), P < 0.05. The invasive capability of CAL-27 cells decreased significantly after knocking down the expression of NORAD (P < 0.05). All the results are indicated in Fig. 5.
3.8 IGF2BP2 effect on the proliferation of cancer cells
In the IGF2BP2 silencing group of HSC3 and CAL-27 cell lines, the expression of IGF2BP2 was greatly decreased. The proliferative ability was measured by the CCK-8 test. The proliferative rate of the IGF2BP2 knockout group decreased significantly (P < 0.05), decreasing to 69. 13% in HSC3 and 74.01% in CAL-27 (P < 0.001). All the results are indicated in Figs. 6 and 7.
3.9 IGF2BP2 effect on the invasive ability of cancer cells
Compared to the negative control group, the invasive ability in IGF2BP2 knockout decreased significantly, which downregulated to 72.85% in HSC3 and 52.44% in CAL-27 (P < 0.001, Fig. 8).
3.10 PDK1 effects on the proliferation of tumor cells
In order to analyze the effect of PDK1 on the proliferation of tumor cells, a CCK8 proliferation assay was adopted to detect the proliferative activity of hypopharyngeal cancer cells. It was found that the proliferative activity of hypopharyngeal cancer cells decreased after interfering with the expression of PDK1, while the proliferative activity of hypopharyngeal cancer cells was significantly enhanced after overexpression of PDK1 (P < 0.05). All the results are indicated in Fig. 9.
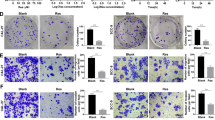
3.11 PDK1 effects on migration and invasion of tumor cells
By adding the target cells to the upper chamber and adding a medium containing serum to the lower chamber, we induced the cells to move to the lower chamber. Finally, crystal violet staining was adopted to count the cells at the bottom of the chamber to evaluate the ability of migration and invasion. The stably silenced/overexpressed PDK1 and control cells were seeded into the superior chamber. After 24 h and 36 h, the cells were fixed and stained with crystal violet, and the images were harvested under a microscope. As indicated in the figure, the number of migrating cells of oral squamous cell carcinoma in the Control group and Lv-PDK1-RNAi group was 234.62 ± 38.27and 141.35 ± 38.72 respectively, and the number of invasive cells was 119.03 ± 19.53, 72.68 ± 11.26 respectively. The number of migrating cells in the Control group and Lv-PDK1-RNAi group was 249.35 ± 46.51 and 553.82 ± 64.78 respectively, and the number of invasive cells was 131.42 ± 26.28 and 229.66 ± 44.58 respectively, indicating that PDK1 promoted the migration and invasion of tumour cells. All the results are indicated in Fig. 10.
4 Discussions
Resveratrol is non-flavonoid polyphenol, which is chemically called cyclopentadienol [8]. Recent studies have shown that resveratrol has antagonistic effects on a variety of tumor cells in multiple systems, such as digestion, hematopoiesis, respiration, reproduction, and so on [7, 9, 10], and has an inhibitory effect on the initiation, promotion, and development of tumor cells [11, 12]. However, there is no agreement on the specific mechanism of apoptosis induced by resveratrol in academic circles. NORAD, also called LINC00657, is a kind of lncRNA located in chromosome 20q11.23, which is 5.3 kb in length and widely exists [13]. NORAD contains 10 repetitive sequences with different structural motifs [14], which is very important for maintaining the stability of the genome. In an anoxic microenvironment, tumor cells enhance the proliferation of tumor stem cells by secreting exocrine bodies containing lncRNA, thus promoting tumor proliferation and metastasis.
Resveratrol exhibits significant therapeutic potential in oral squamous cell carcinoma (OSCC). Our findings reveal that resveratrol inhibits OSCC cell proliferation, induces S and G2/M phase arrest, and downregulates the expression of NORAD, IGF2BP2, and PDK1, key molecules involved in cancer progression. These observations are consistent with previous studies reporting the anti-proliferative and pro-apoptotic effects of resveratrol across various cancer types, including breast, lung, and colorectal cancers [5, 6].
In recent years, some researchers have put forward the theory that lncRNA is involved in a variety of pathological processes of tumors as a competitive endogenous RNA (ceRNA) [15]. According to this theory, lncRNA containing miRNA response elements plays a carcinogenic or inhibitory role by competitively binding with miRNA and modulating the performance of miRNA-specific sequences. Consistent with this theory, NORAD can be adopted as ceRNA to regulate the occurrence and development of tumors. In addition, NORAD can activate a variety of signal pathways of tumorigenesis or induce epithelial-mesenchymal transformation (EMT), thus giving full play to its metastatic characteristics in tumor cells [16]. The proliferative capability of CAL-27 cells decreased largely, later interfering with NORAD expression, and the migratory and invasive capability of YES-2/CDDP-R cells decreased significantly after knocking down NORAD expression [15,16,17].
Consistent with the previous studies, more evidence show that NORAD is in a state of disorder in different types of tumours and contributes to pathological cancerous cellular growth and metastasis. IGF2BP2 is essential for the body's growth and development [18]. Mice with IGF2BP2 knockout showed slower linear growth, higher energy metabolic rate, enhanced capacity for glucose resistance, insulin sensitivities, and tolerance to dietary-related fatness and the development of fatty liver, and even had a longer life span [19]. On the other hand, IGF2BP2 can be expressed as a tumor promoter. High expression of IGF2BP2 is discovered in coupled of cancerous tissues and is related to tumor progression and prognosis [20]. IGF2BP2, known for its tumor-promoting properties, was downregulated by resveratrol treatment. This aligns with the findings that IGF2BP2 knockdown inhibits OSCC cell proliferation and metastasis [21]. Our study extends this understanding by demonstrating that resveratrol not only reduces IGF2BP2 expression but also diminishes its downstream effects on key pathways, such as Mitogen-Activated Protein Kinase (MAPK) signaling.
PDK1 is a protein composed of 556 amino acids. PDK1 is regarded as the main regulator of the related kinase family and is indispensable in cell physiological activities. At present, studies have indicated that PDK1 is considered to be an ideal target for intervention in a variety of tumors. PDK1 is highly expressed in gastric cancer, which is a tumor marker of tumor progression and poor prognosis. In most cases [22], the high expression of PDK1 protein in multitype carcinomas is due to gene amplification. PDK1 transcription in melanoma was significantly larger than that in nevus and further increased in metastatic melanoma. PDK1 is up-regulated in a large number of patients with acute myeloid leukemia, and the therapeutic effect of this part of patients is poor. In cellular functional experiments, overexpression of PDK1 can promote the anchor-independent growth of breast cancer cells, as well as promote tumor growth in nude mice. PDK1 is deeply involved in tumorigenesis. After interfering with PDK1 transcription in breast cancer, the ability of tumor cell migration and metastasis is significantly decreased. Our results confirm that resveratrol suppresses PDK1 expression, leading to reduced cancer cell growth and invasion. These findings suggest that PDK1 may serve as a potential therapeutic target in OSCC management.
In contrast to most studies using CAL-27, it is worth noting that the less sensitive SCC-25 and KB lines showed varying responses to resveratrol. While this study focused on the most sensitive line (CAL-27) to elucidate mechanistic insights, future research should investigate the differential responses of less sensitive lines to optimize resveratrol-based therapies.
5 Conclusion
In conclusion, resveratrol can affect the expression of cell cycle-related protein molecules through many signal cascades, thus down-regulating the expansion of several types of human oral squamous cell carcinoma and malignant cells. Resveratrol has a direct anti-tumor effect, which lays a research foundation for the development of resveratrol as an anti-tumor prescription drug and its application in cancer cell management and treatment.
Data availability
All data supporting the findings of this study are available within the paper.
References
Huang S, Tu Z, Sha X, et al. Fabrication and performance evaluation of pectin–fish gelatin–resveratrol preservative films. Food Chem. 2021;361:191–4.
Xu M, Lian Z, Chen X, et al. Effects of resveratrol on lipid and protein co-oxidation in fish oil-enriched whey protein isolate emulsions. Food Chem. 2021;365:1001–4.
Xu M, Lian Z, Chen X, et al. Effects of resveratrol on lipid and protein co-oxidation in fish oil-enriched whey protein isolate emulsions. Food Chem. 2021;365:13–6.
Monahan DS, Flaherty E, Hameed A, et al. Resveratrol significantly improves cell survival in comparison to dexrazoxane and carvedilol in a h9c2 model of doxorubicin induced cardiotoxicity. Biomed Pharmacother. 2021;140:1332–3.
Xuemei Z, Qian N, Zhimei Z, et al. Resveratrol affects the expression of uric acid transporter by improving inflammation. Mol Med Rep. 2021;24(2):17–9.
Sharifi-Rad J, Quispe C, Alfred MA, et al. Current trends on resveratrol bioactivities to treat periodontitis. Food Biosci. 2021;42:19–20.
Yong W, Jiao J, Kou Z, et al. Resveratrol ameliorates malathion-induced estrus cycle disorder through attenuating the ovarian tissue oxidative stress, autophagy and apoptosis. Reprod Toxicol. 2021;104:15–7.
Zhang Bowei Xu, Yingchuan LH, Wenwen P, et al. Intestinal pharmacokinetics of resveratrol and regulatory effects of resveratrol metabolites on gut barrier and gut microbiota. Food Chem. 2021;357:12–8.
Fu DN, Wang Y, Yu LJ, et al. Silenced long non-coding RNA activated by DNA damage elevates microRNA-495–3p to suppress atherosclerotic plaque formation via reducing Krüppel-like factor 5. Exp Cell Res. 2021;401:203–5.
Luo L, Chen C, He H, et al. Silencing of long non-coding RNA (LncRNA) non-coding RNA activated by DNA damage (NORAD) inhibits proliferation, invasion, migration, and promotes apoptosis of glioma cells via downregulating the expression of AKR1B1. Med Sci Monit. 2020;26:111–4.
Ma Y, Chan F, Wang L, et al. The role of DNA damage-induced non-coding RNA in epithelial mesenchymal transformation in papillary thyroid carcinoma. Chin J Pract Diagn Treatment. 2019;33(05):426–30.
Guo Z, Pei T, Liang D, et al. Research progress on the relationship between insulin growth factor 2mRNA binding protein 3 and tumor. Prog Biotechnol. 2021;11(06):711–7.
Yang Tingkai, Wen Jin, Ji Zhigang. The value of insulin-like growth factor 2 in the diagnosis of bladder urothelial carcinoma [J/OL]. Journal of Concord Medicine:1- 10.
Cao J, Yan W, Ma X, et al. Insulin-like growth factor 2 mRNA-binding protein 2 - a potential link between type 2 diabetes mellitus and cancer. J Clin Endocrinol Metab. 2021;106(10):1023l–3.
Yang Q. Evidence-based medicine study on the prognostic value of long-chain non-coding RNANORAD in multiple tumors. Dali University. 2021.
Zhikun C, Qin C, Chunxue X. NORAD regulates epithelial-mesenchymal transition of non-small cell lung cancer cells via miR-422a. Mol Med Rep. 2021;23(2):764–5.
Wang C, Wu D, He M, Guan L, et al. LncRNA NORAD accelerates the progression of non-small cell lung cancer via targeting miRNA-455/CDK14 axis. Minerva Med. 2021;113:117–9.
Hu C, Chen J, Qin X, et al. Long non-coding RNA NORAD promotes the prostate cancer cell extracellular vesicle release via microRNA-541–3p-regulated PKM2 to induce bone metastasis of prostate cancer. J Exp Clin Cancer Res. 2021;40(1):108–13.
Dong L, Geng Z, Liu Z, et al. IGF2BP2 knockdown suppresses thyroid cancer progression by reducing the expression of long non-coding RNA HAGLR. Pathol Res Pract. 2021;225:117–20.
Cheng HC, Chang Su TKWC, et al. A narrative review of the influence of diabetes mellitus and hyperglycemia on colorectal cancer risk and oncological outcomes. Transl Oncol. 2021;14(7):44–8.
Hu Y, Zhang J. EGFR 2BP2 promotes the progression of oral squamous cell carcinoma by affecting the expression of IGF2BP2 [C]/compilation of papers of the 14th National Conference on Oral Pathology. 2020:51.
Wang L, Xu H, Liang J, et al. An integrated network, RNA sequencing, and experiment pharmacology approach reveals the active component, potential target, and mechanism of Gelsemium elegans in the treatment of colorectal cancer. Front Oncol. 2020;10: 616628.
Funding
Zhejiang Province Traditional Chinese Medicine Science and Technology Program Project. No.2023ZL363
Author information
Authors and Affiliations
Contributions
Authors Contribution Rongrong Zhang1* (study conception, Data Collection, draft manuscript, analysis and interpretation of results) Jinjing Guo1 (study conception, Data Collection) Yicong Lin1 (study conception, Data Collection).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical number: ZCMUHSIRB-2024071509.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Guo, J. & Lin, Y. Resveratrol inhibits the progression of oral squamouscell carcinoma through Norad/Igf2bp2/Pdk1 pathway and remodeling glucose metabolism reprogramming. Discov Onc 16, 603 (2025). https://doi.org/10.1007/s12672-025-02363-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-025-02363-z