Abstract
Introduction
Many countries recommend vaccination against Neisseria meningitidis serogroups A, C, W, and Y in infants and young children to prevent invasive meningococcal disease. We evaluated the immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) booster in children primed with the same meningococcal vaccine 5 years earlier. Immune persistence following priming vaccination was also evaluated, and the study is ongoing to generate 10 years’ post-priming data.
Methods
Healthy children, vaccinated with MenACYW-TT 5 years earlier as toddlers, were enrolled. Participants were randomized to receive MenACYW-TT booster (group 1) or no booster (group 2), stratified by country and meningococcal serogroup C (MenC) vaccination status (primed at age ≤ 1 year vs. naive). Antibodies against each serogroup were measured by serum bactericidal assay using human complement (hSBA). Seroresponse sufficiency at 30 days post-booster was demonstrated if the lower limit of the one-sided 97.5% confidence interval (CI) of the seroresponse rate (proportion of participants with post-vaccination titers ≥ 1:16 when baseline titers were < 1:8 or with a ≥ fourfold increase when baseline titers were ≥ 1:8) was > 75% for each serogroup. Seroprotection rates (proportion with hSBA titers ≥ 1:8) and geometric mean titers (GMTs) for each serogroup were also assessed.
Results
A total of 209 participants were enrolled across 26 sites in Finland, Germany, Hungary, and Spain (group 1, n = 93; group 2, n = 116). Five years post-priming, GMTs, and seroprotection rates were higher than those observed before priming vaccination in both groups, indicating long-term persistence. Booster seroresponse rates in group 1 for all serogroups ranged from 93.2% to 98.9%, with seroresponse sufficiency demonstrated (lower limit of one-sided 97.5% CIs for the seroresponse rates ranging from 85.7% to 93.8%). Seroprotection rates and GMTs post-booster increased across all serogroups, with nearly all participants seroprotected, suggesting adequate booster response. Seroresponse was comparable between MenC-primed and MenC-naive participants. No new safety concerns were identified.
Conclusions
MenACYW-TT provides long-term immune persistence and a robust immune response when administered as a booster in children primed 5 years earlier.
Trial Registrations
Clinicaltrials.gov, NCT04936685; EudraCT: 2021-000104-38; WHO: U1111-1255-4941.
Graphical abstract available for this article.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Why carry out this study? |
Many countries recommend a quadrivalent meningococcal vaccine for infants, toddlers, children, or adolescents; however, long-term immunogenicity and the safety of a booster dose require further evaluation. |
This study assessed antibody persistence and evaluated the immunogenicity and safety of a booster dose of the quadrivalent meningococcal conjugate vaccine, MenACYW-TT, 5 years after priming vaccination with MenACYW-TT as toddlers. |
What was learned from the study? |
Antibody persistence was demonstrated, and a booster dose of MenACYW-TT was immunogenic and well tolerated, with sufficient seroresponse 30 days post-booster, in children primed with MenACYW-TT 5 years earlier. |
This study investigated antibody persistence for a longer time frame (5 years vs. 3 years) than the previous study (MET51, NCT02955797) and is ongoing to generate 10-year persistence data. |
This study confirms that a MenACYW-TT booster dose can elicit a robust immune response against meningococcal serogroups A, C, W, and Y. |
Digital Features
This article is published with digital features, including graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.28400981.
Introduction
Invasive meningococcal disease (IMD), caused by Neisseria meningitidis, is a life-threatening infection [1,2,3]. In 2019, there were an estimated 433,000 people diagnosed globally with IMD caused by N. meningitidis, resulting in approximately 32,100 fatalities; of these, 216,000 cases and 14,400 deaths were among children under 5 years of age [4]. The incidence of IMD is highest in infants and young children, with a second peak incidence observed among adolescents and young adults [1]. In 2022, the IMD notification rates for children aged < 1 year and 1–4 years were 4.3 per 100,000 and 1.8 per 100,000, respectively, in Europe [1]. Although IMD is rare across Europe, it remains a public health concern, especially in children, due to the high risk of mortality, with a case-fatality rate of up to 20% globally, depending on region, age group, and meningococcal serogroup [1, 5]. Additionally, up to 20% of survivors experience long-term sequelae, including neurological and hearing impairments, or amputation [3, 6].
Meningococcal serogroups are classified according to their capsular polysaccharides. Currently, 12 distinct meningococcal serogroups have been identified, but serogroups A, B, C, W, X, and Y are the leading cause of meningococcal disease worldwide [7]. Immunization programs in countries of the European Union (EU) and European Economic Area currently recommend pediatric vaccination against N. meningitidis, with monovalent meningococcal C conjugate vaccines or quadrivalent meningococcal ACYW vaccines [8, 9]. Some countries also recommend additional vaccination against meningococcal serogroup B (MenB) [8, 9]. Meningococcal vaccination against serogroup A, C, W, and Y is also recommended in adolescents [8, 9].
In response to outbreaks of meningococcal serogroup C (MenC) during 1999–2001, a number of European countries initiated immunization programs for conjugate MenC vaccination [9]. As a result of this, many children and adolescents within Europe received MenC vaccination as part of their vaccine program. However, due to the emergence of other serogroups, many countries have replaced the MenC vaccine with a quadrivalent vaccine in infants and/or toddlers and adolescents or in adolescents only [9]. Although routine meningococcal vaccination every 5 years is not currently recommended by national immunization programs, regular booster vaccinations are recommended for individuals at higher risk of IMD [8].
MenACYW-TT (MenQuadfi®; Sanofi Inc., Swiftwater, PA, USA), a tetanus toxoid conjugate quadrivalent meningococcal vaccine, is approved for use as a single dose in individuals aged 12 months and older in the EU and in those aged 2 years and older in the USA [10, 11]. Previous research has demonstrated antibody persistence from MenACYW-TT in children 3 years after priming as toddlers [11,12,13], in adolescents and young adults (aged ≥ 13 to < 26 years) 3 to 6 years after priming, and in older adults (aged ≥ 56 years of age) 3, 5, and up to 7 years after priming [14, 15]. However, while antibody persistence for MenACYW-TT in children has been previously demonstrated 3 years after priming as toddlers, due to the small sample size, those data were descriptive only [12].
The MEQ00073 study aimed to investigate the persistence of MenACYW-TT immune response in children over a 5-to-10-year period and with a larger sample size than previously studied. This study included both MenC-naive children and those who were MenC-primed during the first year of life, reflecting the status of this population in different countries. Additionally, the study aimed to describe the immunogenicity and safety of a booster MenACYW-TT dose. The objective of this interim analysis was to assess whether the booster induced sufficient seroresponse for all serogroups in children primed 5 years earlier with MenACYW-TT as toddlers. Seroresponse sufficiency was demonstrated if the lower limit of the one-sided 97.5% confidence intervals (CIs) for seroresponse rates were > 75% for all serogroups [15]. Additionally, secondary objectives were to describe the seroprotection rates and GMTs for all serogroups, as well as the safety of the booster dose.
This study is ongoing and will also assess the immunogenicity and safety of a second booster dose 5 years after the first booster dose administered as children (10 years after an initial priming vaccine in the MET51 study), as well as the persistence of the immune response 5 years after the first booster (10 years after a priming dose in the MET51 study), and the immune response to a booster dose administered 10 years after a priming dose as toddlers.
Methods
Participants and Study Design
A total of 506 healthy children who were vaccinated with MenACYW-TT (MenQuadfi, Sanofi Inc., Swiftwater, PA, USA) approximately 5 years earlier as toddlers (aged 12–23 months) in the completed MET51 (NCT02955797) study [13] were eligible for this current Phase IIIb, open-label, multicenter study (MEQ00073; NCT04936685). Results from the MET51 study were previously published [13]. Briefly, this phase III, randomized, modified double-blind, active-controlled study enrolled healthy toddlers aged 17–23 months (N = 918) at 34 sites in four European countries (Finland, Germany, Hungary, and Spain). Participants were randomized to receive a single dose of either MenACYW-TT or a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine, MCV4-TT (Nimenrix®, Pfizer Europe, Belgium). MenACYW-TT was well tolerated, and non-inferiority to MCV4-TT was demonstrated with respect to seroprotection rates for all four serogroups in both MenC-naive and MenC-primed participants [13].
Participants for the MEQ00073 study were recruited across 26 sites in Finland, Germany, Hungary, and Spain. Key exclusion criteria included prior meningococcal vaccinations that were not administered as part of the previous MET51 study (excluding MenC and MenB vaccinations received during infancy) or a history of meningococcal infection. Participants were also excluded if they received any other vaccine four weeks prior to the trial vaccine or planned to receive any vaccine 4 weeks following the trial vaccination, excluding influenza vaccination, which could be received at least 2 weeks before or after study vaccination. Participants were also excluded if they had received immunoglobulins and/or blood or blood-derived products in the past 3 months, and antibiotics 72 h prior to blood sampling.
This study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki, the International Council for Harmonisation guidelines for Good Clinical Practice, and all applicable laws, rules, and regulations. The study protocol, informed consent form, and investigator’s brochure were reviewed and approved by the Independent Ethics Committee (IEC) and/or Investigational Review Board (IRB) for each study site before the study was initiated. The IECs/IRBs for all study centers are listed in the supplementary materials (Supplementary Table 1). Parents or other legally acceptable representatives of participants were required to sign a statement of informed consent.
Randomization and Interventions
The study design for the MEQ00073 study is presented in Fig. 1. Eligible participants from the MET51 study were randomly selected for participation in MEQ00073, and randomly assigned to group 1 or group 2, with a balanced distribution between the two groups, and stratified according to country and MenC status (primed [Hungary, Spain] or naive [Finland, Germany]).
Participants in group 1 received an initial booster dose of MenACYW-TT on day (D) 1. Participants in group 2 did not receive any vaccination at this stage of the study. Participants in group 1 will receive a second booster dose of MenACYW-TT after 5 years (approximately 5 years after the first booster dose administered in this study). Participants in group 2 will receive a single booster dose of MenACYW-TT 5 years after recruitment to this study (approximately 10 years after the priming dose as toddlers in the MET51 study). Administration of a single booster dose 10 years after priming in group 2 is consistent with meningococcal vaccination recommendations for a booster dose at 5 years after a toddler dose only for specific at-risk populations.
MenACYW-TT, in liquid solution, was administered at 0.5 ml per dose via intramuscular injection. Each dose contained 10 μg of each of the meningococcal capsular polysaccharide serogroups A, C, W, and Y as well as 55 μg of tetanus toxoid protein carrier.
Immunogenicity
The primary objective was to demonstrate immune sufficiency, using vaccine seroresponse as a parameter, to meningococcal serogroups A, C, W, and Y after administration of MenACYW-TT booster in group 1, determined by serum bactericidal assays using human complement (hSBA). Secondary objectives were to describe antibody persistence to meningococcal serogroups A, C, W, and Y in children who received MenACYW-TT approximately 5 years earlier as toddlers (groups 1 and 2), and to describe the antibody response to these serogroups before and 30 days after MenACYW-TT booster (group 1), measured using hSBA and serum bactericidal assay using rabbit complement (rSBA). For hSBA, vaccine seroresponse was defined as a post-vaccination titer ≥ 1:16 for participants with a pre-vaccination titer < 1:8, or a ≥ fourfold titer increase for participants with a pre-vaccination titer ≥ 1:8, and seroprotection was defined as a titer ≥ 1:8 [16]. For rSBA, seroprotection was defined as a titer ≥ 1:8, and seroresponse was defined as a post-vaccination titer ≥ 1:32 for participants with a pre-vaccination rSBA titer < 1:8, or a ≥ fourfold titer increase for participants with a pre-vaccination titer ≥ 1:8 [16].
Other secondary objectives were to describe tetanus toxoid antibody levels before (D1) and 30 days after (D31) the administration of the first MenACYW-TT booster in group 1, and to describe the antibody responses to meningococcal serogroup C before and 30 days after the MenACYW-TT booster in group 1, according to the MenC priming status in the MET51 study (meningococcal vaccine-naive toddlers and MenC-primed toddlers) 5 years earlier, measured using hSBA and rSBA. The proportion of participants with antibody concentrations against tetanus toxoid ≥ 0.01 IU/ml and ≥ 0.1 IU/ml was measured in group 1.
Blood samples for immunogenicity were collected from all participants on D1 of this study. Group 1 participants also provided a blood sample 30 days post-booster vaccination (D31).
Safety
Participants in group 1 were observed for 30 min after vaccination to assess the occurrence of any immediate unsolicited adverse reactions (ARs) or systemic adverse events (AEs). These events could include dizziness, syncope, and anaphylaxis; however, reporting of immediate ARs or AEs was not limited to specific event types. Parents/legally acceptable representatives were provided with diary cards, digital thermometers, and flexible rulers to record daily body temperature, solicited injection site (pain, erythema, and swelling), and systemic reactions (fever, headache, malaise, and myalgia) up to seven days after vaccination. Unsolicited AEs were recorded up to D31 and serious AEs (SAEs), including adverse events of special interest (AESIs), were recorded throughout the interim study period (August 23, 2022 to March 9, 2023).
The safety objective was to describe the safety profile of MenACYW-TT booster within 30 days (time window of + 14 days) after vaccination in group 1.
Statistical Analyses
The percentage of participants in group 1 who achieved a sufficient hSBA vaccine seroresponse for meningococcal serogroups A, C, W, and Y was evaluated 30 days after booster. Seroresponse was considered sufficient if the lower limit of the one-sided 97.5% CI, calculated using the Exact method (Clopper–Pearson method), for the percentage of participants who achieved hSBA seroresponse against serogroups A, C, W, and Y, was greater than 75%. A sample size of 84 evaluable participants was needed to achieve at least 90.0% power to detect that the lower bound of the one-sided 97.5% CI is greater than 0.75 (proportion under the null hypothesis) for the vaccine seroresponse to each of the four serogroups using a one-sided exact test with a significance level (alpha) of 0.025. Analyses for all secondary endpoints were descriptive; no hypotheses were tested. Categorical variables were summarized and presented by frequency counts, percentages, and CIs. The 95% CIs of percentages were calculated using the exact binomial distribution (Clopper–Pearson method). For antibody geometric mean titers (GMTs) and geometric mean concentrations (GMCs), 95% CIs of the point estimates were calculated using a normal approximation, assuming these were log-normally distributed.
Analysis Sets
Persistence data are reported for the full analysis set (FAS) for persistence, which consisted of participants with a valid baseline serology result (groups 1 and 2). Immunogenicity data post-booster in group 1 are reported for the per-protocol analysis set (PPAS), which consisted of participants who received the booster dose, excluding those with at least one of the pre-defined protocol deviations, and had a valid hSBA or rSBA result on D31. The safety analysis set (SafAS) consisted of participants from group 1 who received booster vaccine and who had safety data available.
Results
Study Participants
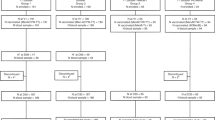
A total of 209 participants were enrolled between August 23, 2022 and February 6, 2023. Overall, there were 114 (54.5%) male and 95 (45.5%) female participants, and the mean age was 6.3 years. Further baseline demographics are reported in Table 1. Overall, 93 participants were assigned to group 1 and 116 participants to group 2. Among participants in group 1, 93 (100%) provided a blood sample and received MenACYW-TT booster on D1 (Fig. 2). On D1, 116 (100%) participants in group 2 provided a blood sample. On D31, 92 (98.9%) participants from group 1 provided a blood sample – one participant discontinued from the study owing to a protocol deviation.
Immunogenicity
Sufficiency of Seroresponse to MenACYW-TT Booster (Group 1)
MenACYW-TT booster (5 years after priming vaccination) induced sufficient seroresponse (Table 2); the primary objective was met, as the lower limit of one-sided 97.5% CI of the hSBA vaccine seroresponse was > 75.0% for each of the four serogroups.
Antibody Persistence of Meningococcal Serogroups A, C, W, and Y (Groups 1 and 2)
Prior to the priming vaccination in the MET51 study (D0 of MET51), hSBA GMTs were low for all serogroups across both study groups (Fig. 3); however, GMTs increased for all serogroups 30 days post-priming vaccination (D30 of MET51) [13] and were comparable between groups 1 and 2 on D1 of MEQ00073. On D1 of the MEQ00073 study, 5 years after the priming vaccination, hSBA GMTs for each serogroup remained higher than MET51 pre-vaccination GMTs in both groups 1 and 2, indicating long-term persistence of immune response. The same trend was observed for rSBA GMTs (Supplementary Table 2). Interestingly, hSBA GMTs for serogroup W on D1 of the MEQ00073 study were similar to those observed on D30 of the MET51 study in both groups (Supplementary Table 2).
Five years after the priming vaccination (D1 of MEQ00073), the proportion of participants with hSBA titers ≥ 1:8 decreased for serogroups C and Y compared with post-priming vaccination (Fig. 4), whereas the proportions for serogroups A and W remained relatively unchanged. As expected, the proportion of participants with hSBA titers ≥ 1:8 was comparable between the two groups for all serogroups (Fig. 4). Similar trends were observed using rSBA (Supplementary Table 2).
Persistence of hSBA antibody response for meningococcal serogroups A, C, W, and Y – percentage of participants with hSBA titers ≥ 1.8 (FAS). D day, FAS full analysis set, GMT geometric mean titer, hSBA serum bactericidal assay using human complement. MEQ00073 study, NCT04936685; MET51 study, NCT02955797
Antibody Response to Serogroups A, C, W, and Y Following MenACYW-TT Booster (Group 1)
Robust increases in GMTs across all serogroups were observed following MenACYW-TT booster compared to pre-booster (Table 3). The proportion of participants with seroprotective hSBA titers ≥ 1:8 post-booster ranged from 97.7% for serogroup C to 100% for serogroups W and Y (Table 3). Seroresponse rates post-booster for each serogroup ranged from 93.2% for serogroup A to 98.9% for serogroups W and Y (Table 3). Similar results were observed using rSBA (Supplementary Table 3).
Antibody Responses to Tetanus Toxoid at D31 (Group 1)
All participants (100%) in group 1 had anti-tetanus antibody concentrations ≥ 0.1 IU/ml post-booster (Table 4). Anti-tetanus antibody GMCs increased from 2.41 (95% CI 1.8, 3.3) on D1 (pre-booster dose) to 13.7 (95% CI 12.0, 15.7) on D31 (post-booster dose) (Table 4).
Serogroup C Antibody Response Following MenACYW-TT Booster by MenC Status Prior to Priming Vaccination
Interestingly, in children who received MenACYW-TT 5 years earlier, hSBA GMTs for serogroup C pre-booster dose tended to be slightly lower in children who were MenC-naive (27.3; 95% CI 16.9, 44.0) compared with children who had been MenC-primed (46.9; 95% CI 27.6, 79.7) at enrolment in the MET51 study. Comparable robust increases in hSBA GMTs for serogroup C were observed for both MenC-naive and MenC-primed participants (Table 5). The proportion of participants with hSBA titers ≥ 1:8 was comparable between MenC-naive and MenC-primed participants before and after MenACYW-TT booster (Table 5). Similarly, seroresponse rates post-booster were comparable between MenC-naive and MenC-primed participants (Table 5). Similar results were observed using rSBA (Supplementary Table 4).
Safety
Overall, no new safety concerns were identified in participants who received MenACYW-TT booster (group 1) (Table 6). There were no immediate unsolicited ARs or AEs (within 30 min after vaccination). Within 7 days of booster vaccination, 72.8% of participants reported at least one solicited injection site reaction and 48.9% reported at least one solicited systemic reaction. During the study, three participants experienced at least one SAE after MenACYW-TT booster (group 1): one participant each experienced giardiasis, which required hospitalization, gastroenteritis, and wrist fracture; none were considered related to the study vaccine. No deaths or AESIs were reported during this period.
Discussion
This interim analysis of the phase IIIb, open-label, multicenter study MEQ00073 demonstrated that a booster dose of MenACYW-TT in children primed 5 years earlier (in the MET51 study) as toddlers with MenACYW-TT was immunogenic and well tolerated, with seroresponse sufficiency demonstrated post-booster vaccination. Moreover, the MEQ00073 study observed a strong persistence of the immune response 5 years after the priming vaccination in toddlers.
Over the last few decades, the overall incidence of IMD has decreased in the EU following the introduction of meningococcal vaccines into national immunization programs; however, the epidemiology of IMD remains highly unpredictable [1]. As such, countries have assumed different practices on meningococcal vaccination, considering the evolution of the local epidemiology [1]. Vaccination programs should consider the antibody persistence of the meningococcal serogroups; however, there are limited data on the long-term protective potential of available vaccines. Additionally, there is no consensus on the need and timing of booster vaccination, with no routine booster recommended in children, except for those at high risk of IMD [8]. Nonetheless, several clinical studies have demonstrated the long-term persistence of the immune response and robustness of booster response with MenACYW-TT across age groups. Previous studies showed persistence of immune response and a robust response with MenACYW-TT booster in children vaccinated 3 years earlier as meningococcal vaccine-naive toddlers [12, 17]. Additionally, the immunogenicity and safety of MenACYW-TT booster has been shown in adolescents and young adults primed with MenACYW-TT (booster dose of MenACYW-TT administered, with or without MenB vaccination) or another quadrivalent meningococcal conjugate vaccine (meningococcal oligosaccharide diphtheria CRM197-conjugate vaccine [MCV4–CRM]; MENVEO®, GSK Vaccines Srl, Sovicille, Italy) 3–6 years earlier, and in older adults (≥ 59 years) primed with MenACYW-TT or the quadrivalent meningococcal polysaccharide vaccine, MPSV4 (Menomune®, Sanofi Pasteur, Swiftwater, PA, USA), and who received a MenACYW-TT booster at least 3 years after priming and up to 7 years after priming [14, 15]. In addition, the immunogenicity of MenACYW-TT booster was previously demonstrated in adults and adolescents (aged ≥ 15 years) who had been primed with other quadrivalent meningococcal conjugate vaccines (meningococcal polysaccharide diphtheria toxoid-conjugate vaccine [MCV4-DT]; Menactra®, Sanofi Pasteur, Swiftwater, PA, USA or MCV4-CRM) 4–10 years earlier [18]. The current study (MEQ00073) showed the persistence of MenACYW-TT in children when administered as a single dose in toddlers for a longer time frame than in previous studies (5 years vs. 3 years) [12]. Moreover, the current study is ongoing in order to generate 10 years’ immune persistence data.
The antibody response to MenACYW-TT booster 5 years post-priming in the current study was similar regardless of MenC priming status during infancy (MenC-primed or meningococcal vaccine naive before priming with MenACYW-TT as toddlers). These findings are consistent with previous observations in another MenACYW-TT conjugate vaccine study [19]. In addition, bactericidal antibody titers elicited by monovalent C and quadrivalent ACWY vaccines have been shown to wane 3–5 years after primary vaccination; this effect is more pronounced in younger children than in older children and adults [20,21,22]. After this initial waning, bactericidal antibody titers remain stable between 6 and 10 years after the priming vaccination [19]. As such, the waning antibody levels observed in this study were therefore expected and are consistent with prior observations following initial priming vaccination with licensed MCV4 vaccines in children and infants [23,24,25]. In these studies, antibody responses waned but remained higher than baseline over 4 and 10 years following primary vaccination with MCV4-TT in toddlers (12–23 months of age) and children (2–11 years of age) [19, 26, 27], and up to 5 years following MCV4-CRM primary vaccination in infants and toddlers (3–5 years) [20, 28].
Interestingly, the hSBA GMT for serogroup W on D1 of the current study (MEQ00073) was similar to that at D30 of the MET51 study in which the participants received the MenACYW-TT priming vaccination [13]. However, while serogroup W was previously responsible for less than 5% of all IMD cases, after a major serogroup W-related outbreak among Hajj pilgrims in 2000, serogroup W has become the dominant cause of IMD across South America, Europe, Australia, and parts of sub-Saharan Africa [29]. Notably, in April 2024, 12 cases of meningococcal disease linked to the Umrah pilgrimage were reported to national public health agencies in the United States, France, and the United Kingdom, 11 of which were caused by serogroup W [30, 31].
No safety concerns were identified with MenACYW-TT booster in children primed 5 years earlier with the same vaccine. The safety profile of MenACYW-TT booster observed in the current study is in concordance with other studies assessing safety and immunogenicity of MenACYW-TT in children, either as a priming vaccine or a booster [12, 13, 17].
This study has some limitations that warrant discussion. Notably, no active comparator was used in this study, as only children vaccinated with MenACYW-TT as toddlers in the MET51 study were enrolled; the current study excluded those primed with the comparator vaccine, MCV4-TT, in the MET51 study. Additionally, only 209 participants were enrolled; however, this was not unexpected as follow-up studies are challenging in terms of willingness to participate in another study. However, it is also important to underline the strengths of this study. For example, both the hSBA and rSBA antibody assays were, and still are, performed in the same laboratories (Global Clinical Immunology [GCI], Sanofi Pasteur, Swiftwater, Pennsylvania, USA, and Public Health England, respectively) throughout the MenACYW-TT development program. This ensured data consistency between the MET51 and MEQ00073 studies [12, 15]. The MET51 and MEQ00073 studies were conducted in Europe only and therefore followed clinical practice recommendations at the time of the study in the countries in which participants were enrolled. Thus, the study findings may not be generalizable to regions in which meningococcal vaccination recommendations differ (for example, where a two-dose MenACWY vaccination series is recommended, with the first dose during the first year of life and a second dose as a toddler).
Conclusions
Overall, these data support the use of MenACYW-TT in children as a priming or booster vaccine, irrespective of MenC priming status in infancy. Further data on the persistence of the immune response of MenACYW-TT 10 years post-priming and following a second booster dose 5 years after a first booster dose will be published upon completion of the MEQ00073 study. Moreover, the findings of the current study, and of other studies conducted with MenACYW-TT in different age groups and with different meningococcal conjugate vaccines for priming vaccination (MenACYW-TT, MCV4-TT or MenC conjugate vaccine in toddlers, MenACYW-TT, MCV4-CRM or MCV4-DT in adolescents/young adults, MenACYW-TT or MPSV4 in older adults), reinforce the evidence that a booster dose of MenACYW-TT can elicit a robust immune response against meningococcal serogroups A, C, W, and Y, irrespective of the meningococcal vaccine used for primary vaccination.
Data Availability
Researchers may request access to patient-level data and related documents, including, for example, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
References
European Centre for Disease Prevention and Control. Invasive meningococcal disease - Annual Epidemiological Report for 2022. Stockholm; 2024.
US Centers for Disease Control and Prevention. Clinical overview of meningococcal disease. https://www.cdc.gov/meningococcal/hcp/clinical/index.html. Accessed 30 Jan 2025.
World Health Organization. Meningitis. https://www.who.int/news-room/fact-sheets/detail/meningitis. Accessed 30 Jan 2025.
GBD 2019 Meningitis and Antimicrobial Resistance Collaborators. Global, regional, and national burden of meningitis and its aetiologies, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2023;22(8):685–711. https://doi.org/10.1016/S1474-4422(23)00195-3.
Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–82. https://doi.org/10.1016/j.vaccine.2019.04.020.
European Centre for Disease Prevention and Control. Factsheet about meningococcal disease. https://www.ecdc.europa.eu/en/meningococcal-disease/factsheet.
McNamara ABA. Meningococcal - Vaccine Preventable Diseases Surveillance Manual | CDC. 2022, https://www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html.
European Centre for Disease Prevention and Control. Vaccine scheduler; Meningococcal disease: recommended vaccinations. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=48&SelectedCountryIdByDisease=-1. Accessed 30 Jan 2025.
Martinón-Torres F, Taha MK, Knuf M, et al. Evolving strategies for meningococcal vaccination in Europe: overview and key determinants for current and future considerations. Pathog Glob Health. 2022;116(2):85–98. https://doi.org/10.1080/20477724.2021.1972663.
European Medicines Agency. MenQuadfi. https://www.ema.europa.eu/en/medicines/human/EPAR/menquadfi.
US Food and Drug Administration. MenQuadfi | FDA. https://www.fda.gov/vaccines-blood-biologics/menquadfi. Accessed 10 Jan 2025.
Piazza FM, Virta M, Paassilta M, et al. Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: a phase III, open-label, multi-center study. Hum Vaccin Immunother. 2021;18(1):1–10. https://doi.org/10.1080/21645515.2021.1902701.
van der Vliet D, Vesikari T, Sandner B, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine-primed toddlers: a phase III randomised study. Epidemiol Infect. 2021;149: e50. https://doi.org/10.1017/s0950268821000261.
Robertson CA, Jacqmein J, Selmani A, Galarza K, Oster P. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: a phase III randomized study. Hum Vaccin Immunother. 2023;19(1):2160600. https://doi.org/10.1080/21645515.2022.2160600.
Zambrano B, Peterson J, Deseda C, et al. Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study. Pediatr Res. 2023;94(3):1035–43. https://doi.org/10.1038/s41390-023-02478-5.
Granoff DM, Pelton S, Harrison LH. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. London: W.B. Saunders; 2013. p. 388–418. https://doi.org/10.1016/B978-1-4557-0090-5.00029-X.
Vesikari T, Borrow R, Forsten A, Findlow H, Dhingra MS, Jordanov E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a phase II randomized study. Hum Vaccin Immunother. 2020;16(6):1306–12. https://doi.org/10.1080/21645515.2020.1733869.
Áñez G, Hedrick J, Simon MW, et al. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a phase III randomized study. Hum Vaccin Immunother. 2020;16(6):1292–8. https://doi.org/10.1080/21645515.2020.1733867.
Vesikari T, Peyrani P, Webber C, et al. Ten-year antibody persistence and booster response to MenACWY-TT vaccine after primary vaccination at 1–10 years of age. Hum Vaccin Immunother. 2020;16(6):1280–91. https://doi.org/10.1080/21645515.2020.1746110.
Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12(5):1300–10. https://doi.org/10.1080/21645515.2015.1136040.
Larrauri A, Cano R, García M, Mateo SD. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23(32):4097–100. https://doi.org/10.1016/j.vaccine.2005.03.045.
Keyserling H, Papa T, Koranyi K, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159(10):907–13. https://doi.org/10.1001/archpedi.159.10.907.
Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014;164(6):1409-15.e4. https://doi.org/10.1016/j.jpeds.2014.02.025.
Dhillon S, Pace D. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix®): a review. Drugs. 2017;77(17):1881–96. https://doi.org/10.1007/s40265-017-0828-8.
Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–7. https://doi.org/10.4161/hv.6.11.12849.
Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34(12):e298-307. https://doi.org/10.1097/inf.0000000000000897.
Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2016;12(1):132–9. https://doi.org/10.1080/21645515.2015.1058457.
Klein NP, Block SL, Johnston W, et al. Persistence of meningococcal bactericidal antibodies and booster response at 60-months of age in children who received infant or toddler doses of MenACWY-CRM conjugate vaccine. Open Forum Infect Dis. 2014;1(suppl_1):S319. https://doi.org/10.1093/ofid/ofu052.793.
Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15(2):470–80. https://doi.org/10.1080/21645515.2018.1532248.
European Centre for Disease Prevention and Control. Cases of invasive meningococcal disease reported in travellers returning from the Kingdom of Saudi Arabia. https://www.ecdc.europa.eu/en/news-events/cases-invasive-meningococcal-disease-reported-travellers-returning-kingdom-saudi-arabia. Accessed 04 Sept 2024.
Vachon MS, Barret AS, Lucidarme J, et al. Cases of meningococcal disease associated with travel to Saudi Arabia for Umrah pilgrimage: United States, United Kingdom, and France, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(22):514–6. https://doi.org/10.15585/mmwr.mm7322e1.
Acknowledgements
The authors would like to thank all participants and their parents/guardians who volunteered to take part in the study. The authors also thank all study investigators: Hanna Karhusaari, Satu Kokko, Susanna Koski, Pauliina Paavola, Oskari Pitkänen, Ilkka Seppä, Elina Sirnelä-Rif, Benita Ukkonen, and Miia Virta (Finland; National Coordinator Investigator: Mika Rämet); Matthias Birke, Katja Denneberg, Matthias Donner, Eivy Franke-Beckmann, Michael Horn, Roland Knecht, Friedrich Kaiser, Ralph Koellges, and Thorsten Krause (Germany; National Coordinator Investigator: Rolf Ebert); Ildiko Batta, Eva Horzsa, Eva Kovacs, and Bernadett Zentai (Hungary; National Coordinator Investigator: Robert Simko); and Cristina Calvo Rey, Maria Luisa Navarro Gomez, and Ignacio Salamanca de la Cueva (Spain; National Coordinator Investigator: Federico Martinón Torres). The authors also wish to acknowledge and thank the Sanofi study team for their support during the conduct of this study.
Medical Writing, Editorial and Other Assistance. Holly McAlister and Steven Goodrick of inScience Communications, Springer Healthcare Ltd, UK, provided medical writing support, which was funded by Sanofi in accordance with Good Publication Practice 2022 guidelines. The authors also thank Isabel Gregoire for editorial assistance and manuscript coordination on behalf of Sanofi.
Funding
The study was funded by Sanofi. The journal’s Rapid Service Fee was funded by Sanofi.
Author information
Authors and Affiliations
Contributions
Conceptualization: Federico Martinón-Torres, Robert Simko, Rolf Ebert, Mika Rämet, Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes. Methodology: Federico Martinón-Torres, Robert Simko, Rolf Ebert, Mika Rämet, Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes. Formal analysis and investigation: Federico Martinón-Torres, Robert Simko, Rolf Ebert, Mika Rämet, Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes. Writing – original draft preparation: Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes. Writing – review and editing: Federico Martinón-Torres, Robert Simko, Rolf Ebert, Mika Rämet, Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes. Supervision: Céline Zocchetti, Olga Syrkina, Siham Bchir, Isabelle Bertrand-Gerentes.
Corresponding author
Ethics declarations
Conflict of Interest
Céline Zocchetti, Olga Syrkina, Siham Bchir and Isabelle Bertrand-Gerentes are employees of Sanofi and may hold shares and/or stock options in the company. Mika Rämet: Finnish Vaccine Research Ltd. (formerly Tampere University Vaccine Research Center) carries out clinical vaccine trials sponsored by all major vaccine manufacturers including Sanofi. Rolf Ebert has no conflicts of interest to disclose. Robert Simko: Scientific lecture fees from Sanofi. Federico Martinón-Torres: Acted as principal investigator in randomized controlled trials of Ablynx, Abbot, Seqirus, Sanofi Pasteur MSD, Sanofi Pasteur, Cubist, Wyeth, Merck, Pfizer, Roche, Regeneron, Jansen, Medimmune, Moderna, Novavax, Novartis, and GSK, with honoraria paid to his institution. FM-T also reports consulting or advisory relationships with GSK Vaccines SRL, Pfizer Inc, Sanofi Pasteur Inc, Janssen Pharmaceuticals Inc, Moderna, MSD, and Seqirus Pty Ltd.
Ethical Approval
This study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki, the International Council for Harmonisation guidelines for Good Clinical Practice, and all applicable laws, rules, and regulations. The study protocol, informed consent form, and investigator’s brochure were reviewed and approved by the Independent Ethics Committee (IEC) and/or Investigational Review Board (IRB) for each study site before the study was initiated. The IECs/IRBs for all study centers are listed in the supplementary materials (Supplementary Table 1). Parents or other legally acceptable representatives of participants were required to sign a statement of informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: Some data from this manuscript were presented previously at the 42nd Annual Meeting of the European Society of Paediatric Infectious Diseases (ESPID), May 20–24, 2024, in Copenhagen, Denmark, and online.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Martinón-Torres, F., Simko, R., Ebert, R. et al. Five-Year Immune Persistence of a Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) and Immunogenicity and Safety of a Booster Dose in Children. Infect Dis Ther 14, 991–1010 (2025). https://doi.org/10.1007/s40121-025-01121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-025-01121-6