Abstract
Introduction
Given the recent approval and recommendation of V116, a 21-valent pneumococcal conjugate vaccine (PCV), in the United States (US), we evaluated the cost-effectiveness of using V116 versus the 20-valent PCV (PCV20) or the 15-valent PCV (PCV15) in series with the 23-valent pneumococcal polysaccharide vaccine (PPSV23) among adults aged ≥ 65 years in the US who had never received a PCV previously.
Methods
A static multi-cohort state-transition Markov model was developed to estimate the lifetime incremental clinical and economic impact of V116 vs. PCV20 or PCV15 + PPSV23 from the societal perspective. All model inputs were based on published literature and publicly available databases and/or reports. Model outcomes included undiscounted clinical cases: invasive pneumococcal disease (IPD), inpatient and outpatient non-bacteremic pneumococcal pneumonia (NBPP), post-meningitis sequelae (PMS), deaths from IPD and inpatient NBPP, discounted quality-adjusted life years (QALYs) as well as the discounted total cost (in 2023 USD), which consisted of vaccine acquisition and administration costs, direct and indirect costs associated with the disease, and travel costs for vaccination. The final summary measure was the incremental cost-effectiveness ratio (ICER), reported as $/QALY gained. Three percent was used for the annual discounting rate.
Results
Based on the inputs and assumptions used, the results indicated that the V116 strategy prevented 27,766 and 32,387 disease cases/deaths and saved $239 million and $1.8 billion in total costs when compared to the PCV20 and PCV15 + PPSV23 strategies, respectively, in vaccine-naïve adults aged ≥ 65 years. The estimated ICERs were cost saving in both regimens (i.e., V116 vs. PCV20 or vs. PCV15 + PPSV23). The scenario analysis and deterministic and probabilistic sensitivity analyses also demonstrated the robustness of the qualitative results.
Conclusions
These results demonstrated that using V116 in adults aged ≥ 65 years in the US can prevent a substantial number of PD cases and deaths while remaining highly favorable economically over a wide range of inputs and scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Why carry out this study? |
In June 2024, the 21-valent pneumococcal conjugate vaccine (PCV) was approved by the US Food and Drug Administration (FDA), and it was recommended by the Centers for Disease Control and Prevention (CDC) for use among US adults about 2 weeks later. |
This study evaluated the cost-effectiveness of PCV21 vs. current recommendations, PCV20/PCV15 + PPSV23, among adults aged ≥65 who were PCV naïve. |
What was learned from the study? |
The use of PCV21 by PCV-naïve adults aged ≥65 would markedly reduce the burden of adult pneumococcal disease, making substantial contributions to public health while saving costs in most situations. |
Introduction
Pneumococcal Disease
Streptococcus pneumoniae (pneumococcus) is a gram-positive facultative anaerobic bacterium [1]. S. pneumoniae is classified into serotypes based on its capsular polysaccharide, an identified virulence factor of pneumococcus [2]. According to published reports, there were estimated to be ≥ 100 pneumococcal serotypes as of 2020 [3,4,5]. S. pneumoniae is transmitted primarily by human contact via respiratory droplets from an individual with pneumococcal colonization in the upper respiratory tract [6]. It is associated with substantial morbidity and mortality worldwide, with the highest burden seen in children < 5 years and elderly adults, and continues to be the main cause of vaccine-preventable pneumonia (or lower respiratory tract infections) globally [7].
Pneumococcal disease (PD) manifestations range in severity from mucosal noninvasive infections, such as otitis media, and other respiratory tract infections to severe and life-threatening infections [8, 9]. Invasive (or bacteremic) pneumococcal disease (IPD) is the most serious manifestation, requiring hospitalization in some cases [8, 9]. IPD includes bacteremic pneumococcal pneumonia, meningitis, bacteremia, septicemia, and other infections of sterile sites [8, 9]. Moreover, patients with pneumococcal meningitis can have the complications of post-meningitis sequelae (PMS), including neurological impairment or hearing loss [10]. In adults, non-invasive PD also significantly contributes to morbidity, especially non-bacteremic pneumococcal pneumonia (NBPP), which accounts for approximately 75% of the cases of pneumococcal pneumonia [11]. The presence of comorbid conditions is associated with a higher risk of PD [12, 13].
Since the introduction of the first pneumococcal conjugate vaccines (PCVs) in children in 2000, there has been a substantial reduction in the incidence of PD among children (direct impact) as well as in adults (indirect impact) in the United States (US) [14]—the incidence of IPD in adults aged ≥ 65 years decreased to 24 per 100,000 population during 2018–2019 in comparison to 58 per 100,000 in 2000 [15]. Despite the positive impact that PCVs have had on the burden of PDs in several countries with established national immunization programs (NIP) [7], there remains a substantial PD burden among adults that is primarily attributable to non-vaccine serotypes (NVTs). It was estimated that, every year prior to the COVID-19 pandemic, there were over 100,000 non-invasive pneumococcal pneumonia hospitalizations, more than 300,000 IPD cases, and about 3,000 IPD deaths among US adults [16, 17]. The risks of disease and severe outcomes are higher among older adults and adults with certain risk conditions. For example, more than 80% of IPD cases occurred among adults with risk-based indications [17]. Over one-third of adults aged 65+ years who were hospitalized with community-aquired pneumonia in Louisville, KY, died within 1 year [17, 18].
Adult Pneumococcal Conjugate Vaccine V116
V116 is a new 21-valent PCV developed by Merck & Co., Inc. (Rahway, NJ, USA) that is specifically designed for adults and contains eight unique serotypes that are not found in any currently licensed vaccine—15A, 15C (from deOAc-15B), 16F, 23A, 23B, 24F, 31, and 35B—in addition to 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15B (only in the IPD indication), 17F, 19A, 20A, 22F, and 33F [19, 20]. According to the most recent Morbidity and Mortality Weekly Report (MMWR) published by the US Centers for Disease Control and Prevention (CDC), PCV21 caused approximately 80% of the IPD cases among all adults (aged 19+) with indications for vaccination, and 20–30% of the IPD cases were due to the eight new serotypes in PCV21 [21]. Furthermore, based on the same source (the CDC’s Active Bacterial Core surveillance (ABCs) data from 2018–2022 [22, 23]), in individuals aged 65+ years in the US, the serotypes covered by V116 are responsible for approximately 85% of IPD cases (31% higher than the 54% coverage by PCV20) and 81% of IPD cases for those aged 19–64 years (23% higher than the 58% PCV20 coverage). From 2018 to 2022, the eight unique serotypes in V116 accounted for a substantial proportion (~ 30%) of the residual IPD burden in US adults aged ≥ 65 years [23].
With its broad serotype coverage, V116 has the potential to substantially reduce the burden of PDs among adults in the US [24]. The Food and Drug Administration (FDA) granted a priority review in December 2023 and approval of V116 in June 2024 [25].
ACIP Recommendations in Adults ≥ 65 Years
In 2021, after giving their approval, the CDC’s Advisory Committee on Immunization Practices (ACIP) recommended using either a 20-valent PCV (PCV20; Prevnar 20™, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) alone or a 15-valent PCV (PCV15; Vaxneuvance™, Merck & Co., Inc., Rahway, NJ, USA) in sequence with a 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23™, Merck & Co., Inc., Rahway, NJ, USA) for all adults aged ≥ 65 years who had not received a pneumococcal vaccine previously [26].
In February 2024, the ACIP Pneumococcal Vaccines Work Group (PVWG) presented three policy questions for consideration by ACIP for the use of V116 in the US adult population. The first policy question was as follows [17, 27]:
Should PCV21 be recommended for US adults aged ≥ 19 years who currently have a recommendation to receive a PCV?
On June 27, 2024, the ACIP unanimously recommended V116 for:
-
1.
All adults aged 65 years and older who have not previously received a pneumococcal conjugate vaccine or whose previous vaccination history is unknown
-
2.
Adults 19–64 years of age with certain underlying medical conditions or other risk factors who have not previously received a PCV or whose previous vaccination history is unknown
-
3.
Adults 19 years of age and older who started their pneumococcal vaccine series with PCV13 but have not received all recommended PPSV23 doses.
Objectives
This study focused on the first population in the recommendation—the PCV-naïve US adults aged ≥ 65 years. The main objectives were to assess the health and economic outcomes of the use of V116 by estimating and comparing the lifetime clinical outcomes, lifetime costs, and the pairwise incremental cost-effectiveness ratios (ICERs) of using V116 vs. PCV20/PCV15 + PPSV23.
The results from the study provide important health and economic information on the population-level resource use of V116 in vaccine-naïve adults aged ≥ 65 years in the US.
Methods
Analytical Approach
This article does not contain any new studies with human participants or animals performed by any of the authors.
Model Type
A state-transition Markov model was developed to conduct this analysis. Following recommendations from previous studies (Stoecker et al. [28] and Chen et al. [29]), a multicohort approach was adopted in this analysis.
Target Population
All populations aged ≥ 65 years (65–99) who were estimated to be PCV naïve were included in this study. Additionally, the study population was stratified into three mutually exclusive risk groups: low-risk (LR), at-risk (AR), and high-risk (HR) adults, based on the age-group-specific proportions and classifications from Pelton et al. [12].
Perspective
The analyses were conducted from the US societal perspective. This perspective included the costs and benefits of vaccination regardless of who paid the costs or who received the benefits. The costs and benefits included the direct costs of vaccination, the direct medical costs, and indirect costs such as productivity costs. Patient travel costs were included in the indirect costs as well. In addition, loss-of-productivity costs was applied to disease-related deaths as a proxy for the cost of death.
Time Horizon
The health and economic outcomes were tracked for all individuals until death or they turned 100 years old (i.e., a lifetime horizon).
Cycle Length
The cycle length of the model was 1 year. However, half-cycle corrections were applied to account for the fact that events and transitions occurred at any point during the cycle, not necessarily at the beginning or end of each cycle. The half-cycle corrections were applied to all cumulative outcomes except clinical outcomes.
Cost Year
All costs were inflated to reflect 2023 US dollars using the medical care component of the CPI index obtained from the US Bureau of Labor Statistics [30].
Discounting
Future quality-adjusted life years (QALYs) and cost outcomes were discounted at an annual rate of 3% [31].
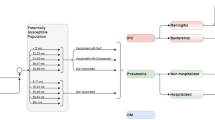
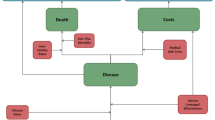
Model Structure
Figure 1 is a schematic diagram of the model. The model has four states—no PD, PD, post-meningitis sequalae (PMS), and death. Among these, three are long-term states (no PD, PMS, and death) at which the cohorts stayed for more than 1 year/cycle, and PD was a short-term state at which the cohorts stayed for one cycle. In other words, it was assumed that all PD manifestations resolved within a year. All cohorts started from the no PD state and were at risk of infection in each cycle. This was achieved by applying the annual incidence rates to the number in the no PD state. Those with PD either had non-meningitis or meningitis IPD or inpatient or outpatient non-bacteremic pneumococcal pneumonia (NBPP). IPD and inpatient NBPP patients were at increased risk of death. Case fatality rates were used to estimate the number of deaths associated with IPD and inpatient NBPP cases. In addition, a proportion of those with meningitis IPD progressed to post-meningitis sequelae (PMS), which was determined from the reported percent of meningitis cases that develop PMS. In our study, PMS was modeled as a long-term disease. In other words, PMS lasts for the rest of the lifetime/time horizon. An annual treatment cost for PMS occurs in each model cycle until death or end of the time horizon. Additionally, PMS patients were at risk of non-meningitis IPD but not meningitis IPD because data on meningitis reinfection for PMS patients are lacking. However, due to the lack of data, the risk of PMS patients developing non-meningitis IPD was the same as that for the general population (i.e., those in the no PD state). Finally, the background (all-cause) age-specific mortality was applied to all states in the model to account for deaths from all other causes annually.
To account for the development of medical conditions with increased age, risk transitioning was implemented in the model as individuals aged. Specifically, LR individuals moved to AR or HR and AR individuals moved to HR in each cycle, as depicted for the no PD and PMS states (Fig. 1). The risk transitions only occurred in the long-term states (no PD and PMS). It was assumed that the risk profiles stayed the same in the short-term state (PD). These movements were achieved by applying the annual transition probabilities among LR, AR, and HR, which were derived from the reported risk distribution and classification data.
The model and the analysis were completed using Microsoft Excel 365® (Microsoft Corporation, Redmond, WA, USA).
Model Inputs
The inputs for the analysis in this study were obtained from the published literature and publicly available reports and databases. Where the required estimates were not available, derived mathematical formulas and/or algorithms were used with existing data together with plausible assumptions to derive appropriate estimates. The major inputs included percentage naïve, IPD and NBPP incidence, vaccine effectiveness, vaccine disease coverage (percent of cases attributable to the serotypes contained in each vaccine), vaccine coverage rate, vaccine acquisition costs, vaccine administration cost, travel cost per vaccination, disease treatment cost, and the utility decrements for each PD. Table 1 presents the details of the major inputs (and their sources) used in the analysis. The full set of inputs and their values are presented in the Supplementary Material.
Vaccine effectiveness (VE) was implemented in the model by applying a reduction factor of (1 − remaining VE) to the baseline annual incidence of PD. The VEs and associated waning functions used in the base case are presented in Table 2 and Fig. 2, respectively. The expected duration of protection (i.e., the area under the curve) for PCVs and PPSVs is 10 years and 7.5 years, respectively. The vaccine coverage rate was assumed to be 55.2% [40]. The proportion of 65+ year-olds that were naïve was assumed to be 34.2% [32].

Based on data from Bonten et al. (2015) [45]
Waning of effectiveness for the single-vaccine strategy. PCV pneumococcal conjugate vaccine, PPSV pneumococcal polysaccharide vaccine.
Model Outcomes
The health outcomes include number of cases of IPD, inpatient and outpatient NBPP, and post-meningitis sequelae (PMS) as well as IPD/NBPP-related deaths. In addition, overall total undiscounted life years and discounted quality-adjusted life years were also presented. Economic results included discounted overall total costs as well as the component costs—vaccine administration cost, vaccine acquisition costs, and direct and indirect treatment costs.
Only pairwise health and economic outcome comparisons were conducted and presented. The estimated population sizes were used for each comparison to provide the expected total population-level outcomes by applying the expected target population size as well as the proportions who were vaccinated with each vaccine. In addition, combined ICERs were calculated by adding the individual health (QALYs) and economic (costs) outcomes.
Sensitivity Analyses
Deterministic sensitivity analyses (DSAs) were conducted to estimate the sensitivity of the ICER to each of the input values. More specifically, within the DSA, the parameters were varied one at a time, based on the associated confidence intervals or plausible ranges presented in the Supplementary Material. Model calculations were performed for each parameter to determine the relative importance of the uncertainty around the parameters in the CEA (cost-effectiveness analysis). The results of DSA were expressed in a tornado diagram. The tornado diagram displays the ICER for each parameter variation from the base value. The parameters with the highest relative importance in the CEA are found at the top of the tornado diagram.
Additionally, probabilistic sensitivity analyses (PSAs) were conducted to assess the robustness of the qualitative results. PSA explores the impact of the simultaneous variation of all the input parameters. Within the PSA, a set of parameter values are drawn by random sampling from the distribution of each parameter around its point estimate to look at the joint impact of the uncertainty around all input parameters. The distribution used for each parameter is presented in the Supplementary Material. One thousand sets of random parameters were drawn, and the outcomes for each random value set were gathered and displayed in a graph as a distribution of outputs.
Lastly, in this sensitivity analysis, only the results for V116 vs. PCV20 were presented, as PCV20 is the key comparator [52].
Scenario Analysis
Four scenario analyses were included in this study:
-
Scenario 1: the health-sector perspective. In the base case, results were reported from the societal perspective. In this scenario, results from the health-sector perspective were reported.
-
Scenario 2: a single-age (65 years old) cohort analysis. In the base case, a multi-age-cohorts approach was used. In this scenario, results from a single-age (65 years old) cohort analysis was conducted.
-
Scenario 3: indirect protection from pediatric vaccination. This scenario incorporated the indirect effect of vaccination with PCV20 in the pediatric population on the disease incidences (of both IPD and NBPP) attributable to the additional seven serotypes (22F, 33F, 8, 10A, 11A, 12F, 15B) not in PCV13. It was accounted for using estimates from a previous analysis [53]. It was also assumed that the equilibrium of the herd effect would be reached gradually over the first 5 years using the yearly percent reduction in disease incidences as follows: 7.8% (year 0), 14.99% (year 1), 21.62% (year 2), 27.74% (year 3), and 33.37% (year 4 and beyond).
-
Scenario 4: alternative vaccine wanings and durations for PCVs. The base-case vaccine waning and durations were chosen to be consistent with the current CDC ACIP research [33, 41, 52]. However, data on the duration of protection conferred by PCVs beyond the 4 years reported by Benton et al. [45] from the CAPiTA study are absent. Analysts have assumed different waning functions, as reported by Treskova et al. [54] after assuming constant vaccine effectiveness for the first 5 years. All the studies assumed quite abrupt duration horizons [54]. In fact, with the exception of Stoecker et al. [28] and van Hoek et al. [55], none of the studies assumed that the vaccine effectiveness would persist beyond the first 5 years, as illustrated by Treskova et al. [54]. For this reason, three waning functions that assumed longer duration horizons than the base-case waning function were examined. The three scenarios were:
-
Scenario 4A: 20-year duration with waning. Constant VE for the first five years followed by a linear decline to zero at year 20 (Fig. 3). The expected duration of protection (i.e., the area under the curve) for this scenario is 12.5 years.
-
Scenario 4B: 15-year duration without waning. Constant VE for 15 years (Fig. 3). The expected duration of protection (i.e., the area under the curve) for this scenario is 15 years.
-
Scenario 4C: 20-year duration without waning. Constant VE for 20 years (Fig. 3). The expected duration of protection (i.e., the area under the curve) for this scenario is 20 years.
-
Model Validation
To ensure that the model was performing as expected and in line with the designed objectives, all the calculations were verified through several tests. Specifically, tests were used to check the three major types of mathematical disease modeling errors—logic, mechanical, and omission [56]. For example, to verify the model logic, the total number of people in each age cohort and risk group was equal to the size of the population for that age group after accounting for deaths.
Given the substantial number of inputs used in the calculations, systematic tests were implemented to confirm that the right inputs (from the right table cells—rows and/or columns) were used in each calculation. Tests for omissions were used to ensure that the theoretical model structure and its component states (as presented in Fig. 1) were accurately represented and accounted for in the calculations [56]. Finally, the model was cross-validated quantitatively and qualitatively with another version of the Markov model developed in Mathematica® 13.1 (Wolfram Research, Champaign, IL, USA).
Results
The detailed base-case, sensitivity, and scenario analysis results are presented below.
Base Case
The age 65+ cohort size is 19,735,405 (= 57,705,864 × 34.20%), which represents the population size of all US individuals aged 65–99 years old (57,705,864) who are PCV naïve (34.20%). Among the target population of 19,735,405 individuals, the vaccination rate is 55.20%. In other words, 55.20% of the 19,735,405 individuals receive the vaccine, and the rest do not receive any vaccine. The estimated clinical and cost outcomes as well as the estimated ICERs when comparing V116 to the current recommendations (PCV20 or PCV15 + PPSV23) for vaccine-naïve adults aged 65+ years (LR/AR/HR) are presented in Table 3.
Compared to PCV20, V116 prevented an additional 27,766 clinical cases/deaths, which included 5204 IPD cases, 63 PMS cases, 8226 NBPP inpatients, 13,172 NBPP outpatients, 751 IPD deaths, and 351 NBPP inpatient deaths. The averted cases and deaths saved 4075 QALYs and resulted in a $239 million reduction in total lifetime medical costs.
Compared to the sequential vaccination strategy PCV15 + PPSV23, V116 prevented an additional 32,387 clinical cases/deaths, which included 4739 IPD cases, 57 PMS cases, 10,183 NBPP inpatients, 16,291 NBPP outpatients, 683 IPD deaths, and 434 NBPP inpatient deaths. The averted cases and deaths saved 4177 QALYs and resulted in a $1.8 billion reduction in total lifetime medical costs.
Sensitivity Analysis
Results of the deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) are presented below.
DSA
The tornado diagrams showing the most influential inputs on the estimated ICER for V116 vs. PCV20 among vaccine-naïve adults aged 65+ years are presented in Fig. 4. Discounting rates and vaccine effectiveness were the most influential inputs for V116 vs. PCV20 (Fig. 4). In all the results, the ICERs remained below zero (i.e., cost saving).
Tornado diagram for V116 vs. PCV20 in vaccine-naïve adults aged 65+ years. AR at risk, HR high risk, IPD invasive pneumococcal disease, NBPP non-bacteremic pneumococcal pneumonia, PCV pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, QALY quality-adjusted life years, ST3 serotype 3, V116 21-valent pneumococcal conjugate vaccine
PSA
The scatter plot showing the incremental costs and QALYs for V116 vs. PCV20 among PCV-naïve adults aged 65+ years are presented in Fig. 5. All of the 500 simulations are in the cost-saving quadrant.
Scenario Analysis
Summary results for all scenarios are presented in Table 4. Detailed clinical and economic outcomes are shown in the Supplementary Material. All the ICERs for V116 versus the comparators are cost savings, as in the base case, with the exception of V116 vs. PCV20 under scenario 1—the health-sector perspective, with an ICER of $3,873/QALY gained. In scenario 2, a single-age (65 years old) cohort analysis was also shown to be cost saving. Additionally, in scenario 3, the inclusion of the herd immunity effect from pediatric vaccination with PCV20 was found to be more cost saving—there were slightly higher incremental QALYs and more cost savings (i.e., higher incremental costs)—compared to the base case. For the three longer protection-duration horizons analyzed in scenario 4, as expected, all ICERs were cost savings, as in the base case. However, the detailed results (in Table 4 and in the Supplementary Material) indicated that, compared to the base case, a longer duration of protection provided additional QALYs and resulted in more cost savings with V116 vs. comparators.
Discussion
In this study, a state-transition Markov model was used to estimate and compare the lifetime health and economic outcomes of the use of V116 in vaccine-naïve adults aged ≥ 65 years in the US compared to the current recommendations (PCV20/PCV15 + PPSV23). The study adopted a multicohort approach, including adults aged 65–99 years, and utilized a lifetime time horizon. The results indicated that the use of V116 resulted in improved health outcomes, with fewer PD cases and associated deaths compared to the current recommendations. The estimated pairwise ICERs were both favorable and cost saving.
The results from these analyses align with findings reported in recently published or reported V116 cost-effectiveness analyses [37, 52, 57, 58]. The only exception was the favorable results for V116 vs. PCV20 found in this study when the herd effect from the pediatric vaccination with PCV20 was included. However, upon careful investigation of the implementation of the herd effect from pediatric vaccination in the model, the herd effect results were deemed reasonable. Since PCV13 has been used in both pediatric and adult populations for several years [59], the current burden of PCV13 serotypes among adults already accounts for the herd effect from pediatric vaccination. As a result, the incremental herd effect from PCV20 can be assumed to occur in relation to the additional seven PCV20-nonPCV13 serotypes (i.e., 8, 10A, 11A, 12F, 15B, 22F, and 33F). However, all seven serotypes are included in V116 and are also covered by PCV15 + PPSV23. Therefore, a reduction in the burden of the common serotypes among the adult population has a minimal effect on model comparisons because all regimens (V116, PCV20, and PCV15 + PPSV23) provide similar protections against all those serotypes. Nonetheless, additional investigation using a dynamic transmission model that accounts for the herd effect as well as other plausible serotype-level dynamics (such as serotype replacement and co-colonization) is needed to gain a better understanding of the impact of the indirect protection conferred by pediatric immunization.
Strengths
As indicated by the studies of Stoecker et al. (2016) [28] and Chen et al. (2018) [29], the type of model used (single cohort vs. multicohort) can have a substantial impact on the results when the time-varying serotype epidemiology is considered. The multicohort approach used in the study, which evaluates health and economic benefits across numerous age cohorts, was found to be more appropriate in capturing the heterogeneity in age cohorts, which is represented by the age-dependent inputs in the model. As a result, using the multicohort approach accounted for all the different costs and benefits for the whole age group, which would not be captured with single-cohort analysis.
Another strength of this study is that the model was built to analyze outcomes at the serotype level. As reported in the previous cost-effectiveness studies (Treskova et al. [54]), very few studies have examined the important serotype-level impact on outcomes.
In addition, this study accounted for risk groups as well as immunosenescence—the changing risk profile as the cohorts aged during the time horizon of the analysis. This feature is critical because the at-risk and high-risk categories are associated with a higher IPD burden and higher costs [12, 13]. Including the risk-transitioning feature in the model led to a better representation of the expected outcomes over time for each age cohort.
Another major strength of this is the investigational analysis (scenario 4) of a longer vaccine effectiveness duration horizon for the PCVs. Although the ICERs for all the scenarios stayed cost saving, as in the base case, the improvements in the incremental QALYs and costs indicated that the assumptions about the vaccine effectiveness duration horizon have a substantial impact on the health and economic outcomes of PCV use and the resulting (numerical) ICERs. The findings suggested that there is a strong need for additional clinical studies to provide more robust and long-term evidence on the duration of vaccine effectiveness. Treskova et al. [54] showed that previous modeling studies assumed relatively short vaccine effectiveness duration horizons, potentially leading to a substantial underestimation of the societal benefits of PCVs.
Finally, scenario analysis and both of the recommended sensitivity analyses (DSA/PSA) were performed to determine the influential parameters as well as to assess the robustness of the base-case results presented in this study.
Limitations
Model Assumptions
Due to the complexity of PD and the associated lack of reliable data, three main simplifying assumptions were made in the model and analysis. First, the maximum time horizon was set to 35 years because the earliest starting age was 65 years and people were tracked until they died or turned 100 years old. However, it is a typical approach to assume a lifetime time horizon in models of vaccines. Second, PMS patients were still at risk of developing another episode of non-meningitis IPD or NBPP but not a recurrent episode of meningitis IPD (due to lack of data). This is a conservative assumption for V116 because the benefit with vaccination would increase if we had data to support PMS patients developing recurrent meningitis IPD. Third, when more than one vaccine was applied or was in the cohort’s vaccine history at any point in time, the serotype-specific vaccine effectiveness (VE) was assumed to be the one with the higher VE at each time point. Since VE is an impactful parameter on the analysis results (e.g., ICER), as demonstrated by the DSA results, future studies on VE from a single vaccine or sequential vaccination and its waning would significantly enhance the study results.
Analysis and Modeling Limitations
Although the model in this study accounted for several important factors, such as waning effects, heterogeneity by age and vaccine status, and immunosenescence, the model is a static model. This means that real-life processes such as herd immunity and serotype replacement were not adequately accounted. However, the approach employed to account for herd effects of pediatric vaccination on the adult population somewhat mitigates the impact of this limitation on the results. This limitation is shared across all Markov/static models. To address this, one approach is to utilize dynamic transmission models (DTMs). As of now, there are no published cost-effectiveness studies on V116 with DTMs. Once they becomes available in the near future, a comparison of the results between different models would provide insights and possibly quantify the impact of this limitation.
The other limitation in this analysis is the source of representative data. Most of the inputs used were either derived or obtained from sources that may not be considered nationally representative. As an example, the risk categories, risk ratios, costs of IPD/NBPP, and incidence of inpatient/outpatient NBPP were obtained from databases that may not be nationally representative of the US population. However, these are the best data available and are also aligned with the inputs used in the CDC models [33, 40, 52] informing national recommendations. However, our scenario analysis and sensitivity analysis have shown the robustness of the results. Therefore, if more nationally representative inputs were available, the study would have shown similar results and conclusions to those we have shown here. Regardless, any future studies to gather evidence among broader population would further strengthen the extent to which the results are generalizable.
Conclusions
To provide insights on the efficiency of resource use for V116 in vaccine-naïve US adults aged ≥ 65 years, a state-transitioning Markov model was used to track health and economic outcomes of the use of V116 among eligible US adults. The analysis demonstrated that V116 strategies resulted in fewer PD cases and associated deaths compared to the current recommendations of PCV20/PCV15 + PPSV23. Pairwise comparisons indicated quite favorable ICERs and showed that V116 vaccination was cost saving. These results demonstrated that V116 vaccination in vaccine-naïve US adults aged ≥ 65 years can substantially reduce the burden of PD among US adults while saving costs.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kobayashi M, Pilishvili T, Farrar JL, et al. Pneumococcal vaccine for adults aged ≥19 years: recommendations of the advisory committee on immunization practices, United States, 2023. MMWR Recomm Rep. 2023;72(3):1–39.
Gingerich AD, Mousa JJ. Diverse mechanisms of protective anti-pneumococcal antibodies. Front Cell Infect Microbiol. 2022;12: 824788.
Ganaie F, Maruhn K, Li CX, et al. Structural, genetic, and serological elucidation of serogroup 24 serotypes: discovery of a new serotype, 24C, with a variable capsule structure. J Clin Microbiol. 2021. https://doi.org/10.1128/JCM.00540-21.
Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020. https://doi.org/10.1128/mBio.00937-20.
Werren JP, Troxler LJ, Oyewole OR, et al. Carbon source-dependent changes of the structure of Streptococcus pneumoniae capsular polysaccharide with serotype 6F. Int J Mol Sci. 2021;22(9):4580.
Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–67.
Global Burden of Disease 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Gierke R, McGee L, Beall B, Pilishivili T. Chapter 11: Pneumococcal. In: Centers for Disease Control and Prevention, editor. Manual for the surveillance of vaccine-preventable diseases. 2020. Available from: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html. Accessed May 2023.
Ogilvie I, Khoury AE, Cui Y, et al. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine. 2009;27(36):4891–904.
van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Current concepts: community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354(1):44–53.
Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4): e60273.
Pelton SI, Bornheimer R, Doroff R, et al. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clin Infect Dis. 2019;68(11):1831–8.
Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–9.
Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(9):1–35.
Centers for Disease Control and Prevention. ABCs Bact Facts Interactive Data Dashboard. Atlanta, GA: Centers for Disease Control and Prevention; 2024.
Kobayashi M, Stoecker C, Xing W, Cho BH, Pilishvili T. Cost-effectiveness of implementing 13-valent pneumococcal conjugate vaccine for U.S. adults aged 19 years and older with underlying conditions. Hum Vaccin Immunother. 2021;1–9.
Kobayashi M. Preliminary work group interpretations of EtR and next steps. Presentation to the ACIP, February 29, 2024. Atlanta, GA: Centers for Disease Control and Prevention; 2024.
Arnold FW, Reyes Vega AM, Salunkhe V, et al. Older adults hospitalized for pneumonia in the United States: incidence, epidemiology, and outcomes. J Am Geriatr Soc. 2020;68(5):1007–14.
Merck & Co. Safety and immunogenicity of V116 in pneumococcal vaccine-naïve adults (V116-003, STRIDE-3). Rahway, NJ: Merck & Co.; 2022. https://classic.clinicaltrials.gov/show/NCT05425732. Accessed 20 June 2024.
Food and Drug Administration. CAPVAXIVE™ (pneumococcal 21-valent conjugate vaccine): United States prescription information. June 17, 2024. Available from: https://www.fda.gov/media/179426/download. Accessed 20 June 2024.
Kobayashi M, Leidner AJ, Gierke R, et al. Use of 21-valent pneumococcal conjugate vaccine among U.S. adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(36):793–798.
Gierke R. Current epidemiology of pneumococcal disease among adults, United States. Presentation to the ACIP, February 29, 2024. Atlanta, GA: Centers for Disease Control and Prevention; 2024.
Centers for Disease Control and Prevention. 1998–2021 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance. Atlanta, GA: Active Bacterial Core Surveillance; 2023.
Yi Z, Johnson K, Owusu-Edusei K. Lifetime health and economic burden of invasive pneumococcal diseases attributable to V116 serotypes among adults in the United States. Infect Dis Ther. 2024. https://doi.org/10.1007/s40121-024-00988-1.
Merck & Co. U.S. FDA approves CAPVAXIVE™ (pneumococcal 21-valent conjugate vaccine) for prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults. Rahway, NJ: Merck & Co.; 2024.
Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbi Mortal Wkly Rep. 2022;71(4):109–17.
Loehr J. Pneumococcal vaccines. Presentation to the ACIP, February 29, 2024. Atlanta, GA: Centers for Disease Control and Prevention; 2024.
Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–8.
Chen C, Wood J, Beutels P, et al. The role of timeliness in the cost-effectiveness of older adult vaccination: a case study of pneumococcal conjugate vaccine in Australia. Vaccine. 2018;36(10):1265–71.
Bureau of Labor Statistics. CPI for All Urban Consumers (CPI-U): medical care in U.S. city average, all urban consumers, not seasonally adjusted. Washington, DC: Bureau of Labor Statistics; 2023.
Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8.
Hun M-C, Srivastav A, Lu P-J, et al. Vaccination coverage among adults in the United States. National Health Interview Survey, 2021. 2022. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-coverage-adults-2021.html. Accessed 20 June 2024.
Stoecker C. Economic assessment of PCV20 for adults vaccinated with PCV13. Presented at ACIP, October 19, 2022. Atlanta, GA: Centers for Disease Control and Prevention; 2022.
Isturiz RE, Ramirez J, Self WH, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–61.
Self WH, Johnson KD, Resser JJ, et al. Prevalence, clinical severity, and serotype distribution of pneumococcal pneumonia among adults hospitalized with community-acquired pneumonia in Tennessee and Georgia, 2018–2022. Clin Infect Dis. 2024. https://doi.org/10.1093/cid/ciae316.
Centers for Disease Control and Prevention. CDC vaccine price list. 2024. Available from: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html. Accessed 20 July 2024.
Altawalbeh SM, Wateska AR, Nowalk MP, et al. Cost-effectiveness of an in-development adult-formulated 21-valent pneumococcal conjugate vaccine in US adults aged 50 years or older. Vaccine. 2024. https://doi.org/10.1016/j.vaccine.2024.04.002.
Micromedex Solutions. Capvaxive. Red Book online. 2024. https://www.micromedexsolutions.com/home/dispatch
Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am J Prev Med 2006;31(1):72–9. https://doi.org/10.1016/j.amepre.2006.03.008.
Stoecker C. Economic assessment of PCV15 & PCV20. Presentation to the ACIP, June 25, 2021. Atlanta, GA: Centers for Disease Control and Prevention; 2021.
McLaughlin JM, McGinnis JJ, Tan L, Mercatante A, Fortuna J. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–73.
Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–43.
Grosse SD, Krueger KV, Pike J. Estimated annual and lifetime labor productivity in the United States, 2016: implications fro economic evaluations. J Med Econ. 2019;22(6):501–8.
Mangen M-JJ, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–16.
Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25.
Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21.
Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–8.
Rudnick W, Liu Z, Shigayeva A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine. 2013;31(49):5863–71.
Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50.
Suaya JA, Jiang Q, Scott DA, et al. Post hoc analysis of the efficacy of the 13-valent pneumococcal conjugate vaccine against vaccine-type community-acquired pneumonia in at-risk older adults. Vaccine. 2018;36(11):1477–83.
Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 2020;17(10): e1003326.
Stoecker C. Economic assessment of PCV21 in U.S. adults. Atlanta, GA: Centers for Disease Control and Prevention; 2024.
Stoecker C, Hampton LM, Link-Gelles R, et al. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132(2):e324–32.
Treskova M, Scholz SM, Kuhlmann A. Cost effectiveness of elderly pneumococcal vaccination in presence of higher-valent pneumococcal conjugate childhood vaccination: systematic literature review with focus on methods and assumptions. PharmacoEconomics. 2019. https://doi.org/10.1007/s40273-019-00805-5.
van Hoek AJ, Miller E. Cost-effectiveness of vaccinating immunocompetent 65 year olds with the 13-valent pneumococcal conjugate vaccine in England. PLoS One 2016;11:e0149540. https://doi.org/10.1371/journal.pone.0149540.
Dasbach EJ, Elbasha EH. Verification of decision-analytic models for health economic evaluations: an overview. Pharmacoeconomics. 2017;35(7):673–83.
de Boer PT, van Werkhoven CH, van Hoek AJ, et al. Higher-valency pneumococcal conjugate vaccines in older adults, taking into account indirect effects from childhood vaccination: a cost-effectiveness study for the Netherlands. BMC Med. 2024;22(1):69.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of an in-development adult-formulated pneumococcal vaccine in older US adults. Vaccine. 2023;41(30):4431–7.
Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5.
Funding
This study and the rapid service fee were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
All the authors—Zinan Yi (ZY), Kwame Owusu-Edusei (KOE), Elamin H. Elbasha (EHE)—conceptualized and determined the scope of the study. ZY performed the study analyses and developed the first draft of the manuscript. All the authors (ZY, KOE, and EHE) reviewed the inputs, results, and the interpretation of the results. All the authors (ZY, KOE, and EHE) critically reviewed and revised the manuscript for intellectual content. All the authors (ZY, KOE, and EHE) approved the submission of this manuscript to the journal. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
This study was performed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Zinan Yi, Kwame Owusu-Edusei, and Elamin Elbasha are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may hold stock or stock options in Merck & Co., Inc., Rahway, NJ, USA. V116 was developed by Merck & Co., Inc., Rahway, NJ, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in—or a financial conflict with—the subject matter or materials discussed in this manuscript apart from those disclosed herewith.
Ethical Approval
This article does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yi, Z., Owusu-Edusei, K. & Elbasha, E. Cost-Effectiveness Analysis of the Use of V116, a 21-Valent Pneumococcal Conjugate Vaccine, in Vaccine-Naïve Adults Aged ≥ 65 Years in the United States. Infect Dis Ther 13, 2597–2615 (2024). https://doi.org/10.1007/s40121-024-01067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-01067-1
Keywords
Profiles
- Zinan Yi View author profile