Abstract
Introduction
This study evaluates the financial impact on healthcare payers when chronic pain patients initiate peripheral nerve stimulation (PNS) with a 60-day percutaneous PNS (60-Day PNS) treatment versus a conventional brief PNS trial (PNS-BT) with possible follow-on of a permanently implanted PNS system (PNS-PI).
Methods
Centers for Medicare & Medicaid Services (CMS) fee-for-service (FFS) data were analyzed to identify patients with at least 12 months of follow-up (median 26.4 months) who initiated PNS treatment with: (1) 60-Day PNS or (2) PNS-BT. An economic decision tree model assessed the cost to payers in each cohort. Clinical response to 60-Day PNS was estimated by retrospectively reviewing anonymized outcomes from a national real-world database, focusing on patients ≥ 65 years of age who were implanted with a 60-day percutaneous PNS system. For the economic model, a Monte Carlo simulation with 10,000 iterations was used to generate 95% confidence intervals, considering variability in treatment outcome probability and costs.
Results
Based on CMS data, among 60-Day PNS patients, 18% (229/1265) proceeded to a permanently implanted PNS system with a 4% explant rate (10/229). Among PNS-BT patients, 41% (1140/2811) received a permanent implant with a 7% rate of explant (77/1140). Estimated PNS-related weighted average costs for the 60-Day PNS cohort [US$17,344; 95% confidence interval (CI): $16,168–$18,527] were lower than the PNS-BT cohort ($24,392; 95% CI $22,865–$25,941) when considering the percent of patients who advanced to a permanently implanted PNS system. The total cost per successful outcome also favored 60-Day PNS ($25,228 per success for the 60-Day PNS cohort vs. $64,502 per success for the PNS-BT cohort) as a first-line approach in PNS treatment.
Conclusions
The findings suggest that, when PNS for chronic pain is warranted, initiating PNS with a 60-day treatment is more cost-effective than utilizing a brief conventional trial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Why carry out this study? |
The healthcare costs associated with the treatment of chronic pain with either conventional permanent peripheral nerve stimulation (PNS) preceded by a brief trial (PNS-BT) or temporary 60-day PNS are not well understood. |
This study evaluates expected reimbursement costs in chronic pain patients who initiate PNS treatment with a permanent PNS treatment approach (PNS-BT) versus a percutaneous 60-Day PNS treatment. |
What was learned from the study? |
Estimated PNS-related weighted average costs for the 60-Day PNS cohort [US$17,344; 95% confidence interval (CI) $16,168–$18,527] were lower than the PNS-BT cohort ($24,392; 95% CI $22,865–$25,941). |
When PNS for chronic pain is warranted, initiating PNS with a 60-day treatment is cost saving relative to a brief conventional PNS trial. |
Introduction
Peripheral nerve stimulation (PNS) is a non-opioid, non-pharmacological solution for pain relief that has helped address a wide range of pain conditions, such as lower back pain, joint pain, post-traumatic and neuropathic pain, post-operative pain, complex regional pain syndrome, oncologic pain, and others [1,2,3]. Traditionally, patients with chronic pain received a conventional brief PNS trial typically lasting approximately 7 days (PNS-BT). Patients who respond favorably to the brief trial have the trial leads removed and may then proceed to receive a permanent implant.
More recently, a percutaneous PNS system was introduced that includes fine-wire, open-coil leads developed with the goal of reducing infection risk, such that they can be percutaneously implanted for treatment periods lasting up to 60 days (60-Day PNS). [4,5,6] The 60-Day PNS treatment has demonstrated the potential to produce substantial pain relief, leading to enhanced quality of life, functionality, and diminished opioid usage [7,8,9]. Clinical trials, including multiple randomized controlled trials (RCTs), have substantiated the safety and efficacy of this treatment, demonstrating significant pain relief during the treatment and sustained relief following the end of treatment (EOT), including studies targeting the lumbar medial branch, axillary, femoral, and sciatic nerves for various chronic pain conditions in the back and extremities [5, 8,9,10,11]. These studies have indicated the potential for 60-Day PNS to produce sustained relief without requiring a permanent implant.
Therefore, patients with chronic pain being treated with PNS have two primary temporary stimulation approaches: a percutaneous PNS treatment (60-Day PNS) or the conventional brief PNS trial with the goal of advancing to permanent implant. However, no study has directly compared the reimbursement costs to payers associated with these two alternative approaches.
Study Objective
The present study evaluates reimbursement costs when chronic pain patients initiate PNS treatment with PNS-BT versus 60-Day PNS using a healthcare economic simulation model based on Medicare data.
Methods
Data Source
The primary source of data was Centers for Medicare & Medicaid Services (CMS) files on 100% of beneficiaries enrolled in the traditional (fee-for-service, FFS) US Medicare program. Reviewed data from 2017 through 2022 were drawn from the Medicare beneficiary summary file and outpatient facility and carrier claims [4].
Economic Analysis Cohort Definitions
The CMS FFS data were analyzed to identify two cohorts: (1) patients who started with a conventional brief PNS trial (PNS-BT) or (2) those who started with a 60-Day PNS treatment. Patients were identified by Current Procedural Terminology (CPT) codes and matching National Provider Identifier (NPI) numbers in the relevant time frames (discussed below). Patients were required to have a minimum of 12 months of follow-up (median follow-up across the study population was 26.4 months). Medicare patients were selected in the database with a record of CPT code 64555, percutaneous implantation of peripheral nerve neurostimulator electrode array (denoted as “time zero”). Only the first occurrence of 64555 was considered to avoid those with multiple PNS trials or treatments, and the occurrence of 64555 was not eligible for inclusion if there was also a CPT code of 64590 (implantation of peripheral neurostimulator pulse generator) on the same day or visit indicating no trial prior to implantation.
PNS-BT Cohort
The PNS-BT cohort was defined as patients following time zero who then had either (1) a subsequent CPT Code 99212–99215 (evaluation and management, established patient office visit) up to 20 days from time zero or (2) a subsequent CPT Code 99024 (global postoperative visit) up to 10 days from time zero (suggesting trial lead removal) with physician matching NPI number to ensure the visit was with the same physician from time zero. At present, CPT 99024 is only required for reporting physician visits related to post-operative follow-ups within the 10-day global period following PNS lead placement in practices of 10 or more practitioners and in nine states (Florida, Kentucky, Louisiana, Nevada, New Jersey, North Dakota, Ohio, Oregon, and Rhode Island) [13]. As such, cases using exclusively 99024 and not 99212–99215 accounted for only 12% of the PNS-BT cohort as recorded in the Medicare FFS dataset. The majority of PNS-BT cases (88%) were identified by the CPT 99212–99215 code.
Permanent implantation was inferred if the patient had a record of CPT 64590 + CPT 64555/64575 (pulse generator and lead implantation) or the presence of CPT 95971 or 95972 (device programming) following the qualifying trial-related codes. Failure to proceed to permanent implant was assumed anytime following time zero if the patient had no record of CPT 64590 + CPT 64555/64575 or the presence of CPT 95971 or 95972 alone. Explant was implicated if anytime following permanent implantation the patient had a record of CPT code 64595 (revision or removal of pulse generator).
60-Day PNS Cohort
The 60-Day PNS cohort was defined as patients following time zero who then had a subsequent CPT Code 99212–99215 (suggesting office visit for lead removal) between 50 and 70 days from time zero with physician matching NPI number to ensure the same physician from time zero. After the 60-Day PNS treatment period (> 70 days from time zero), permanent implantation and explant of permanent PNS systems were determined in the same way as the PNS-BT cohort.
Other potential applications of peripheral nerve stimulation, such as sacral stimulation and gastric stimulation, were not included as they are typically referenced with separate CPT codes (e.g., 64561, 43647).
Real-World Evidence (RWE) Outcomes Analysis
This study also used data from real-world clinical outcomes to inform some treatment probability inputs for the economic model. Outcomes data were summarized for 60-Day PNS patients aged 65 or older in a sub-analysis of the recent report by Huntoon et al. [12] This institutional review board (IRB)-approved study (WIRB-Copernicus Group IRB) evaluated anonymized data from a national real-world database of patients who were implanted with a 60-Day PNS system (SPRINT® PNS System, SPR Therapeutics, Cleveland, OH, USA) between August 2019 and August 2022. A subset of the patients in the study were ≥ 65 years of age. Patients in the study gave written approval at the time of lead implantation for the manufacturer to collect treatment-related data, provided baseline and end of treatment data sufficient to assess the primary outcome, and had moderate to severe average pain before beginning PNS (NRS pain score ≥ 4). See Supplemental Material, Tables S.1–S.3 Results from Sub-Analysis of Real-World Clinical Outcomes in Patients ≥ 65 years of age (n = 1763) [12].
Based on the clinical outcomes of anonymized patients in the RWE data, responders to 60-Day PNS treatment at the EOT were defined as those with (1) ≥ 50% pain relief (based on patient reported percentage of pain relief relative to baseline) and/or (2) clinically significant improvement in quality of life (≥ 1) as measured on the Patient Global Impression of Change (PGIC). PGIC is a global measure of quality of life (QoL) assessed on a 7-point scale (− 3 to + 3, or very much worse to very much improved). Safety was analyzed by compiling product complaints from the manufacturer’s database that were associated with the patients included in the review.
Decision Tree Model Structure and Inputs
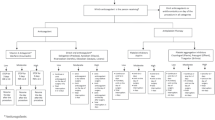
An economic decision tree simulation focused on the subset of pain patients receiving PNS treatment and compared costs to payers (reimbursement) when chronic pain treatment began with 60-Day PNS versus PNS-BT. The model estimated treatment successes and failures in each cohort and accounted for probabilities of initial and follow-on temporary or permanent PNS treatments (including explant) and the attendant costs (Fig. 1). This cost offset model was a purely financial model as it focused on cost of treatment and did not account for the value of quality-adjusted life years (QALYs). The model perspective was a US government payer [Medicare Ambulatory Surgery Center (ASC) reimbursement], but the results are informative for commercial payers (which generally pay larger amounts).
Care pathway diagram from Medicare Fee-For-Service (FFS) Database. (a) Medicare patients 2017–2022 in the database with a first (incident) record of Current Procedural Terminology (CPT) code 64555. Any patient with a CPT code of 64590 on that day or visit would indicate they were not an incident patient and were therefore removed. (b) Analysis of pre-enrollment period for patient baseline characteristics to avoid bias in cohort construction – patients were followed at least 12 months. (c) Following time zero patient has a record of any one of the CPT Codes 99212–99215 up to 20 days from Time Zero (CPT 64555) with physician matching National Provider Identifier (NPI) number or CPT Code 99024 up to 10 days from time zero (CPT 64555) with physician matching NPI number. (d) Following time zero patient has a record of any one of the CPT Codes 99212–99215 50–70 days from Time Zero (CPT 64555) with physician matching NPI number. (e) Any time following time zero patient has no record of 64590 + 64555/64575 or the presence of 95971 or 95972 alone. (f) Greater than 20 days from time zero patient has a record of 64590 + 64555/64575 or the presence of 95971 or 95972. (g) Greater than 70 days from time zero patient has a record of 64590 + 64555/64575 or the presence of 95971 or 95972. (I) Anytime following perm implant patient has a record of CPT code 64595 indicating an explant. *Cell is reported as “ < 11” in Medicare analysis, the most conservative number (n = 10) was used. PNS peripheral nerve stimulation, PNS-BT PNS preceded with a conventional brief trial
Numbers of patients receiving PNS-BT, 60-Day PNS, permanent implantation and explant were based on the CMS data. Input probabilities for one-year treatment success (which was used to estimate cost per successful outcome) were derived for each cohort. In the PNS-BT arm, patients with record of permanent implantation after the brief trial were considered long-term treatment successes unless there was a subsequent record of permanent PNS explant. This represents a conservative assumption of 100% success rate for permanently implanted patients unless explant occurs. Patients with no record of permanent implantation were considered trial failures. In the 60-Day PNS arm, treatment success was derived from the real-world clinical outcomes data, which identified treatment responders at the end of treatment. Because 60-Day PNS is associated with a potential for sustained pain relief after the end of the treatment, one-year treatment success was then informed by published clinical trials and based on a conservative adjustment of the EOT responder rate assuming return of pain in a subset of patients (see Results). The 60-Day PNS cohort also included some patients with subsequent permanent implantation; those with a permanent PNS implant and no explant were assumed to have a successful outcome as in the PNS-BT cohort.
Cost inputs were based on reimbursements for 60-Day PNS treatment, conventional PNS trial, neurostimulator implant, and neurostimulator explant (Table 1). Cost data were determined based on national Medicare reimbursements for relevant CPT codes in ASC settings.
Model Outcomes and Economic Analysis
The key outcome of interest for this analysis was the reimbursed cost difference to the payer when comparing the cohort that started with 60-Day PNS versus the cohort that initiated treatment with PNS-BT. Net costs or savings to the payer associated with 60-Day PNS treatment relative to the PNS-BT strategy were calculated as the expected costs of the 60-Day PNS cohort minus the expected costs of the PNS-BT cohort. A Monte Carlo simulation with 10000 iterations accounted for variability in treatment probability and costs and generated 95% confidence interval estimates. All cost inputs were assumed to have a symmetrical triangle distribution (± 10%) and treatment and efficacy probabilities had beta distributions [13]. Triangle and beta distribution functions enable the model inputs to account for variance in treatment probabilities and costs across different simulation iterations. For example, the distribution functions have a peak probability equivalent to the expected (mean) value of the cost inputs and allow for cost inputs to vary higher or lower than the expected value according to the distribution function. Table S.4 in the Supplementary Material summarizes the event probabilities and distribution parameters for the Monte Carlo simulation.
In addition to calculating the reimbursement savings and differences in reimbursed cost per successful treatment outcome in the two cohorts, stochastic sensitivity analyses were used to identify the most influential inputs that drove the results. This was achieved by estimating the mean outcome measures (such as savings between cohorts) for the lowest decile and highest decile for each simulated input. The inputs with the largest spans were considered the most influential and were graphically represented in tornado diagrams. Those measures on the top of the diagram were the most influential. Standardized regressions of the financial output measure were also conducted on each of the input measures. The resulting coefficients for each input measure reflected both the direction and relative magnitude of each input measure. Those inputs with positive coefficients were positively correlated with the financial outcome measure and the inputs with relatively large coefficients had more influence on the outcomes.
Ethical Approval
The study was conducted in accordance with relevant sections of the U.S. Code of Federal Regulations, the Declaration of Helsinki, and International Conference on Harmonization guidelines for good clinical practice. The analysis of anonymized data from Centers for Medicare & Medicaid Services (CMS) files was exempted from review by the WIRB-Copernicus Group IRB under 45 CFR 46.104(d) [4]. The study also included anonymized outcomes data reviewed retrospectively under a waiver of consent in an IRB-approved study (WIRB-Copernicus Group IRB). No patient-identifying information is included in this manuscript.
Results
60-Day PNS Response Rate for Patients Aged 65 or Over
Of the original 6160 patients included the retrospective review by Huntoon et al. [12], 1763 with known age were ≥ 65 years old. There were no significant differences in baseline characteristics and average and worst pain at baseline in the ≥ 65 cohort compared to the full study cohort (See Supplementary Material, Table S.1). In the retrospective analysis, 70% [1226/1763, 95% confidence interval (CI) 67–72%[ of patients aged 65 and over responded with ≥ 50% pain relief and/or clinically significant improvement in quality of life as measured by PGIC (≥ 1) at the end of the PNS treatment period. The mean percentage of pain relief was 67% [standard deviation (SD): 24%] among responders and 48% (SD 35%) overall. Among patients receiving at least 45 days of the PNS treatment (i.e., as an indicator of treatment compliance), the overall success rate was 73% (1127/1535). The mean average pain score and mean worst pain score at end of treatment were also reduced in the ≥ 65 cohort relative to baseline.
While the subsequent healthcare economic analyses do not stratify by body region, clinical outcomes were stratified with particular interest in the sites that align most closely with the most common areas of chronic pain reported by Medicare beneficiaries: back, shoulder, knee, and foot/ankle (see Supplementary Material, Table S.2). Safety data are reported in Table S.3 of the Supplementary Material.
Model Cohort Characterization, Treatment Probabilities and Event Probabilities from Medicare Data
In the Medicare FFS sample, 1265 patients received 60-Day PNS treatment and 2811 received a conventional brief trial (PNS-BT). Both cohorts were comparable with no differences in average age, sex, race/ethnicity, or comorbidity count (Table 2). While the search window for the PNS-BT cohort was up to 20 days, the most frequent duration of the conventional brief trial was 7 days (i.e., days between the 64555 code and subsequent post-operative physician visit presumed to be for trial lead removal).
Analysis of the Medicare FFS records helped identify the proportion of patients who, after receiving either 60-Day PNS treatment or PNS-BT, proceeded to a permanently implanted PNS system and the proportion of patients subsequently explanted. Among 60-Day PNS patients, 18% (229/1265) proceeded to a permanently implanted PNS system with a 4% explant rate (10/229). As a model input, the success rate at the end of the 60-Day PNS treatment was set to match the real-world clinical outcomes analysis (70%, 880/1265). Based on published literature, long-term success rates of 60-Day PNS may be up to ~ 70% [9, 10, 14, 15]. As 18% (229/1265) of the group that starts with 60-Day PNS was found to proceed to permanent PNS, the overall long-term success rate of 60-Day PNS in the model was therefore conservatively estimated to be 51% (651/1265, i.e., patients who began with 60-Day PNS and may be considered treatment responders long-term due to 60-Day PNS and not permanent PNS, Fig. 1).
Among PNS-BT patients, 41% (1140/2811) received a permanent implant with a 7% rate of explant (77/1140). This corresponds to an overall success rate of 38% in the PNS-BT group (1063/2811). Among those who advanced to a permanently implanted system, median time to implant was statistically significantly shorter for PNS-BT versus 60-Day PNS (42 days vs. 147 days, p < 0.001). Figure 1 summarizes the patient progression probabilities and absolute number of patients who proceeded to each terminal node of the decision tree model.
Decision Tree Simulation Cost Outcomes
The economic simulation model found that reimbursement costs for the cohort which initially used 60-Day PNS treatment ($17,344; 95% CI $16,168–$18,527) were lower than for the cohort that began with PNS-BT ($24,392; 95% CI $22,865–$25,941). The net reimbursement savings were $7048 per patient (95% CI $5342–$8776). The total reimbursement cost per successful outcome (i.e., one-year success from permanent PNS implant or 60-Day PNS with sustained relief) also favored initiating with 60-Day PNS: $25,228 (95% CI $23,324–$27,191) per success for the 60-Day PNS cohort versus $64,502 (95% CI $60,454–$68,687) per success for the PNS-BT cohort (Fig. 2A and B). Figures S.1 to S.4 in the Supplementary Material display the distribution (n = 10,000 draws) for the 60-Day PNS and the PNS-BT reimbursement cost per patient and reimbursement cost per successful treatment outcome.
A Estimated PNS costs per patient; B estimated cost per successful outcome; 95% confidence intervals in parentheses (based on Monte Carlo simulation 2.5 percentile and 97.5 percentile estimates); PNS peripheral nerve stimulation, PNS-BT PNS preceded with a conventional brief trial, 60-Day PNS 60-Day percutaneous PNS treatment
Simulated probabilistic sensitivity analyses identified the most important inputs that impact the magnitude of the reimbursement savings and difference in reimbursement cost per successfully treated patient associated with an initial 60-Day PNS treatment approach. On net, the reimbursement payment for the temporary lead placements, the probabilities of permanent PNS implant and reimbursement payment for the permanent PNS implant were the key determinants that drove reimbursement savings in the model.
With respect to overall savings (Supplementary Material Figure S.5), the reimbursement payment for PNS-BT was the most important factor that was positively correlated (standardized regression coefficient = + 0.66) with savings. The second most influential driver for savings was the reimbursement payment for the 60-Day PNS system (standardized regression coefficient = – 0.54) which reduced savings when the reimbursement amount was larger. In the model the 60-Day PNS and PNS-BT trial/lead placement were coded separately; given the magnitude of the two measures they would likely offset each other with slight positive impact on savings if jointly coded in the model (both inputs are based on the same CPT code). The probability of a permanent PNS implant for either the 60-Day PNS cohort (− 0.38 regression coefficient) or PNS-BT cohort (+ 0.33 regression coefficient) were the next two most important determinants of savings. The fifth most important input for determining reimbursement savings was the reimbursement payment for implantation of the permanent PNS device. It was positively correlated with savings (regression coefficient = + 0.20). The remaining four variables had less impact on the overall savings as all regression coefficients were very close to 0.
With regard to the reimbursed cost difference per successfully treated patient between the 60-Day PNS cohort and the PNS-BT cohort (Figure S.6 in Supplementary Material), the reimbursement payment for the PNS trial/leads (+ 0.75 standardized regression coefficient) and the reimbursement payment for the permanent PNS implant procedure (+ 0.29) were both positively correlated with a larger difference and represent the top two most influential inputs that increase the difference. The probability of a permanent PNS implant after the PNS-BT was negatively correlated with the cost difference per success (− 0.32 coefficient). While this probability increased the cost per patient it also increased the likelihood that a PNS-BT patient had successful outcome, suggesting improved clinical efficiency. The reimbursement cost of 60-Day PNS was negatively associated with cost per success (− 0.32) as expected since a higher cost of implant increases the overall cost of the 60-Day PNS approach (numerator) without any improvement in success rate (denominator) for the 60-Day PNS cohort. Three other probability measures had a moderate impact on the difference in reimbursed cost per success, including share of permanent PNS explants in the PNS-BT cohort (+ 0.24), probability of receiving a permanent PNS device in the 60-Day PNS cohort (− 0.23) and share of 60-Day PNS patients non-responsive to treatment (− 0.18). The relatively tight output ranges in both the savings and cost per success outcomes suggest that model outcomes are robust to input values.
In addition to the simulation sensitivity analysis using standardized regressions, a break-even analysis was conducted to help understand at what point the reimbursed cost per 60-Day PNS success would reach parity with the PNS-BT cohort as the probability of long-term treatment success following 60-Day PNS decreases. As described, the base case assumed a 70% success rate at end of treatment for 60-Day PNS patients based on the real-world clinical outcomes analysis and 51% long-term success rate based on a conservative estimate of sustained clinical efficacy. These success rates would have to drop significantly from 70 to 28%, or from 51 to 10%, respectively, before the reimbursement cost per success in the 60-Day PNS cohort matched the cost per success in the PNS-BT cohort ($64,502).
Overall, across both outcome measures, the model was quite sensitive to the impact of reimbursement payments, while select probability estimates also had a significant, but more muted influence on the economic value of each treatment strategy.
Discussion
This study evaluated data from two sources representing the Medicare patient population to evaluate the cost savings of beginning with a 60-Day PNS treatment versus PNS-BT when PNS is prescribed for Medicare beneficiaries with chronic pain. Findings suggest that when physicians prioritize beginning with a 60-Day PNS treatment over a conventional PNS trial there is a projected savings on average of $7,048 per Medicare patient (95% CI $5,342–$8,776) and a total savings of $39,274 per successful outcome based on total cost per success of $25,228 for 60-Day PNS versus $64,502 for PNS-PI.
The CMS data indicated that patients receiving 60-Day PNS were less likely than those receiving PNS-BT (18% vs. 41%) to go on to receive a permanent implant (Fig. 1). While the cost for the initial phase of 60-Day PNS and PNS-BT were equivalent ($11,681), patients in the PNS-BT cohort were over twice as likely to proceed to Permanent PNS which is almost three times as expensive as the initial procedures ($31,176; $11,681 for lead placement + $19,495 for neurostimulator implant). This migration of patients away from the relatively expensive permanent PNS implant was an important driver of value associated with the 60-Day PNS cohort. Differential explant rates also may have a secondary effect on savings. For those who did proceed to permanent implant, 60-Day PNS patients were less likely to be explanted (4% vs. 7%). Although there may be other factors that lead to explant like need for advanced imaging incompatible with the implant, mechanical failures, etc., the comparatively lower rate of explant, 4% versus 7%, suggests that 60-Day PNS may improve patient selection. A 60-day PNS treatment may increase the ability to identify delayed non-responders – those patients who have a positive early response to PNS within the first several days of treatment that eventually wanes to minimal relief. In the PNS-BT paradigm, delayed non-responders may appear to be responsive to stimulation, and appropriate identification over a longer temporary PNS treatment period may help prevent the decision to implant a permanent PNS system that is more likely to be explanted due to lack of efficacy. These findings are consistent with a prior study demonstrating that 7% of the patients treated with 60-Day PNS were delayed non-responders [16].
While long-term success rates following 60-Day PNS have been demonstrated in prospective clinical trials in multiple regions of the body and types of pain, the present model used a conservative estimate of long-term success to ensure that any economic findings were robust and relevant to real-world clinical practice. The reimbursement cost per success break-even analysis also suggests that the long-term success rate for 60-Day PNS could be substantially lower than the base case input estimate (51% long-term efficacy in the base case versus 10% in the break-even analysis) before the reimbursed cost per success between the two cohorts reaches parity. This demonstrates that even if the real-world effectiveness of 60-Day PNS were substantially lower over the course of a year than published prospective clinical studies have indicated, the cost effectiveness would likely remain higher in the 60-Day PNS cohort.
Study Limitations
There are several important caveats to consider when interpreting the direction and magnitude of the findings. The study relied on Medicare FFS data to categorize patients based on patterns of CPT codes, but these codes lack the information to verify treatments and procedures used. The model was constructed conservatively to capture the most likely PNS-BT and 60-Day PNS patients but may nonetheless contain some mis-categorizations and/or exclude some patients who received PNS-BT or 60-Day PNS. For example, the model may exclude situations such as same-day implants (e.g., no trial or intraoperative trial only), or implant on the same day as trial lead removal such that CPT Code 99212–99215 or 99024 is not used. There are also cases where temporary leads may be removed by a provider with a different NPI than the implanting physician. While not included in this analysis, this practice is more than likely equally distributed between cohorts, and this restriction helped ensure that those who were in the sample were ‘true positives’ for their respective cohorts rather than ‘false positives’ who may have seen unrelated providers in the pertinent timeframe.
Another potential limitation is the study’s use of a retrospective analysis of clinical effectiveness data for 60-Day PNS. This data was derived from the use of a device manufacturer’s database consisting of treatment-related and outcomes data originally recorded by device representatives in their role supporting patient education, treatment optimization, and technical troubleshooting. Secondary analyses of such data are subject to potential selection and reporting bias, though standardized data collection instruments like average NRS pain score, patient-reported percentage pain relief, and PGIC were used to help minimize bias in administration. However, the modeled success rates with 60-Day PNS were consistent with previously published literature [8, 9, 14, 15] and sensitivity and break-even analyses explored the robustness of the findings relative to the assumptions regarding clinical outcomes. Other conservative assumptions were made in lieu of clinical data to inform the model, like assuming a 100% success rate for permanently implanted patients that have a record of permanent implant and no record of explant thus suggesting the present findings may be underestimating the cost savings for 60-Day PNS. Recent data published in a randomized controlled trial of permanently implanted PNS suggest that the success rate after implant is approximately 85–90%. [17]. The assumptions in the model that all implanted PNS which were not explanted were successful is therefore conservative, meaning if the success rate of PNS-BT treatment is reduced to less that 100%, larger savings per successful outcome would accrue for the 60-Day PNS cohort.
This study was modeled exclusively using Medicare National Average ASC payment data. The study did not consider payments in the hospital outpatient setting, or commercial insurance payments which may be substantially higher than the Medicare payments for the same service. As such, the savings calculated in our Medicare-based payment model may underestimate the savings that commercial payers would realize for the same types of patients. In order to focus on pure financial impacts from the health payer perspective, impacts on productivity, quality of life and broader social costs were not considered within this model. Future data which focus on additional procedural settings and payer profiles, as well as broader cohorts beyond the Medicare population, may provide more generalizable estimates of the value of 60-Day PNS.
The model also focused solely on the sub-group of patients receiving PNS treatment for their pain therapy. There are several other potential interventions to treat chronic pain that this analysis did not aim to capture. The findings might be best interpreted as savings accruing to payers during a period when the primary intervention for pain is focused on PNS. However, subsets of patients in both cohorts would likely accrue additional non-PNS treatment costs after PNS trial/treatment failure (including a higher proportion of patients in the PNS-BT cohort due to trial failure) and therefore, based on the large difference in cost per successful treatment, it is likely that incorporating other treatment costs in the same timeframe would still result in lower overall costs in the 60-Day PNS cohort.
Lastly, although the costs of the PNS systems which drive the modeling in the present study are agnostic to pain indication, there was not sufficient sample size to conduct indication-specific analyses. Outcomes and reimbursement data in which the specific pain indications are consistently reported may help illustrate how dynamics differ by chronic pain type. Additionally, in some cases, there were multiple pain sources and it was difficult to discern the primary source. Future analyses may leverage longer time horizons and more carefully delineate events related to chronic pain treatment in greater detail to allow for more precise estimation with time-dependent models.
Conclusions
The findings suggest that, when PNS is warranted, beginning with a 60-Day PNS treatment is more cost-effective (projected per patient savings $7048) compared to starting with a brief conventional PNS trial. When considering the cost per successful outcome, the savings associated when initiating PNS with a 60-Day PNS treatment are even more significant, with projected savings of $39,274 per patient. The significantly lower rate of conversion to a permanently implanted PNS system following a 60-Day PNS treatment, and the lower rate of explantation, have significant economic implications for healthcare payers and points to the preferential utilization of a 60-Day PNS treatment prior to considering permanent implantation. Given that 60-Day PNS treatment and a brief trial are reimbursed equally under the same CPT code, initial care using the 60-Day PNS treatment offers significant potential savings for healthcare payers.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. The Medicare data analyzed in this study is not publicly available due contractual requirements from CMS. The real-world evidence data is also not available to maintain confidentiality of patients.
References
Deer TR, Esposito MF, McRoberts WP, et al. A systematic literature review of peripheral nerve stimulation therapies for the treatment of pain. Pain Med 2020;21(8):1590–603.
Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics 2008;5(1):100–6.
Xu J, Sun Z, Wu J, et al. Peripheral nerve stimulation in pain management: a systematic review. Pain Physic 2021;24(2):E131.
Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med 2019;44(6):637–45.
Gilmore CA, Desai MJ, Hopkins TJ, et al. Treatment of chronic axial back pain with 60-day percutaneous medial branch PNS: Primary end point results from a prospective, multicenter study. Pain Pract 2021;21(8):877–89.
Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2017;17(6):753–62.
Deer TR, Eldabe S, Falowski SM, et al. Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J Pain Res 2021;14:721.
Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2020;45(1):44–51.
Gilmore CA, Kapural L, McGee MJ, Boggs JW. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with 1 year of sustained relief following short-term implant. Pain Pract 2020;20(3):310–20.
Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared to usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabilit Assoc Academ Physiatr 2014;93(1):17.
Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodul Technol Neur Interf 2014;17(8):771–776.
Huntoon MA, Slavin KV, Hagedorn JM, Crosby ND, Boggs JW. A Retrospective review of real-world outcomes following 60-day peripheral nerve stimulation for the treatment of chronic pain. Pain Phys 2023;26(3):273.
Evans M, Hastings N, Peacock B. Statistical distributions. Meas Sci Technol 2001;12(1):117–117.
Chae J, David TY, Walker ME, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil 2005;84(11):832–42.
Gilmore CA, Deer TR, Desai MJ, et al. Durable patient-reported outcomes following 60-day percutaneous peripheral nerve stimulation (PNS) of the medial branch nerves. Intervent Pain Med 2023;2(1):100243.
Naidu R, Li S, Desai MJ, Sheth S, Crosby ND, Boggs JW. 60-day PNS treatment may improve identification of delayed responders and delayed non-responders to neurostimulation for pain relief. J Pain Res. 2022;15:733.
Hatheway J, Hersel A, Song J, et al. Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomised controlled trial. Reg Anesth Pain Med 2024;
Acknowledgements
The authors thank the participants of the study.
Funding
This study was funded by SPR Therapeutics (Cleveland, OH, USA).
Author information
Authors and Affiliations
Contributions
David Dickerson, Hemant Kalia, Kevin Vorenkamp, Konstantin Slavin, Jonathan M. Hagedorn, Candace Gunnarsson, Eric Keuffel, Andrew Epstein, Mark Stultz and Nathan Crosby contributed to the study conception and design. Material preparation, data collection and analysis were performed by Eric Keuffel, Andrew Epstein, Candace Gunnarsson and Nathan Crosby. The first draft of the manuscript was written by Eric Keuffel and Candace Gunnarsson and David Dickerson, Hemant Kalia, Kevin Vorenkamp, Konstantin Slavin, Jonathan M. Hagedorn, Candace Gunnarsson, Eric Keuffel, Andrew Epstein, Mark Stultz and Nathan Crosby commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dave Dickerson is a consultant to Abbott, Vertos, Biotronik, and SPR Therapeutics, and his institution has received research funding from Abbott. Hemant Kalia is a consultant to Abbott, Averitas Pharma, Nalu, and SPR Therapeutics, and serves on advisory boards for Curonix and Abbott. Kevin Vorenkamp reports no disclosures. Konstantin Slavin and/or his institution have received consulting income, research and educational grants from Medtronic, Abbott, Boston Scientific, ATI, MSEI and Neuros; he has minor ownership or ownership options in Neuramodix, Thermaquil, and Higgs Boson. Jonathan Hagedorn reports no disclosures. Candace Gunnarsson, Eric Keuffel, and Andy Epstein are consultants to SPR Therapeutics. Mark Stultz and Nathan Crosby are employees of SPR Therapeutics and hold stock options in SPR Therapeutics.
Ethical Approval
The study was conducted in accordance with relevant sections of the U.S. Code of Federal Regulations, the Declaration of Helsinki, and International Conference on Harmonization guidelines for good clinical practice. The analysis of anonymized data from Centers for Medicare & Medicaid Services (CMS) files was exempted from review by the WIRB-Copernicus Group IRB under 45 CFR 46.104(d)[4]. The study also included anonymized outcomes data reviewed retrospectively under a waiver of consent in an IRB-approved study (WIRB-Copernicus Group IRB). No patient-identifying information is included in this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dickerson, D.M., Kalia, H., Vorenkamp, K.E. et al. Cost Savings in Chronic Pain Patients Initiating Peripheral Nerve Stimulation (PNS) with a 60-Day PNS Treatment. Pain Ther 14, 269–282 (2025). https://doi.org/10.1007/s40122-024-00677-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00677-4