Abstract
The formation of purinosomes, dynamic complexes involved in de novo purine biosynthesis, has been recognized as a critical process for cell growth. Although their upregulation in cancer cells suggests their potential as a therapeutic target, the specific role of purinosomes in hepatocellular carcinoma (HCC) remains uncertain. The purinosome score was found to have prognostic value. Enrichment analyses indicated a connection between purinosome-related genes and cell cycle regulation. Moreover, our research has demonstrated a correlation between the upregulation of genes associated with purinosomes and the enhanced formation of purinosomes in Huh-7 cells. Pyrimethamine has been identified as a promising therapeutic option for targeting purinosome to exert anti-cancer effects. Furthermore, the purinosome score exhibited an positive relationship with the response to immunotherapy. It may guide the stratification of liver cancer patients and screen for populations that may benefit from immunotherapy. This study examines the prognostic and predictive value of purinosome in liver cancer, suggesting that targeting purinosome formation with pyrimethamine or immunotherapy could benefit patients with high purinosome scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Hepatocellular carcinoma (HCC) remains the most common type of primary liver cancer and is a prominent global health issue [1, 2]. Despite advancements in liver cancer treatment in recent years [3], traditional cytotoxic drugs frequently demonstrate limited efficacy, underscoring the need for innovative and efficacious therapeutic strategies for patients with advanced-stage HCC [3]. The development of such treatments is imperative, regardless of genotype [4].
The regulation of purine metabolism is essential in the biological functions of cancer cells, influencing key processes such as cellular energetics, proliferation, invasiveness, metastasis, and immune evasion [5]. Aberrant purine metabolism has been documented in a range of cancers, including hepatocellular carcinoma (HCC) [5,6,7]. The targeting of purine metabolism has been identified as a potentially effective therapeutic approach for individuals with HCC [8, 9]. Mammalian cells predominantly rely on two pathways for purine metabolism: the purine de novo biosynthesis pathway (DNBP) and the salvage pathway. While the salvage pathway typically meets the majority of cellular purine needs by recycling degraded bases [10], rapidly dividing and cancerous cells exhibit heightened requirements for purines, leading to the upregulation of the purine de novo biosynthesis pathway.
Prior research on de novo purine biosynthesis has elucidated the intricate multienzyme purinosome complex known as the "purinosome," comprising six enzymes (PPAT, GART, FGAMS, PAICS, ADSL, and ATIC) that play a crucial role in the conversion of phosphoribosylpyrophosphate to inosine 5ʹ-monophosphate [11]. Purinosomes are crucial in mediating purine metabolic flux, as evidenced by their pivotal role in this process [5]. Furthermore, research has shown that hypoxia triggers the formation of the multienzyme purinosome complex, suggesting its importance for cell viability under adverse conditions or stress experienced by cancer cells [12]. Nevertheless, the mechanisms underlying purinosome assembly in cancer cells are not fully understood.
Recent studies have illuminated the significance of aberrant nucleotide metabolism in expediting tumor progression and modulating the immune response in the tumor microenvironment. This implies the promising prospect of targeting nucleotide metabolism to augment immunotherapy [13,14,15]. In a prior investigation, we illustrated the correlation between xanthine dehydrogenase, a pivotal modulator of purine metabolism, and tumor-infiltrating lymphocyte levels, immune response, and overall survival in patients with liver cancer [16]. Purinergic signaling interacts with and regulates immune-related signaling in hepatic disease [17]. Additionally, research has conclusively demonstrated that purine metabolism plays a pivotal role in modulating the expression of the immune checkpoint PD-L1, thereby facilitating tumor immune evasion [17, 18]. In light of the constraints of existing immune-based treatments, it is imperative to delve into this intricate metabolic interplay and its connection to immunotherapy [4, 19].
The objective of this study was to examine the expression of purinosome-related genes in HCC and evaluate their prognostic and therapeutic implications. Purinosome activity was assessed by determining the purinosome score through single-sample gene-set enrichment analysis (ssGSEA) and calculating the ssGSEA score for each tumor sample. Additionally, an investigation was conducted to establish a correlation between purinosome score and response to immunotherapy. These findings suggest potential for the development of combination strategies for the treatment of HCC.
2 Materials and methods
2.1 Data acquisition
The training dataset consisted of mRNA expression data from 374 patients diagnosed with HCC, as well as their associated clinical trait information. This dataset was obtained from the University of California Santa Cruz (UCSC) xena database (http://xena.ucsc.edu/) [20]. The validation datasets for gene expression and clinical trait data from GSE14520 and GSE10130 were acquired from the Gene Expression Omnibus (GEO) database [21]. The Liver Cancer-RIKEN JP cohort was obtained from the International Cancer Genome Consortium (ICGC) database (https://icgc.org/) [22]. Furthermore, scRNA-seq data from GSE125449 were retrieved from the National Center for Biotechnology Information-Gene Expression Omnibus (NCBI-GEO) database (https://www.ncbi.nlm.nih.gov/geo/). The validation set for the 10 × Genomics platform consisted of 19 liver cancer tissues [23].
2.2 Survival analysis
The survival curves of six purinosome-related genes were acquired from the GEPIA2 website and analyzed using the log-Rank test [24]. The prognostic significance of purinosome-related signaling was assessed through univariate Cox regression and presented using the R package "forestplot". Additionally, multiple Cox survival analyses were conducted to assess overall survival (OS), disease-specific survival (DSS), progression-free interval (PFI), and disease-free interval (DFI), while considering age, gender, and stage as covariates [25].
2.3 Estimation of purinosome score in patient samples
The ssGSEA algorithm was utilized to evaluate the purinosome score of individual samples across various types of cancer by analyzing gene expression levels. ssGSEA analysis is a rank-based approach that calculates the degree of overexpression for a specific geneset compared to other genes in the genome [26]. Previous bioinformatic studies have acknowledged the algorithm's effectiveness in estimating signaling activity based on transcriptome data [27]. In this research, the median ssGSEA score was utilized as the threshold to categorize tumor samples into low and high purinosome score groups within each cohort. Subsequently, Pathway enrichment analysis was conducted to compare the gene expression profiles between the high and low purinosome activity groups By using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) [28]. To avoid database selection bias, a GSEA analysis was performed using gene sets from the REACTOME and BIACARTA databases[29]. The top enriched pathways were visualized using the R package "clusterprofiler." [28]. Protein–protein interaction (PPI) analysis was conducted by STRING database[30].
2.4 Cell culture experiments and human liver cancer tissue Immunostaining
Huh-7 HCC cell lines were procured from the China Center for Type Culture Collection (CCTCC) and cultured in Dulbecco's Modified Eagle medium (Gibco, DMEM, Cat. #C11885500BT) supplemented with 100 ml/l of fetal bovine serum (FBS, GENOM, Inc. Cat. #GNMFBUP5), 100 IU/ml penicillin and 10 μg/ml streptomycin (Biosharp, Cat. #BL505A) at 37°C in a 5% CO2, 95% air environment. The cells were cultured in both purine-free medium and normal medium separately. Following this, Huh-7 cells were individually collected for RNA extraction and analysis of gene expression profiles. The relative expression levels were standardized against GAPDH as reference and evaluated using the 2−ΔΔCT method. All experiments were performed in triplicate and repeated three times. The primer sequences can be found in Table 1. Written informed consent was obtained from all patients for immunostaining of human liver cancer tissue. The HCC liver samples were acquired in compliance with the approved protocol of the ethics committee at Fudan University. For fluorescence immunostaining, slides were initially treated with 0.2% Triton X-100, followed by blocking with 5% normal serum control after antigen retrieval using a citrate buffer (10 mM Citric Acid, 0.05% Tween 20, pH 6.0). The slides were then incubated with specific antibodies (ADSL from Santa Cruz Biotechnology, Inc. #sc-365623; Ki67 from MXB Biotechnologies, #RMA-0731) and covered with a DAPI mounting medium (Beyotime Biotechnology, Inc. Cat. #C1002). Imaging was performed using a Nikon A1R confocal microscope, equipped with CrestOptics X-Light V3 Confocal Microscope System and NIS-Elements AR software.
2.5 Immunotherapy and chemotherapy response prediction
The Tumor Immune Dysfunction and Exclusion (TIDE) scores for patients with Liver Hepatocellular Carcinoma (LIHC) were acquired from the TIDE website (http://tide.dfci.harvard.edu) [31]. In order to evaluate the predictive capacity of the model for clinical responses to liver cancer treatment, the IC50 values of commonly used chemotherapeutic and targeted agents were computed. Pearson correlation analysis was employed to assess the relationships between the activity levels and IC50 values of each compound. The IC50 differences between high and low-activity groups were compared using the Wilcoxon signed-rank test and visualized with box plots in R [32]. The Genomics of Drug Sensitivity in Cancer program aims to identify molecular features that predict response to anti-cancer drugs, validating the anti-cancer role of pyrimethamine [33].
2.6 Statistical analysis
Statistical analyses were performed utilizing R Statistical Software version 4.0.4, with significance determined by a p-value less than 0.05.
3 Results
3.1 Enhanced expression of enzymes involved in de novo purine biosynthesis pathway is correlated with a negative prognosis in individuals diagnosed with liver cancer
The establishment of purinosomes has been recognized as a pivotal and dynamic assembly in the pathway of de novo purine synthesis. To explore the transcriptional activity of genes associated with de novo purine biosynthesis, we initially conducted a comparative analysis of mRNA expression levels of six essential enzymatic genes implicated in purinosome formation (ADSL, ATIC, PFAS, PAICS, PPAT, and GART) between hepatic cancer tissues and their respective healthy counterparts. Figure 1A illustrates the significantly elevated mRNA levels of the six purinosome-related genes in tumor tissues compared to normal tissues. Furthermore, immunohistochemistry data obtained from the Human Protein Atlas (HPA) website (Fig. 1B) indicated an upregulation of protein expression of these components in the majority of liver cancer tissues. To assess the prognostic significance of these genes, we conducted additional analysis using The Cancer Genome Atlas (TCGA) dataset. Univariate Cox regression analysis and log-rank test are often complementary in survival analysis and can be used together to gain a more comprehensive understanding of the impact of purinosome-related genes on prognosis.Significantly, univariate Cox regression analysis revealed a notable correlation between elevated mRNA expression of the six genes and unfavorable outcomes in the LIHC cohort (Fig. 1C). Additionally, in the log-rank test, five of the six purinosome-related genes were identified as significant risk factors for individuals with liver cancer (Supplementary Fig. 1). These findings strongly indicate that augmented de novo purine biosynthesis is crucial in facilitating the proliferation and advancement of human liver cancer.
The landscape and the prognostics value of six purinosome-related genes. A The mRNA expression levels of ADSL, ATIC, PFAS, PAICS, PPAT, and GART in liver tumor tissue compared to their corresponding normal tissue. B The protein levels of the six purinosome-related genes in liver cancer tissues were determined using immunostaining data obtained from The Human Protein Atlas (HPA). C The forest plot displays the Hazard Ratio for patients in the liver hepatocellular carcinoma (LIHC) cohort from The Cancer Genome Atlas (TCGA), indicating the association between the expression of the six genes and patient outcomes. The Hazard Ratio was determined using a unique cox regression model
3.2 Purinosome score as a prognostic factor in cancer patients
In order to examine the prognostic implications of purinosome-related genes in HCC, we employed the ssGSEA algorithm to compute a purinosome score derived from the mRNA expression levels of the six principal enzymatic genes implicated in purinosome assembly (Fig. 2A). The findings from survival analysis indicate a correlation between higher purinosome scores and poorer patient outcomes (Fig. 2B). The utility of the purinosome score in predicting clinical outcomes was evaluated using ROC curves, yielding AUC values of 0.751, 0.703, and 0.662 for 1-year, 3-year, and 5-year overall survival, as illustrated in Fig. 2C. The prognostic value of the purinosome score was further validated in three independent cohorts, namely GSE14520, GSE10130, and ICGC cohorts, as illustrated in Fig. 2D–F. Furthermore, a multivariate Cox regression analysis was conducted to control for potential confounding clinical variables including OS, DSS, PFI, and DFI, ultimately validating the purinosome score as a standalone prognostic indicator for patients with HCC (Fig. 2G–J). Moreover, the prognostic value of the purinosome score was explored across multiple cancer types. Notably, an increased purinosome score was associated with worse outcomes in uveal melanoma (UVM), kidney renal papillary cell carcinoma (KIRP), mesothelioma (MESO), pancreatic adenocarcinoma (PAAD), sarcomas (SARC), LIHC, skin cutaneous melanoma (SKCM), and lung adenocarcinoma (LUAD), while exhibiting exceptional prognostic value in rectum adenocarcinoma (READ) and thymoma (THYM) (Supplementary Fig. 2). In summary, the purinosome score exhibits significant prognostic potential in various cancer types, including HCC.
Quantification of the purinosome score and its prognostic value in hepatocellular carcinoma. A Risk distribution of the purinosome score calculated using the ssGSEA algorithm. B Survival analysis demonstrating the prognostic value of the purinosome score in patients with HCC. C ROC curve analysis evaluating the accuracy of the purinosome score in predicting 1-year, 3-year, and 5-year overall survival (OS) in HCC patients. D–F Survival curves illustrating the prognostic significance of the purinosome score in three independent cohorts: GSE14520, GSE10130, and ICGC cohorts. G–J Relationship between purinosome score and clinical outcomes, including OS (G), DSS (H), PFI (I), DFI (J) in a dataset comprising 33 tumors from The Cancer Genome Atlas (TCGA). The forest plot presents the results of multiple Cox regression analysis
3.3 The expression of purinosome-related genes is crucial for cancer cell proliferation.
To enhance our comprehension of the impact of abnormal purinosome-related genes on downstream pathways in cancer cells, we stratified the liver cancer patient cohort into two groups according to their purinosome score and examined the variance in gene expression. Following this, we performed a series of enrichment analyses to investigate the potential involvement of purinosome-associated genes in cellular processes. Notably, we observed a substantial enrichment of cell cycle-related entities across various databases, such as GO, KEGG, Reactome, and Biocarta (Fig. 3A–D). To confirm the involvement of purinosome-related genes in liver cancer, we analyzed single-cell RNA sequencing data from GSE125449. Among 9946 cells, we identified six cell types (Fig. 3E), with tumor cells showing significantly higher expression of GART, PAICS, ATIC, and ADSL compared to other cell types (Fig. 3F). This finding was supported by the AUCell method, which allows for the identification of cells with active gene sets (e.g., purinosome) in single-cell RNA-seq data(Fig. 3G), indicating a correlation between high purinosome score and tumor cells. Furthermore, our observations indicated that tumor cells with elevated purinosome scores demonstrated a greater prevalence in the G2 and S phases, which are pivotal stages in DNA synthesis and the onset of mitosis (Fig. 3H). To further explore the regulatory network, genes related to the cell cycle occupy a central position in the PPI network by STRING database (Fig. 3I). Moreover, the clustering of interacting genes is primarily annotated on the cell cycle (Fig. 3J).
The functional role of purinosome-related genes in the cell cycle. A, B Functional enrichment analysis of differentially expressed genes using Gene Ontology-Biological Process (GO-BP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways conducted with the "clusterprofiler" package. C, D Gene Set Variation Analysis (GSVA) based on Reactome and Biocarta databases. E t-SNE visualization displaying cell types represented by different colors. F Identification of purinosome-related genes in six major cell types investigated in this study. G Mapping of the purinosome score onto the t-SNE map of cell subclusters. H Distribution of G2M score and S score in low-purinosome and high-purinosome socre cell groups. I PPI analysis of differentially expressed genes by using STRING database.The genes related to the cell cycle are marked in red. J Clustering annotation of interacting genes
We confirmed the role of purinosome in the cell cycle by conducting experiments on Huh-7 cells in purine-free and normal medium. qPCR analysis showed increased gene expression of purinosome-related, cell cycle, and proliferation genes over time (Fig. 4A). The results of the correlation analysis indicated a significant relationship between purinosome-related genes, cell cycle progression, and cellular proliferation, as illustrated in Fig. 4B. Additionally, the protein expression of ADSL, a known marker of purinosome activity, was evaluated using immunofluorescent staining. Notably, enhanced fluorescence signals were observed in cells cultured in a purine-free medium at 2 and 3 days, suggesting an increase in purinosome formation (Fig. 4C, D). In order to substantiate the correlation between purinosome and cellular proliferation in vivo, confocal imaging was conducted to observe the co-localization of ADSL and Ki67 (a proliferation marker) as illustrated in Fig. 4E. These results collectively indicate that heightened purinosome levels functionally impact the cell cycle, thereby facilitating the accelerated growth of cancer cells.
Validation of the role of purinosome in the cell cycle through cell experiments. A mRNA profiles were obtained from Huh-7 cells treated with purine-free medium at different timepoints. The heatmap represents the mRNA expression levels of genes associated with purinosome, cell cycle, and proliferation. B Correlation analysis of these genes using the corplot package. C, D Immunofluorescent staining of Huh-7 cells showing the protein expression of ADSL (visualized in green fluorescence) at 2 and 3 days. E Confocal overview image of a liver cancer tissue section, showing DAPI labeling (blue) to highlight nuclei, and immunostaining with antibodies against ADSL (green) and Ki67 (red)
3.4 Enhancing treatment responses by targeting purinosome formation in HCC
The increased expression of genes related to the purinosome is linked to cancer prognosis and plays a critical role in the regulation of the cell cycle. Therefore, it is imperative to discover efficacious drugs capable of inhibiting the heightened levels of the purinosome score in cancer cells. Utilizing the pRRophetic approach, pyrimethamine was identified as the most effective medication for targeting the purinosome score, displaying a low IC50 value in cancer cells exhibiting a high purinosome score (Fig. 5A–D). To confirm the clinical potential of pyrimethamine, we analyzed data from the GDSC database, which identifies molecular features of cancers that predict response to drugs. Our findings showed a negative relationship between pyrimethamine effectiveness and purinosome-related gene expression in different cancer types, supporting our initial hypotheses (Supplementary Fig. 3). Immunotherapy shows promise for treating HCC. Our analysis found a negative link between the purinosome score and TIDE score in HCC tissue (Fig. 5E). To confirm this discovery, we analyzed the mRNA levels of immune checkpoint genes like CD274, CD276, LGALS9, TNFRSF14, CD40, and CD70, which showed a connection with purinosome-related genes (Fig. 5F). Interestingly, CD276 and TNFRSF14 expression in Huh-7 cells increased gradually over time in a purine-free medium (Fig. 5G, H). Owing to the relatively few cases in the HCC immunotherapy database, an urothelial cancer dataset (IMvigor210) where samples received anti-PD-L1 therapy was selected as the test set. The distribution of purinosome score decreases simultaneously with the different therapeutic effect evaluation indexes, including complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) (Fig. 5I). Meanwhile, the purinosome score of patients with CR and PR were much higher than those with PD and SD (Fig. 5J). The high CRRS patients possess a higher percentage of SD/PD, and CR/PR more happened in patients with low CRRS (Fig. 5K). The survival times of patients with low purinosome score were much higher than those with low purinosome score (Fig. 5L).These findings indicate that individuals with elevated purinosome scores may potentially derive therapeutic benefits from pyrimethamine treatment or may experience systemic effects through immunotherapy targeting purinosome formation.
Prediction of treatment response for chemotherapy and immunotherapy in hepatocellular carcinoma. A Volcano plot displaying the distribution of various drugs in terms of response prediction. B Chemical structure of pyrimethamine, the most sensitive medication for the purinosome score. C, D Correlation analysis and bar plot depicting the relationship between the IC50 of pyrimethamine and the purinosome score. E Correlation plot illustrating the negative correlation between the TIDE score and the purinosome score in HCC tissue. F Heatmap showcasing the mRNA expression levels of immune checkpoint genes and their correlation with purinosome-related genes. G, H Bar plot representing the mRNA expression levels of CD276 and TNFRSF14 in Huh-7 cells cultured in purine-free medium over an increased incubation time. I, J The distribution of purinosome score among response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) in IMvigor210. K Bar graph illustrated the treatment response CR/PR and SD)/PD to immunotherapy in high and low purinosome subtypes. L Kaplan–Meier curve of OS for patients with high and low purinosome subtypes for IMvigor210
4 Discussion
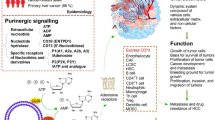
The purinosome, a complex of metabolic enzymes involved in de novo purine biosynthesis, has been associated with heightened purine demand and a hypoxic microenvironment [12]. These observations underscore the potential functional significance of the purinosome in cancer biology. In our study, we discovered elevated expression levels of de novo purine biosynthesis enzymes in liver cancer, which demonstrated substantial prognostic value. Furthermore, we identified a significant association between purinosome-associated genes and crucial aspects of cancer biology, including cell cycle regulation, cell survival dependency, and treatment response(Fig. 6). These findings strongly suggest that directing interventions towards purinosome formation represents a promising and potentially effective therapeutic approach for combating cancer.
Cancer cells exhibit an elevated demand for purines to sustain their rapid proliferation, predominantly met through the upregulation of the de novo purine biosynthesis pathway [34, 35]. Central to this pathway is the purinosome, a well-established model of BioPhase separation [36], which facilitates efficient purine metabolic flux [11]. Recent studies have elucidated the involvement of the Hsp70/Hsp90 chaperone machinery and GPCRs in regulating purinosome assembly [37,38,39], while the Akt-independent PDK1 signaling pathways and AMPK pathway are known to modulate its spatial dynamics [40]. Despite these advancements in the understanding of purinosome physiology, its implications in cancer patients have received limited attention. In our investigation of the LIHC cohort, we observed markedly increased expression of the six key enzymatic genes responsible for purinosome formation in liver cancer tissues, underscoring the pivotal role of the purinosome in HCC progression. Immunostaining further confirmed heightened levels of these six key enzymatic proteins in HCC. Notably, survival analysis demonstrated the purinosome score to be a promising prognostic biomarker in HCC.
Previous research has established a link between the purinosome and the cell cycle [41]. The purinosome is not only a key feature of liver cancer but also a well—recognized target for cancer therapy [42].Moreover, it has been found that individual components of the purinosome can influence tumor progression. The carcinogenic effect of ADSL on prostate cancer development and progression occurs via the cell cycle pathway [43]. Additionally, the bifunctional enzyme ATIC facilitates the propagation of hepatocellular carcinoma by regulating the AMPK-mTOR-S6K1 signaling pathway [44]. In our study, comprehensive analyses including GO, KEGG, and GSEA confirmed the significant involvement of purinosome in cell cycle processes.
HCC represents the majority of liver cancer cases, and chemotherapy, targeted therapy and immunotherapy remain the primary treatment modalities [45]. Thus, it is crucial to identify effective biomarkers that can predict treatment response and aid in therapy selection. Based on the aforementioned research, medications specifically targeting purinosomes hold considerable potential in inhibiting cancer progression.Notably, our study also suggests that patients with elevated purinosome scores may derive substantial clinical benefit from pyrimethamine-based therapy. Previous research has demonstrated the inhibitory effects of pyrimethamine on cell growth by inducing cell senescence and enhancing CD8 + T-cell-mediated cytotoxicity in colorectal cancer [46]. In contrast to traditional chemotherapy regimens, which often yield limited efficacy against HCC [45], pyrimethamine, a dihydrofolate reductase inhibitor involved in purine and thymidylate synthesis [47], holds considerable promise as a therapeutic target for HCC [48]. Our study lays the groundwork for future clinical trials exploring the potential of pyrimethamine in HCC patients. Recent advancements in immunotherapy have shown promise in certain immunogenic cancers, including liver cancer [49]. However, immunotherapy does not prove efficacious for all liver cancer patients. Our findings reveal a robust positive correlation between the purinosome score and the predicted response to immunotherapy in patients with liver cancer. The application of purinosome activity scoring may hold potential as a valuable tool for identifying patients who are likely to exhibit a favorable response to immunotherapy. Furthermore, an independent immunotherapy patient cohort (IMvigor210) was used to validate the predictive capacity of purinosome. Nevertheless, we also observed that liver cancer with high expression of purinosome may benefit from the aforementioned treatments, yet fail to gain benefits in terms of prognosis. This could be attributed to the fact that patients' prognosis is influenced by a multitude of factors. In addition to the efficacy of immunotherapy itself, these factors encompass the type and stage of the tumor, the patients' physical condition, and genetic characteristics, among others. Consequently, the purinosome score could serve as a tool for stratifying liver cancer patients, thereby enhancing individualized treatment strategies.
It is crucial to acknowledge the limitations inherent in our study. Firstly, our research would benefit from the incorporation of a larger volume of retrospective data to mitigate the potential biases that may arise from an insufficient sample size. Secondly, further validation through prospective studies is deemed necessary to strengthen our findings. Thirdly, there is a need to conduct immune cell co-culture experiments, along with a series of related experiments, in order to delve into the intricate relationship between purinosome activity and immune cell-mediated cytotoxicity. Despite these limitations, our study effectively validated the biological function of purinosome-related genes in the cell cycle and illustrated the prognostic significance of the purinosome score in HCC. Furthermore, our results offer strong evidence for the interconnectedness of purinosome-related genes, cell survival dependency, and the tumor immune microenvironment, highlighting the potential of targeting purinosome formation as a promising therapeutic approach for cancer management.
5 Conclusion
In summary, our study has revealed a significant association between elevated levels of purinosome and unfavorable prognosis in liver cancer. Furthermore, the purinosome score shows promise as a reliable biomarker for predicting the effectiveness of pyrimethamine as a treatment option. We also identified a correlation between the purinosome score and sensitivity to immune checkpoint inhibitor therapy in liver cancer patients. Importantly, While experimental validation and prospective clinical trials are necessary, our findings strongly suggest that the purinosome score is a promising indicator of prognostic value in hepatocellular carcinoma. It holds substantial potential for therapeutic stratification, thereby guiding personalized treatment in cancer patients and ultimately contributing to enhanced clinical outcomes.
Availability of data and materials
The data that support the findings of this study are openly available in TCGA and GEO data portal.
Abbreviations
- TCGA:
-
The Cancer Genome Atlas
- GEO:
-
Gene Expression Omnibus
- HPA:
-
Human Protein Atlas
- ICGC:
-
International Cancer Genome Consortium
- UCSC:
-
University of California Santa Cruz
- GSEA:
-
Gene Set Enrichment Assay
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Ontology
- ssGSEA:
-
Single sample gene set enrichment analysis
- TIDE:
-
Tumor immune dysfunction and exclusion
- ADSL:
-
Adenylosuccinate lyase
- ATIC:
-
5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase
- PFAS:
-
Phosphoribosylformylglycinamidine Synthase
- PAICS:
-
Phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoimidazole succinocarboxamide synthetase
- PPAT:
-
Phosphoribosyl Pyrophosphate Amidotransferase
- GART:
-
Phosphoribosylglycinamide Formyltransferase, Phosphoribosylglycinamide Synthetase, Phosphoribosylaminoimidazole Synthetase
- OS:
-
Overall survival
- DSS:
-
Disease-specific survival
- PFI:
-
Progression-free interval
- DFI:
-
Disease-free interval
- GDSC:
-
Genomics of drug sensitivity in cancer
References
Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1). https://doi.org/10.1038/s41572-021-00245-6.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. https://doi.org/10.1056/NEJMoa1915745.
Zhou W, Yao Y, Scott AJ, et al. Purine metabolism regulates dna repair and therapy resistance in glioblastoma. Nat Commun. 2020;11(1):3811. https://doi.org/10.1038/s41467-020-17512-x.
Yin J, Ren W, Huang X, Deng J, Li T, Yin Y. Potential mechanisms connecting purine metabolism and cancer therapy. Front Immunol. 2018;9:1697. https://doi.org/10.3389/fimmu.2018.01697.
Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5(3):546–57. https://doi.org/10.1002/cam4.601.
Chen GL, Ye T, Chen HL, et al. Xanthine dehydrogenase downregulation promotes tgfbeta signaling and cancer stem cell-related gene expression in hepatocellular carcinoma. Oncogenesis. 2017;6(9): e382. https://doi.org/10.1038/oncsis.2017.81.
Chong YC, Toh TB, Chan Z, et al. Targeted inhibition of purine metabolism is effective in suppressing hepatocellular carcinoma progression. Hepatol Commun. 2020;4(9):1362–81. https://doi.org/10.1002/hep4.1559.
Jiang T, Sanchez-Rivera FJ, Soto-Feliciano YM, et al. Targeting the de novo purine synthesis pathway through adenylosuccinate lyase depletion impairs liver cancer growth by perturbing mitochondrial function. Hepatology. 2021;74(1):233–47. https://doi.org/10.1002/hep.31685.
Pedley AM, Benkovic SJ. A new view into the regulation of purine metabolism: the purinosome. Trends Biochem Sci. 2017;42(2):141–54. https://doi.org/10.1016/j.tibs.2016.09.009.
An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320(5872):103–6. https://doi.org/10.1126/science.1152241.
Doigneaux C, Pedley AM, Mistry IN, Papayova M, Benkovic SJ, Tavassoli A. Hypoxia drives the assembly of the multienzyme purinosome complex. J Biol Chem. 2020;295(28):9551–66. https://doi.org/10.1074/jbc.RA119.012175.
Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–9. https://doi.org/10.1126/science.abc3421.
Vigano S, Alatzoglou D, Irving M, et al. Targeting adenosine in cancer immunotherapy to enhance t-cell function. Front Immunol. 2019;10:925. https://doi.org/10.3389/fimmu.2019.00925.
Wu HL, Gong Y, Ji P, Xie YF, Jiang YZ, Liu GY. Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy. J Hematol Oncol. 2022;15(1):45. https://doi.org/10.1186/s13045-022-01263-x.
Lin Z, Xie YZ, Zhao MC, Hou PP, Tang J, Chen GL. Xanthine dehydrogenase as a prognostic biomarker related to tumor immunology in hepatocellular carcinoma. Cancer Cell Int. 2021;21(1):475. https://doi.org/10.1186/s12935-021-02173-7.
Linden J, Koch-Nolte F, Dahl G. Purine release, metabolism, and signaling in the inflammatory response. Annu Rev Immunol. 2019;37:325–47. https://doi.org/10.1146/annurev-immunol-051116-052406.
Lv H, Lv G, Chen C, et al. Nad(+) metabolism maintains inducible pd-l1 expression to drive tumor immune evasion. Cell Metab. 2021;33(1):110–27. https://doi.org/10.1016/j.cmet.2020.10.021.
Satriano L, Lewinska M, Rodrigues PM, Banales JM, Andersen JB. Metabolic rearrangements in primary liver cancers: cause and consequences. Nat Rev Gastroenterol Hepatol. 2019;16(12):748–66. https://doi.org/10.1038/s41575-019-0217-8.
Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the xena platform. Nat Biotechnol. 2020;38(6):675–8. https://doi.org/10.1038/s41587-020-0546-8.
Barrett T, Wilhite SE, Ledoux P, et al. Ncbi geo: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41(Database issue):D991–5. https://doi.org/10.1093/nar/gks1193.
Zhang J, Baran J, Cros A, et al. International cancer genome consortium data portal—a one-stop shop for cancer genomics data. Database. 2011;2011:26. https://doi.org/10.1093/database/bar026.
Ma L, Hernandez MO, Zhao Y, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418–30. https://doi.org/10.1016/j.ccell.2019.08.007.
Tang Z, Kang B, Li C, Chen T, Zhang Z. Gepia2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–60. https://doi.org/10.1093/nar/gkz430.
Abd ES, D'Arrigo G, Leonardis D, Fusaro M, Tripepi G, Roumeliotis S. Methods to analyze time-to-event data: the cox regression analysis. Oxidative Med Cell Longev. 2021; 1302811. https://doi.org/10.1155/2021/1302811.
Barbie DA, Tamayo P, Boehm JS, et al. Systematic rna interference reveals that oncogenic kras-driven cancers require tbk1. Nature. 2009;462(7269):108–12. https://doi.org/10.1038/nature08460.
Jin Y, Wang Z, He D, Zhu Y, Chen X, Cao K. Identification of novel subtypes based on ssgsea in immune-related prognostic signature for tongue squamous cell carcinoma. Cancer Med. 2021;10(23):8693–707. https://doi.org/10.1002/cam4.4341.
Wu T, Hu E, Xu S, et al. Clusterprofiler 4.0: a universal enrichment tool for interpreting omics data. Innovation-Amsterdam. 2021;2:100141. https://doi.org/10.1016/j.xinn.2021.100141.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–50. https://doi.org/10.1073/pnas.0506580102.
Szklarczyk D, Kirsch R, Koutrouli M, et al. The string database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(D1):D638–46. https://doi.org/10.1093/nar/gkac1000.
Jiang P, Gu S, Pan D, et al. Signatures of t cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–8. https://doi.org/10.1038/s41591-018-0136-1.
Geeleher P, Cox N, Huang RS. Prrophetic: an r package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE. 2014;9(9): e107468. https://doi.org/10.1371/journal.pone.0107468.
Yang W, Soares J, Greninger P, et al. Genomics of drug sensitivity in cancer (gdsc): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(1):D955–61. https://doi.org/10.1093/nar/gks1111.
Y. Ariav, J.H. Ch'Ng, H.R. Christofk, N. Ron-Harel, A. Erez, Targeting nucleotide metabolism as the nexus of viral infections, cancer, and the immune response. Sci Adv. 2021;7 (21). https://doi.org/10.1126/sciadv.abg6165.
Robinson AD, Eich ML, Varambally S. Dysregulation of de novo nucleotide biosynthetic pathway enzymes in cancer and targeting opportunities. Cancer Lett. 2020;470:134–40. https://doi.org/10.1016/j.canlet.2019.11.013.
Pedley AM, Pareek V, Benkovic SJ. The purinosome: a case study for a mammalian metabolon. Annu Rev Biochem. 2022. https://doi.org/10.1146/annurev-biochem-032620-105728.
Pedley AM, Boylan JP, Chan CY, Kennedy EL, Kyoung M, Benkovic SJ. Purine biosynthetic enzymes assemble into liquid-like condensates dependent on the activity of chaperone protein hsp90. J Biol Chem. 2022;298: 101845. https://doi.org/10.1016/j.jbc.2022.101845.
Pedley AM, Karras GI, Zhang X, Lindquist S, Benkovic SJ. Role of hsp90 in the regulation of de novo purine biosynthesis. Biochemistry. 2018;57(23):3217–21. https://doi.org/10.1021/acs.biochem.8b00140.
Fang Y, French J, Zhao H, Benkovic S. G-protein-coupled receptor regulation of de novo purine biosynthesis: a novel druggable mechanism. Biotechnol Genet Eng Rev. 2013;29:31–48. https://doi.org/10.1080/02648725.2013.801237.
Schmitt DL, Sundaram A, Jeon M, Luu BT, An S. Spatial alterations of de novo purine biosynthetic enzymes by akt-independent pdk1 signaling pathways. PLoS ONE. 2018;13(4): e195989. https://doi.org/10.1371/journal.pone.0195989.
Chan CY, Zhao H, Pugh RJ, et al. Purinosome formation as a function of the cell cycle. Proc Natl Acad Sci USA. 2015;112(5):1368–73. https://doi.org/10.1073/pnas.1423009112.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Liao J, Song Q, Li J, et al. Carcinogenic effect of adenylosuccinate lyase (adsl) in prostate cancer development and progression through the cell cycle pathway. Cancer Cell Int. 2021;21(1):467. https://doi.org/10.1186/s12935-021-02174-6.
Li M, Jin C, Xu M, Zhou L, Li D, Yin Y. Bifunctional enzyme atic promotes propagation of hepatocellular carcinoma by regulating ampk-mtor-s6 k1 signaling. Cell Commun Signal. 2017;15(1):52. https://doi.org/10.1186/s12964-017-0208-8.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta-Rev Cancer. 2020;1873(1): 188314. https://doi.org/10.1016/j.bbcan.2019.188314.
Dong H, Hu L, Li W, et al. Pyrimethamine inhibits cell growth by inducing cell senescence and boosting cd8(+) t-cell mediated cytotoxicity in colorectal cancer. Mol Biol Rep. 2022;49(6):4281–92. https://doi.org/10.1007/s11033-022-07262-y.
Ramchandani S, Mohan CD, Mistry JR, et al. The multifaceted antineoplastic role of pyrimethamine against human malignancies. IUBMB Life. 2022;74(3):198–212. https://doi.org/10.1002/iub.2590.
Schweitzer BI, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990;4(8):2441–52. https://doi.org/10.1096/fasebj.4.8.2185970.
Kole C, Charalampakis N, Tsakatikas S, et al. Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers. 2020;12(10):2441. https://doi.org/10.3390/cancers12102859.
Acknowledgements
The authors would like to thank the TCGA databases for the availability of the data.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81802362).
Author information
Authors and Affiliations
Contributions
ZL and GLC created the idea for the paper. ZL, JWL, MCZ, PG, JW and GLC performed the collection and assembly of data, conducted the analysis, drafted the manuscript, and prepared the figures. JW and GLC revised the manuscript. All authors have seen and approved the submitted version of this manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All patients provided their written informed consent. HCC liver samples were obtained according to a protocol approved by the ethics committee of Fudan University, Shanghai Cancer Center (No. 050432-4-1212B). Consent for participation for all patients was obtained through The Cancer Genome Atlas Project. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12672_2025_2366_MOESM1_ESM.docx
Supplementary Material 1: Supplementary Fig. 1: Survival curve of the six purinosome-related genes analyzed using the log-Rank test. Survival analysis grouped by the median of gene expression.The log-Rank test indicates the association between the expression of the six genes and patient outcomes. Supplementary Fig. 2: Hazard ratio of the purinosome score across 33 different cancer types. The prognostic value of the purinosome score was explored by Cox regression analysis across multiple cancer types in TCGA. Supplementary Fig. 3: Correlation analysis of pyrimethamine effectiveness and purinosome-related gene expression across diverse cancer types. Correlation analysis between the IC50 of pyrimethamine and purinosome-related gene expression in different cancer types in GDSC database.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, Z., Long, JW., Zhao, Mc. et al. Purinosomes as a therapeutic target in hepatocellular carcinoma: insights and opportunities. Discov Onc 16, 564 (2025). https://doi.org/10.1007/s12672-025-02366-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-025-02366-w