Abstract
Introduction
Microvascular and macrovascular complications in type 1 diabetes (T1D) may be linked to endothelial stress due to glycaemic variability. Continuous glucose monitoring systems (CGMs) provide new opportunities to quantify this variability, utilising the amplitude of glucose change summated over time. The aim of this study was to examine whether this determination of glucose variability (GV) is associated with microvascular clinical sequelae.
Methods
Continuous glucose monitoring values were downloaded for 89 type 1 diabetes mellitus (T1D) individuals for up to 18 months from 2021 to 2023. Data for patient demographics was also taken from the patient record which included Sex, Date of Birth, and Date of Diagnosis. The recorded laboratory glycated haemoglobin (HbA1c) test results were also recorded. The glucose management index (GMI) was calculated from average glucose readings for 18 months using the formula GMI (%) = (0.82−(Average glucose/100)). This was then adjusted to give GMI (mmol/mol) = 10.929 * (GMI (%) − 2.15). Average Glucose Fluctuation (AGF) was calculated by adding up the total absolute change value between all recorded results over 18 months and dividing by the number of results minus one. The % Above Critical Threshold (ACT) was calculated by summing the total number of occurrences for each result value. A cumulative 95% limit was then applied to identify the glucose value that only 5% of results exceeded in the overall population. Using this value, we estimated the percentage of total tests that were above the Critical Threshold (ACT).
Results
Results for the 89 individuals (44 men and 45 women) were analysed over 18 months. The mean age of participants was 43 years and the mean duration of diabetes was 18 years. A total of 3.22 million readings were analysed, giving an average of 10.3 mmol/L blood glucose. Those with the largest change in glucose from reading to reading, summated over time, showed the greatest change in eGFR of 3.12 ml/min/1.73 m2 (p = 0.007). People with a higher proportion of glucose readings > 18 mmol/L showed a fall in eGFR of 2.8 ml/min/1.73 m2 (p = 0.009) and experienced higher rates of sight-threatening retinopathy (44% of these individuals) (p = 0.01) as did 39% of individuals in the highest tertile of glucose levels (p = 0.008).
Conclusion
Those individuals with T1D in the highest tertile of reading-to-reading glucose change showed the greatest change in eGFR. Those with a higher proportion of glucose readings > 18 mmol/L also showed a fall in eGFR and experienced higher rates of sight-threatening retinopathy, as did people with higher mean glucose. Discussions with T1D individuals could reflect on how the percentage recorded glucose above a critical level and degree of change in glucose are important in avoiding future tissue complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Why carry out this study? |
Microvascular and macrovascular complications in type 1 diabetes (T1D) may be linked to endothelial stress due to glycaemic variability |
Continuous glucose monitoring systems (CGMS) provide new opportunities to quantify this variability, utilising the amplitude of glucose change summated over time |
What was learnt from this study? |
Those individuals with T1D in the highest tertile of reading-to-reading glucose change showed the greatest change in estimated glomerular filtration rate (eGFR). |
Those individuals with a higher proportion of glucose readings > 18 mmol/L also showed a fall in eGFR and experienced higher rates of sight-threatening retinopathy, as did people with higher mean glucose |
Discussions with individuals with T1D could reflect on how percentage recorded glucose above a critical level and degree of change in glucose are important in avoiding future tissue complications |
Introduction
Following the introduction of insulin therapy for the treatment of diabetes, it became increasingly apparent that type 1 diabetes (T1D) is accompanied by long-term microvascular and macrovascular complications, leading to associated morbidity and shortened life expectancy.
The Diabetes Control and Complications Trial (DCCT) provided compelling evidence of the importance of glycaemic control in reducing the risk of diabetic complications [1, 2]. Ever since these results became available, monitoring of glycated haemoglobin (HbA1c) has been a cornerstone of diabetes management [3], as it reflects the time-averaged glucose exposure. However, the DCCT also demonstrated that the risk of diabetes complications cannot be fully explained by HbA1c alone (reflecting average blood glucose levels) [4]. In particular, HbA1c measurements provide no information on glycaemic variability [4, 5].
Acute hyperglycaemic fluctuations, as seen in those with the greatest glycaemic variability, may contribute to increased oxidative stress, inflammation, endothelial dysfunction and altered gene expression [6,7,8]. Consequently, glycaemic variability has been implicated in the development of microvascular disease, and its measurement has assumed greater importance.
The idea that glucose = glycaemic variability contributes additional risk over and above that captured by HbA1c can be termed the ‘glucose variability hypothesis’ [9]. Two types of short-term glucose variability are recognised: (1) ‘within-day’ glucose variability, which is variability greater than the expected oscillations in blood glucose in excess of normal physiological variation within a 24-h period and (2) ‘between-day’ glucose variability, which is defined as glucose levels close to target on some days but far from target on others [10, 11], or as glucose levels that exhibit greater ‘spread’ at specific times of the day. In reality, both types of short-term glucose variability are frequently seen in the same individual.
In 2014, the FreeStyle Libre Flash Glucose Monitoring System (Abbott Diabetes Care, Oxon, UK) became available as a potential alternative to self-monitoring of capillary glucose [12], facilitating the clinical application of continuous glucose monitoring (CGM) and flash glucose monitoring, thus allowing for detailed assessment of glycaemic variability.
In the study reported here, we examined the relation between novel indices derived from CGM and microvascular outcomes. Specifically, we investigated the degree to which glucose variability (GV)—denoted here as average glucose fluctuation (AGF)—was associated with microvascular clinical sequelae in the context of standard derived parameters.
Methods
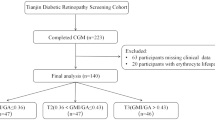
This was an observational, retrospective real-world study involving a consecutive sample of individuals with T1D who used the Freestyle Libre 2 continuous glucose monitoring system linked to the Freestyle Libre app on smartphones. All participants were being treated with a basal-bolus insulin regimen.
Glucose values were recorded at 15-min intervals for up to 18 months for any individual with T1D, with a total of 3.22 million glucose values analysed. Data were collected between 1 January 2021, and 31 July 2023, with the precise 18-month time window varying between individuals. HbA1c test results and demographic details, including sex, date of birth and date of diabetes diagnosis, were obtained from patient records.
To evaluate blood glucose control during the study period, we used three indices: the glucose management indicator (GMI) [13], change in blood glucose and time above high blood glucose. The results were summated over 18 months for each person.
Glucose management indicator
This metric provides an estimation of HbA1c, and is based on the correlation between mean glucose and HbA1c reported in large studies. It was calculated from the average interstitial glucose using the formula GMI (%) = (0.82 - [Average glucose/100]); the product was then adjusted to give GMI (mmol/mol) = 10.929 × (GMI [%] – 2.15) [10].
Change in blood glucose
Average glucose fluctuation between glucose readings was calculated by the summating the total absolute change between recorded results over time for the whole recording period, divided by the number of results minus one. This gave the mean change over the total period examined (up to 18 months).
Time above high blood glucose: above critical threshold
The total for each result value was consolidated for over up to 18 months, and a cumulative 95% limit was applied to establish the glucose value at which only 5% of results were greater overall. Applying this value to determine the percentage of the total number of tests above this value gave an estimated percentage above the critical threshold (ACT) for any given period.
Clinical outcomes included: (1) renal function as measured by the estimated glomerular filtration rate (eGFR), calculated from creatinine, measured at the end of the period taking age and sex into consideration; (2) average annual change in eGFR since a previous reading at least 4 years previously; and (3) current retinopathy status assessed by digital retinal screening or in ophthalmology clinic and determined as requiring treatment with either local injections or laser.
The patient group was divided into three tertiles according to GMI, AGF and ACT, with the clinical outcomes observed in each tertile then compared to each other.
This study was a service evaluation exercise in a single clinic.
Statistics
The records of each individual patient’s blood sugar values were downloaded at 15-min intervals from the FreeStyle Libre Flash Glucose Monitoring System ((Abbott Diabetes Care) as CSV files, then anonymised ID labelled and consolidated. The overall dataset was imported into Excel equipped with the PowerPivot feature (Microsoft Inc., Seattle, WA, USA) and analysed, which included dividing each patient’s total period into 100-day (as HbA1c) sequential segments, thereby establishing a fluctuation value as the absolute change from the previous value and determining whether the record was above the critical threshold (18 mmol/L).
Other patient data, including sex, date of birth, date of diagnosis, eGFR values and retinopathy score (none detected, detected but no treatment needed, detected and treatment required) were extracted from clinical records and labelled with anonymised ID to link to their blood glucose monitoring (BGM) values within PowerPivot.
Excel was then used to calculate how the patient cohorts with different levels of observed average blood glucose, AGF and percentage ACT (%ACT) reflected on their eGFR, both as measured last value and change since previous reading, and retinopathy score.
Ethics
All participants were attending the clinic of one of the authors (AHH). The FreeStyle Libre glucose monitoring data are available in its entirety as part of their usual care for analysis by the clinical team. All patients were given the option to opt out of their data being analysed. Such data analysis in relation to clinical care is acceptable under UK research ethics guidance, and the patients whose data were analysed were kept fully informed in accordance with the UK guidance at https://digital.nhs.uk/services/national-data-opt-out/understanding-the-national-data-opt-out/protecting-patient-data. All data was fully anonymised prior to analysis.
Results
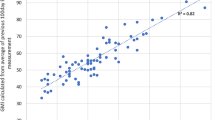
Results for 89 individuals (44 men and 45 women) collected over an 18-month data collection period were analysed. The mean age of the study population was 42.6 (standard deviation [SD] 12.7 years), and the mean duration of diabetes was 18.4 (SD 11.8) years. We collected a total of 3.22 million glucose values and determined that the mean blood glucose value was 10.3 mmol/L, reflecting a mean GMI of 56.9 mmol/mol. Of all the glucose results, 5% were higher than 18 mmol/L which was deemed to be the critical threshold. A summary of the descriptive data is provided in Table 1. There was a strong correlation between GMI and laboratory-measured HbA1c (r2 = 0.82).
Our comparison of patients across tertiles of the selected measures with respect to clinical outcomes is provided in Table 2, which shows that patients with higher average glucose readings had a greater likelihood of developing retinopathy requiring treatment.
Average glucose levels (summated over time) did not demonstrate a clear trend of association with either measured eGFR or the highest change in eGFR. However, those with the largest change in glucose from reading to reading summated over time showed the greatest change in eGFR, with a mean change of 3.12 ml/min/1.73 m2 (p = 0.007). Individuals with a higher proportion of glucose readings > 18 mmol/L showed a mean fall in eGFR of 2.8 ml/min/1.73 m2 (p = 0.009) and experienced higher rates of sight-threatening retinopathy (44% of these individuals) (p = 0.01), as did 39% of patients in the highest tertile of glucose levels (p = 0.009).
When we conducted a sensitivity analysis excluding overnight readings from midnight to 0700 hours, there was no material change in the associations seen.
There was no association between any of the indices with change in the urine albumin/ creatinine ratio (UACR). The measurement of UACR was not universal in the 89 individuals, with serial UACR measurements missing for three of the 89 individuals.
Discussion
We have described that both the likelihood of requiring treatment for diabetes-related sight-threatening retinopathy and the annual change in eGFR (decrement) are related to the percentage of readings above the critical threshold of 18 mmol/L (top 5% of all glucose readings). Furthermore, those individuals with the highest reading-to-reading glucose change showed the greatest mean change in eGFR, with a decrease of 3.12 ml/min/1.73 m2.
The aim of the study was to highlight that additional measures derived from the CGM data may be useful in predicting the likelihood of an individual with T1D developing diabetes-related retinopathy and/or diabetes-related renal problems. While we accept that based on the results of our small study population (89 individuals with T1D) we cannot conclude that the factors described are independently associated with diabetes-related complications, the results our this study do indicate that a larger study is needed to investigate the relations reported here. Furthermore, the study gives a precise focus for discussion in relation to the interpretation of the FreeStyle Libre glucose monitoring data (or other CGM data) that can be helpful in appreciating which features are the most important and should be focused on in relation to the communication of risk implications for both retinal and renal health.
In light of these findings, we propose that discussions with people with T1D should reflect on both the percentage of glucose records above a critical level and the degree of fluctuation in glucose level through the day, as these are as important an aspect of glucose control as average blood glucose, as measured by GMI for any period. These data can contribute to achieving effective coordination in the timing of nutrition, exercise and medication to optimise the stability of glucose control.
It is important to consider underlying pathogenetic mechanisms in relation to our findings. In the presence of high tissue glucose levels, there is overproduction of superoxide by the mitochondrial electron-transfer chain [14], which in turn results in the production of a cascade of deleterious effects due to enhanced polyol activity, increased formation of advanced glycation end products, activation of protein kinase C (PKC) and nuclear factor-kappa B (NF-κB) and increased hexosamine pathway flux. Through these pathways, increased intracellular reactive oxygen species (ROS) cause defective angiogenesis in response to ischaemia, activate several pro-inflammatory pathways and cause long-lasting epigenetic changes that drive persistent expression of pro-inflammatory genes even after glycaemia is normalised; this is termed ‘hyperglycaemic memory’ [14]. In this context the results of a study performed by Quagliaro et al. are relevant: these authors reported that human umbilical vein endothelial cells exposed to intermittent high glucose exhibited apoptosis, in contrast to cells exposed to a stable high glucose environment [15].
Regarding susceptibility to diabetes-related retinopathy, Skol et al. in the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) trials implicated the FLCN gene as a putative diabetic retinopathy susceptibility gene in the presence of high glucose levels/glucose peaks [16]. It is likely that more extensive molecular profiling of the cellular response to glucose in conjunction with work on other cell lines may yield further insights into the underlying genetic basis of diabetic retinopathy [17].
Although most of the relevant literature relates to glycaemic spikes as being the pathogenetic feature of glucose variability, there is also evidence linking fasting glycaemic variability and hypoglycaemia to adverse cardiovascular outcomes, although whether hypoglycaemia is causal remains unclear [18, 19]. In the DCCT, optimal glycaemic control was shown to delay the progression of chronic kidney disease and reduce the death rate in diabetes: 1441 individuals with T1D were randomised to receive intensive or conventional insulin treatment. The risk of microalbuminuria was reduced by 34% in the intensive treatment group after at least 4 years of follow-up [20]. Although these findings date back more than 30 years, they remain highly relevant.
Variability in fasting plasma glucose has also been correlated with an increased risk for moderate to severe diabetic nephropathy in several prospective studies, synergising with our findings in the present study [21, 22]. In a Swedish prospective cohort study over 11 years that involved 100 people with T1D, GV at baseline (SD of blood glucose levels over a 4-week period) was associated with incident peripheral neuropathy [23]. However, no associations were detected between GV and either nephropathy or retinopathy, in contrast the findings of the present study.
GV can be as much of an issue for people with type 2 diabetes (T2D)who require insulin treatment as it is for those with T1D. There is observational evidence that postprandial and/or post-load blood glucose is associated with all-cause mortality [24], and more limited evidence that it is associated with cardiovascular events [11].
Structured education and low carbohydrate diets can reduce glycaemic variability [25]. Regular educational updates for people with diabetes either in person or ‘online’ could provide the opportunity for glycaemic variability and glucose spikes to be better addressed [26].
Limitations
This study utilised data from intermittent scanning CGM (flash glucose monitoring), which required participants to swipe a reader over the sensor. This need to swipe may have introduced bias as there may be a greater propensity to swipe at extreme levels of glucose. However, the sensor records the glucose reading every minute and stores a glucose reading every 15 min for the previous 8 h. These data are then captured after each swipe. In August 2023, the Libre 2 software was replaced with a fully automatic CGM facility.
The CGM analysis was undertaken over an 18-month period, which is considerably shorter than the amount of time needed for microvascular complications to develop. Nonetheless, the patterns in glycaemia may well reflect longer-term trends. Regarding eGFR, we accept that the changes were relatively small. Also, we did not have data on insulin dosing, carbohydrate intake or structured education. The latter has been shown to improve biochemical indices, although its effect on microvascular outcomes is unclear. We did not have detailed data on the rate of retinopathy change.
Finally, the number of patients included in the study was relatively small. Nevertheless, a large number of glucose results were analysed for each individual.
Conclusion
A key aspect of the study reported here is that we have summated glucose data over a long period of up to 18 months, rather than over relatively shorter periods found in most studies. Thus, a measure of GV based on the amplitude of glucose change summated over time (AGF) and the percentage above a critical threshold (%ACT), for specific times of the day, could be used to provide valuable clinical insights into complication risk, with the aim to focus measures to achieve optimisation of insulin regime in people with T1D. The increasing use of CGM provides an ongoing opportunity to test this assertion in a more definitive way [27].
In this regard, those individuals with the largest change in glucose from reading to reading summated over time showed the greatest change in eGFR, namely 3.12 ml/min/1.73 m2 (p = 0.007). Individuals with a higher proportion of glucose readings > 18 mmol/L showed a fall in eGFR of 2.8 ml/min/1.73 m2 (p = 0.009) and experienced higher rates of sight-threatening retinopathy (44% of these individuals) (p = 0.01) as did 39% of individuals in the highest tertile of glucose levels (p = 0.008).
We suggest that discussions with patients should reflect both how the percentage of glucose readings recorded above a critical level can be reduced and the degree of change in glucose measured over time, as these are key components in the strategy to reduce the likelihood of developing tissue complications in people with T1D.
Data Availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. Survival, causes of death, and complications. Diabetologia. 1978;14(6):363–70.
Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40:994–9.
Heinemann L, Freckmann G, Müller-Wieland D, Kellerer M. Critical reappraisal of the time-in-range: alternative or useful addition to glycated hemoglobin? J Diabetes Sci Technol. 2020;14(5):922–7.
The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–83.
Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32(10):1901–3.
FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016;39:973–81.
FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016;39:973–81.
Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–82.
Cavalot F. Do data in the literature indicate that glycaemic variability is a clinical problem? Glycaemic variability and vascular complications of diabetes. Diabetes Obes Metab. 2013;15(Suppl 2):3–8.
Leelarathna L, Evans ML, Neupane S, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. 2022;387(16):1477–87.
Bergenstal RM, Beck RW, Close KL, et al. Glucose Management Indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–80.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–804.
Skol AD, Jung SC, Sokovic AM, et al. Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes. Elife. 2020;9:e59980.
Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–6.
Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61:48–57.
Pieber TR, Marso SP, McGuire DK, on behalf of the DEVOTE StudGroup, et al. DEVOy TE3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–65.
Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Zhou JJ, Coleman R, Holman RR, Reaven P. Long-term glucose variability and risk of nephropathy complication in UKPDS, ACCORD and VADT trials. Diabetologia. 2020;63(11):2482–5.
Scott E, Januszewski A, O’Connell R, et al. Long-term glycaemic variability and vascular complications in Type 2 diabetes: post-hoc analysis of the FIELD study. J Clin Endocrinol Metab. 2020;105(10):e3638–49.
Bragd J, Adamson U, Bäcklund LB, Lins PE, Moberg E, Oskarsson P. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008;34:612–6.
The DECODE study group on behalf of the Europe an Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:P617–21.
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomized controlled trial. BMJ. 2002;325(7367):746. https://doi.org/10.1136/bmj.325.7367.746.
https://www.england.nhs.uk/london/wp-content/uploads/sites/8/2019/07/dia-FreeStyle-Libre-training-pack-for-HCP-and-patients-052018.pdf. Accessed 30 April 2024
Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with Type 1 diabetes? Diabet Med. 2020;37:513–21.
Acknowledgements
Vernova Healthcare is the provider of the diabetes service that all the participants attend.
Funding
No external funding was used for this study. The rapid service fee has been covered by the University of Manchester.
Author information
Authors and Affiliations
Contributions
Adrian Heald and Mike Stedman conceived the study. Mike Stedman led the data analysis. Julian Warner Levy and Lleyton Belston supported data management and contributed to data analysis. Angela Paisley provided expert input in relation to T1D management as did Reena Patel and Alison White from a nursing point or view. Martin Gibson and Ed Jude provided invaluable insight in relation to the context of the study while Hellena Habte-Asres, Martin White and Angus Forbes led on the interpretation of the glucose monitoring data and the implications of the findings for people with T1D.
Corresponding author
Ethics declarations
Conflict of interest
Adrian H Heald, Mike Stedman, John Warner Levy, Lleyton Belston, Angela Paisley, Reena Patel, Alison White, Edward Jude, J Martin Gibson, Hellena Habte-Asres, Martin Whyte and Angus Forbes have nothing to declare.
Ethical Approval
All participants were attending the clinic of one of the authors (Adrian H Heald). The FreeStyle Libre data are available in its entirety as part of their usual care for analysis by the clinical team. All patients were given the option to opt out of their data being analysed. Such data analysis in relation to clinical care is acceptable under UK research ethics guidance. Patients whose data were analysed were kept fully informed, in accordance with the UK guidance at https://digital.nhs.uk/services/national-data-opt-out/understanding-the-national-data-opt-out/protecting-patient-data. All data were full anonymised prior to analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Heald, A.H., Stedman, M., Levy, J.W. et al. The Relation of Diabetes Complications to a New Interpretation of Glycaemic Variability from Continuous Glucose Monitoring in People with Type 1 Diabetes. Diabetes Ther 15, 2489–2498 (2024). https://doi.org/10.1007/s13300-024-01648-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01648-w