Abstract
Background
Huntington disease (HD) is a progressive neurodegenerative disease that causes psychiatric and neurological symptoms, including involuntary and irregular muscle movements (chorea). Chorea can disrupt activities of daily living, pose safety issues, and may lead to social withdrawal. The vesicular monoamine transporter 2 inhibitors tetrabenazine, deutetrabenazine, and valbenazine are approved treatments that can reduce chorea.
Objective
This post hoc analysis was conducted to evaluate safety and efficacy among participants who received high-dosage deutetrabenazine treatment (> 48 mg/d) in ARC-HD, an open-label study that assessed long-term safety and efficacy of deutetrabenazine for the treatment of chorea in HD in adults.
Methods
ARC-HD was a single-arm, two-cohort, open-label study. Participants either successfully completed the First-HD study or switched overnight from tetrabenazine to deutetrabenazine. Participants were dosed with deutetrabenazine in a response-driven manner (maximum 72 mg/d allowed). For the current analysis, exposure-adjusted incidence rates (EAIRs) for adverse events of interest were analyzed according to daily dosage (≤ 48 mg/d versus > 48 mg/d), and total maximal chorea (TMC) scores were analyzed by cohort during the stable-dose period.
Results
In total, 116 of the 119 participants enrolled in ARC-HD entered the stable-dose period, where no apparent differences were seen in EAIRs when receiving deutetrabenazine dosages ≤ 48 mg/d (exposure = 177.7 person-years) compared with > 48 mg/d (exposure = 74.1 person-years). Similar results were found among the subset of participants who received deutetrabenazine dosages > 48 mg/d at least once during the study (n = 49, 42%) when their dosage was ≤ 48 mg/d (exposure = 37.9 person-years) versus > 48 mg/d (74.1 person-years). Efficacy analyses were conducted for participants who had TMC scores available (rollover cohort, n = 77; switch cohort, n = 35). For most participants, the lowest deutetrabenazine dosage needed to achieve a TMC response (≥ 30% improvement from baseline) was between 24 and 48 mg/d in both the rollover (n = 57, 74.0%) and switch (n = 16, 46.0%) cohorts. Whereas the dosage needed for a TMC response was independent of baseline TMC score in the rollover cohort, participants with higher baseline TMC scores in the switch cohort required higher dosages to achieve a TMC response during the trial.
Conclusions
In this open-label, long-term study, some participants received deutetrabenazine dosing > 48 mg/d to achieve adequate chorea control. There was no new safety concern or incremental change in the safety profile between dosages of ≤ 48 mg/d and > 48 mg/d. These results include dosages that have not been approved for clinical use, however, they increase our understanding of safety and tolerability of deutetrabenazine doses.
Clinical Trials Registration
ARC-HD (ClinicalTrials.gov identifier: NCT01897896); First-HD (ClinicalTrials.gov identifier: NCT01795859).
Plain Language Summary
ARC-HD was a medical study in which participants took a medicine called deutetrabenazine. Deutetrabenazine is used to treat unplanned movements known as chorea, which happen in participants who have Huntington disease. At the start of the ARC-HD study, all participants took 6 milligrams per day (mg/d) of the medicine. Each week, the doctor could give them more medicine, but not more than 72 mg/d. The doctor checked if the medicine helped to lower their chorea and if any safety concerns occurred. The highest amount of the medicine that is allowed in the USA is 48 mg/d. Some participants took more than that. We wanted to learn more about the safety (side effects) and the chorea in participants who took more than 48 mg/d. Participants who took more than 48 mg/d had no new side effects of interest. They also reported the same number of side effects as participants who took 48 mg/d or less. For most participants, the lowest amount of medicine they needed to lower their chorea was between 24 and 48 mg/d.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
The findings of this post hoc analysis of the ARC-HD open-label study suggest that for some participants (42%) with chorea in Huntington disease who received a deutetrabenazine dosage above the approved maximum of 48 mg/d, higher dosages were not associated with increase of exposure-adjusted incidence rates of adverse events of interest. |
Participants who took deutetrabenazine in ARC-HD were able to achieve a response [on the basis of total maximal chorea (TMC) score], regardless of their baseline score; however, those who switched from tetrabenazine required higher dosages of deutetrabenazine if they had high baseline TMC scores. |
Titrating to higher dosages within the approved dose range (maximum dosage 48 mg/d) while closely monitoring for safety and tolerability can help ensure that participants receive the full benefit of treatment with deutetrabenazine. |
1 Introduction
Huntington disease (HD) is a hereditary neurodegenerative disorder that affects motor and cognitive function and causes behavioral/emotional disturbances [1,2,3,4,5]. Chorea is the hallmark visible motor symptom of HD, affecting approximately 90% of adult patients over the course of their disease [6,7,8], and can potentially increase the risk of injury, cause social isolation, and impede daily function [2, 7, 8]. Currently, no treatment exists for the underlying disease process in HD, but chorea can be managed with approved treatments such as the vesicular monoamine transporter 2 (VMAT2) inhibitors tetrabenazine, deutetrabenazine, or valbenazine [9,10,11], and off label with treatments as published in the scientific literature [12].
Deutetrabenazine, a novel, highly selective VMAT2 inhibitor, is approved by the United States Food and Drug Administration (US FDA) and countries such as Australia, Brazil, Israel, and China for the treatment of adults with chorea in HD or tardive dyskinesia (TD). The recommended maximum dosage is 48 mg/d (24 mg twice daily), or 36 mg/d (18 mg twice daily) if patients are poor metabolizers of cytochrome P450 2D6 (CYP2D6) or are receiving concomitant, strong CYP2D6 inhibitors [9, 13].
In the randomized, double-blind, placebo-controlled First Time Use of Deutetrabenazine in Huntington Disease (First-HD; ClinicalTrials.gov Identifier: NCT01795859) study [14], deutetrabenazine significantly improved motor signs as measured by Unified Huntington’s Disease Rating Scale® total maximal chorea (TMC) scores. Results from First-HD also demonstrated an overall improvement in quality of life compared with placebo, as measured by Patient Global Impression of Change (PGIC), Clinician Global Impression of Change (CGIC), and 36-Item Short Form–physical functioning subscale scores (SF-36) at 12 weeks, and had a comparable safety profile with placebo. The Alternatives for Reducing Chorea in Huntington Disease (ARC-HD; ClinicalTrials.gov Identifier: NCT01897896) open-label study [15] evaluated the long-term safety and efficacy of deutetrabenazine dosed in a response-driven manner up to 72 mg/d for the treatment of chorea in HD. ARC-HD comprised two cohorts: participants who switched overnight from tetrabenazine (the switch cohort) and those who were previously taking deutetrabenazine as part of the First-HD study (the rollover cohort). Deutetrabenazine maintained and improved chorea control with a favorable benefit–risk profile across the dosage range (6–72 mg/d) in both cohorts.
This post hoc analysis of the ARC-HD study was performed to investigate differences, if any, in deutetrabenazine safety and efficacy between dosages > 48 mg/d and ≤ 48 mg/d among participants who received higher dosages. This analysis can support decision-making on risk/benefit assessment when determining dose adjustments.
2 Methods
2.1 Study Design, Participants, and Interventions
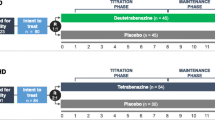
ARC-HD was an open-label, single-arm, two-cohort, multicenter study. It was conducted from November 2013 to August 2017 at 37 sites in the USA, Canada, and Australia and consisted of two cohorts: the rollover cohort and the switch cohort. The study design and inclusion criteria for both studies have been described previously [14,15,16]. Briefly, the Rollover cohort comprised participants who had successfully completed First-HD [14], including participants randomly assigned to placebo and those randomly assigned to deutetrabenazine for 12 weeks. Key inclusion criteria for First-HD were age ≥ 18 years, HD diagnosis with at least moderate chorea (TMC score ≥ 8 and a total functional capacity [TFC] score ≥ 5), and no tetrabenazine use for ≥ 6 months prior to screening. Participants were required to have daily access to a caregiver, or a live-in caregiver in more advanced cases (TFC score 5–7). In ARC-HD, participants in the rollover cohort completed a 1-week washout period before initiating deutetrabenazine (6 mg/d), followed by re-titration for 8 weeks. Dosages could be titrated up or down in weekly increments of 6 mg/d up to 48 mg/d. After a dosage of 48 mg/d was reached, weekly changes of 6 or 12 mg/d [up or down, up to a maximum of 72 mg/d (36 mg twice daily)] were permitted at the investigator’s discretion to reach an optimal, individualized dosage on the basis of efficacy and tolerability. Dosages of 6 mg/d were administered once daily in the morning, and dosages ≥ 12 mg/d twice daily, approximately 10 h apart. If participants were receiving a strong CYP2D6 inhibitor (e.g., paroxetine, bupropion, or fluoxetine), the maximum dosage of deutetrabenazine was 42 mg/d (21 mg twice daily).
The switch cohort included participants with chorea in HD who enrolled in ARC-HD de novo and had been on a stable dosage of tetrabenazine with therapeutic benefit (although the benefit may have been suboptimal) for ≥ 8 weeks. These participants were converted overnight from tetrabenazine to deutetrabenazine. Initial deutetrabenazine dosages were calculated with a protocol-specified algorithm hypothesized to provide comparable systemic exposure to their previous stable tetrabenazine dosage [15, 16]. Participants in the switch cohort remained on their initial deutetrabenazine dosages for 1 week, after which dose adjustments could be made up to week 4, with an extended dose-adjustment period up to week 8. Similar to the rollover cohort, weekly adjustments (up or down) in increments of 6 mg/d up to 48 mg/d and of 6 or 12 mg/d up to 72 mg/d were permitted. For both cohorts, the stable-dose period was defined as week 8 through the last dose of study drug.
2.2 Safety Assessments
Safety and tolerability were assessed during in-clinic study visits and telephone study visits from the time informed consent was received through the end of follow-up (4 weeks after last dose of study drug) [15]. For this post hoc analysis, adverse events (AEs) of interest were evaluated using the following groupings of preferred terms: akathisia (including akathisia, hyperkinesia, psychomotor hyperactivity, restlessness, and agitation [preferred terms]), parkinsonism (including akinesia, bradykinesia, cogwheel rigidity, freezing phenomenon, hypertonia, masked facies, muscle rigidity, on and off phenomenon, parkinsonian crisis, parkinsonian gait, parkinsonian rest tremor, parkinsonism, Parkinson disease, and resting tremor [preferred terms]), fall, dysphagia (including aphagia and dysphagia [preferred terms]), somnolence, insomnia, anxiety, depression (including all preferred terms containing “depression”), and suicidality (including completed suicide, suicidal depression, intentional overdose, intentional self-injury, deliberate poisoning, self-injurious ideation, suicidal behavior, suicidal ideation, and suicide attempt [preferred terms]). Participant data (from both cohorts) were divided into two sets according to daily dosage: all participants, and participants who ever received dosages > 48 mg/d during the stable-dose period. AEs of interest reported during the stable-dose period (where dosages > 48 mg/d would be expected) were analyzed according to daily dosage (≤ 48 mg/d and > 48 mg/d) at the time of the AE report. Additionally, the temporal relationships (if any) between AE reports and dosage increases for participants who had dosage increases > 6 mg/d (at the discretion of the investigator) were evaluated.
2.3 Efficacy Assessments
In ARC-HD, changes in TMC and TMS scores from baseline to week 8 (titration period) and during the stable-dose period were evaluated [15]. Because baseline observations for participants who switched overnight from tetrabenazine to deutetrabenazine (switch cohort) were made while the participants were on different stable dosages of tetrabenazine, post hoc efficacy analyses were performed separately for the rollover and switch cohorts for the entire study period (titration and stable-dose periods). The lowest dosage needed for a TMC response (defined as ≥ 30% improvement from baseline in TMC score) was determined and plotted against baseline TMC score. Additionally, for each dosage level, the number of participants for whom that dosage was the lowest dosage sufficient to elicit a TMC response during the entire study period was assessed, and the cumulative proportions of participants who achieved a TMC response, by dose, were calculated. These analyses were also performed for participants with baseline TMC scores above and below the median value to determine whether there was a relationship between baseline TMC score and the dosage needed to achieve a TMC response.
2.4 Statistical Analysis
Exposure-adjusted incidence rates (EAIRs) for AEs reported during the stable-dose period (starting at visit 4/week 8) were used for analysis. EAIRs were calculated as the ratio of the number of individuals with the given AE divided by the total exposure time up to the end of follow-up (AEs per person-year) [17]. Comparisons between dosages are summarized as rate ratio (95% CI). The 95% CIs were calculated separately for each AE, assuming a uniform Poisson rate among participants while receiving the lower deutetrabenazine dosage and a different rate while receiving the higher dosage. Only participants who had baseline and post-baseline TMC scores were included in the efficacy analyses.
3 Results
3.1 Participants' Characteristics
In total, 136 participants were screened in the ARC-HD study, of whom 119 were administered the study drug (82 in the rollover cohort and 37 in the switch cohort); of those, 116 entered the stable-dose period. In the rollover and switch cohorts, 77 and 35 participants, respectively, had both baseline and post-baseline TMC scores and were included in the efficacy analyses. When comparing baseline characteristics between the two participants groups (all participants and those who had ever received dosages > 48 mg/day during the stable-dose period, respectively), the average (SD) ages of participants were 53.3 (12.0) and 50.5 (12.3) years, respectively. The majority of participants were male (56% and 57%), white (94% and 96%), and had normal body mass indexes (BMIs) (mean [SD]) at screening (25.2 [4.70] and 24.8 [5.1] kg/m2) (Table 1). The average (SD) ages of participants in the rollover and switch cohorts were 53.7 (12.3) and 52.4 (11.5) years, respectively. The majority of participants were male (rollover cohort, 55%, and switch cohort, 59%), white (93% and 97%), and had normal BMIs at screening (25.8 kg/m2 and 23.9 kg/m2) (Table 1). Overall, 100 participants (84%) completed ≥ 1 year of deutetrabenazine treatment, and 81 participants (68%; 56 in the rollover cohort and 25 in the switch cohort) completed the study [15].
3.2 AEs of Interest
Of the 116 participants who entered the stable-dose period, 49 had ever received (at least once during the stable-dose period) a deutetrabenazine dosage > 48 mg/d. EAIRs for AEs of interest were compared in the following two analyses: (1) among all participants, when receiving ≤ 48 mg/d (exposure = 177.7 person-years) versus when receiving > 48 mg/d (exposure = 74.1 person-years) and (2) among participants who had ever received dosages > 48 mg/d, when receiving ≤ 48 mg/d (exposure = 37.9 person-years) versus > 48 mg/d (exposure = 74.1 person-years). In general, no apparent differences in EAIRs were observed for participants who received deutetrabenazine dosages ≤ 48 mg/d compared with > 48 mg/d among the full set of participants at the time of AE occurrence, or in those who ever received a dosage > 48 mg/d during the stable-dose period (Fig. 1). Among participants who ever received a dosage > 48 mg/d during the stable-dose period, the highest rate ratio observed was for akathisia (rate ratio, 2.56 [95% CI 0.29–121.14]), suggesting that akathisia might be more common when receiving dosages > 48 mg/d; however, the CI ranges reported are wide.
3.3 Safety for Participants with Dosage Increases of More Than 6 mg/d
After a daily dosage of 48 mg was reached, further weekly changes of 6 or 12 mg/d (up or down) were permitted at the investigator’s discretion, up to a maximum dosage of 72 mg/d (36 mg twice daily). Out of the 49 participants who were ever assigned daily dosages of > 48 mg/d, 37 participants received only dosage increases of 6 mg/d and 12 participants received a mixture of dosage increases (6 mg/d and > 6 mg/d); no participants received only > 6-mg/d increases. Two participants reported AEs (akathisia and insomnia) within 2 days of a dosage increase from 48 to 60 mg/d. In each of these cases, the investigator considered the AE to be at least possibly related to study drug, but not serious, and thus continued to increase the dosage by an additional 12 mg/d 5 days later, although the AEs were still present.
In a separate case, a participant reported intermittent gagging and intermittent insomnia (both considered possibly related to study drug but not serious) 13 and 41 days, respectively, after an increase from 42 to 48 mg/d; the dosage was further increased by 12 mg/d 46 days later, although the AEs were still present. A total of 26 days later, the participant reported additional intermittent worsening anxiety (considered possibly study-drug related but not serious), but the dosage was not adjusted further. Another participant’s dosage was increased from 30 to 48 mg/d on day 41 because of a dosing titration error (documented as a minor protocol deviation). No AEs were reported following this increase; however, the dosage was subsequently decreased by 12 mg/d 11 days later. The remainder of AEs (e.g., insomnia, somnolence, falls, depression, and irritability) that were reported as soon as 6 days after a 12-mg/d dosage increase were not considered serious and did not prompt a further dosage decrease, regardless of their duration or relationship to study drug.
3.4 Efficacy
Changes in efficacy, based on TMC response from baseline throughout the study (week 0 to week 145), were compared within the rollover cohort and switch cohort separately to avoid potential confounding of the results from previous exposure to tetrabenazine for participants in the switch cohort. For the majority of participants in the rollover cohort, the lowest dosage sufficient to show a TMC response was between 24 and 48 mg/d (n = 57 [74.0%]) (Fig. 2). For some participants (n = 6 [7.8%]), this dosage was lower (between 6 and 18 mg/d), whereas others (n = 9 [11.7%]) received higher dosages (between 54 and 72 mg/d) (Fig. 2).
Lowest deutetrabenazine dosage needed for a TMC responsea. a Rollover cohort, b switch cohort. TMC total maximum chorea. aTMC response was considered a ≥ 30% improvement from baseline and was assessed in participants with both baseline and post-baseline TMC scores (rollover cohort, n = 77; switch cohort, n = 35)
Most (> 50%) participants needed to reach a dosage of ≥ 36 mg/d to achieve at least a 30% improvement in TMC (Fig. 3). At a dosage of ≤ 48 mg/d, more than 80% of participants achieved a TMC response, and at ≤ 72 mg/d, the proportion of TMC responses was more than 90%. No meaningful differences were observed between participants with baseline TMC scores above and below the median value (11.0). Participants were able to achieve TMC responses regardless of their baseline TMC score, and no relationship between the two factors was observed (Fig. 4).
Cumulative proportion of participants with a TMC responsea by deutetrabenazine dosage. a rollover cohort, b switch cohort. TMC total maximum chorea. aTMC response was considered a ≥ 30% improvement from baseline and was assessed in participants with both baseline and post-baseline TMC scores (rollover cohort, n = 77; switch cohort, n = 35). Baseline TMC score median value = 11.0 (rollover cohort) and 12.5 (switch cohort)
Lowest dosage needed for a TMC responsea by baseline TMC score, a rollover cohort, b switch cohort. TMC total maximum chorea. aTMC response was considered a ≥ 30% improvement from baseline and was assessed in participants with both baseline and post-baseline TMC scores (rollover cohort, n = 77; switch cohort, n = 35). Dot size on the chart is representative of the number of participants at that point
Although the switch cohort comprised a smaller number of participants, similar results were observed as with the rollover cohort, except for a slight trend where participants with baseline TMC scores above the median of 12.5 received higher dosages (median dosage, 60 mg/d) to achieve TMC response (Figs. 2, 3, 4).
4 Discussion
This post hoc analysis was conducted to evaluate safety and efficacy among participants who received deutetrabenazine treatment with dosages above the maximum approved dosage of 48 mg/d in the ARC-HD study, where titration to dosages up to 72 mg/d was allowed at the investigator’s discretion on the basis of efficacy and tolerability. In the ARC-HD study, participants who received deutetrabenazine dosages > 48 mg/d showed no apparent increases in EAIRs of AEs of interest compared with dosages ≤ 48 mg/d. There were no observed patterns between the timing of AEs and dosage increases of ≥ 12 mg/d. While a minority of participants in this study achieved their therapeutic response goal with a lower dosage, most participants (74.0%) achieved a TMC response (≥ 30% improvement from baseline) when taking deutetrabenazine dosages between 24 and 48 mg/d. Similar minimal efficacious dose ranges were observed for most participants in the pivotal (First-HD) [14] and long-term (ARC-HD) [15] deutetrabenazine studies. However, some participants (11.7%) in the ARC-HD study achieved a TMC response at higher dosages (54–72 mg/d). Overall, these data highlight the importance of adequate titration to an effective and tolerable dosage.
The limited use of the 42-mg/d dosage in this study may be explained by the convenience of the lower number of tablets that need to be taken to arrive at daily dosages of 36 or 48 mg compared with 42 mg, which required the highest number of pills of the dosages evaluated in the study. Convenience, along with safety of the dosing range, may have moved participants to doses lower or higher than 42 mg. Some patients may not experience full efficacy at specific doses, but the flexibility of the deutetrabenazine dose range allows physicians to modify the treatment to a dose that is both tolerable and most efficacious for the patient.
These data do not indicate baseline TMC score as a predictor of deutetrabenazine dose required for response, but rather that stable doses differ between individuals and that patients should be treated on the basis of their individualized response and treatment history, which may be influenced by factors such as concomitant medication and overall HD stage [9].
TMC is a useful tool for measuring overall severity of involuntary movements, however, the location and impact of these movements may vary between patients [18]. To supplement TMC data, future research may consider more granular and/or composite measures that encompass additional aspects of the patient experience, such as patients finding ways to control their involuntary movements and thereby decreasing their impact. Some limitations of the analyses presented here may be that ARC-HD was an open-label study that did not include a control group, and that these analyses are post hoc and not powered for comparison. In addition, study participants may have received dosages > 48 mg/d for a limited period of time.
A slight trend was observed for the switch cohort, where participants with higher baseline TMC scores required higher dosages to achieve TMC responses. This could be explained by the fact that baseline TMC values for these participants were recorded while the participants were being treated with tetrabenazine (in contrast to the rollover cohort, where participants had a 1-week washout period). Participants were able to achieve TMC response regardless of baseline TMC score. These results are similar to those in the Reducing Involuntary Movements in Participants With Tardive Dyskinesia (RIM-TD; ClinicalTrials.gov Identifier: NCT01795859) study, where participants with TD benefited from deutetrabenazine in the same way, regardless of baseline Abnormal Involuntary Movement Scale (AIMS) scores [19].
Because of the complexity and lack of treatment options available for neurodegenerative diseases, there is a clear need for novel strategies for treatment of the symptoms. Individualized treatment to help patients achieve optimal response (accomplished by response-driven dosing) is growing in importance for both clinicians and patients as part of seeking overall improvement in the quality of life of patients [20]. Achieving this improvement is complex, considering that HD and chorea affect patients in different ways. Optimal dosages cannot be generalized across a patient population for which chorea is among a triad of symptoms [21], although, in the context of HD, the presence of chorea itself affects emotional, interpersonal, and professional functioning [22]. This study provides clinicians and patients with information about the safety and efficacy of higher deutetrabenazine dosages. In this study, dosages above the maximal approved therapeutic dosage (48 mg/d) were explored. The data reinforce the safety and tolerability of the different deutetrabenazine dosages, including higher ones. Treating patients with dosages higher than the approved therapeutic dosage may offer greater benefits while retaining the tolerability profile. It may also mitigate early treatment discontinuation and non-adherence because of a lack of efficacy. Additionally, these long-term safety and efficacy results from ARC-HD can enhance physicians’ and patients’ understanding of possible chorea improvement and AEs on higher deutetrabenazine dosages while adhering to the approved dosing regimen.
5 Conclusions
The results of these post hoc analyses of the ARC-HD open-label study show the efficacy and safety profile of participants with chorea in HD who received doses higher than the ones currently approved. Patients who receive higher deutetrabenazine dosages should be closely monitored for safety and tolerability. Response-driven and flexible dosing may increase the number of patients with chorea in HD who achieve therapeutic benefit.
References
Foroud T, Gray J, Ivashina J, Conneally P. Differences in duration of Huntington’s disease based on age at onset. J Neurol Neurosurg Psychiatry. 1999;66(1):52–6. https://doi.org/10.1136/jnnp.66.1.52.
Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8(9):844–56. https://doi.org/10.1016/S1474-4422(09)70183-8.
Testa CM, Jankovic J. Huntington disease: a quarter century of progress since the gene discovery. J Neurol Sci. 2019;396:52–68. https://doi.org/10.1016/j.jns.2018.09.022.
Carlozzi NE, Schilling SG, Lai J-S, Paulsen JS, Hahn EA, Perlmutter JS, et al. HDQLIFE: development and assessment of health-related quality of life in Huntington disease (HD). Qual Life Res. 2016;25(10):2441–55. https://doi.org/10.1007/s11136-016-1386-3.
Schwab LC, Garas SN, Drouin-Ouellet J, Mason SL, Stott S, Barker RA. Dopamine and Huntington’s disease. Expert Rev Neurother. 2015;15(4):445–58. https://doi.org/10.1586/14737175.2015.1025383.
Anderson KE. Huntington’s disease. Handb Clin Neurol. 2011;100:15–24. https://doi.org/10.1016/B978-0-444-52014-2.00002-1.
Burgunder JM, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey-based algorithm for the pharmacologic treatment of chorea in Huntington’s disease. PLoS Curr. 2011;3: RRN1260. https://doi.org/10.1371/currents.RRN1260.
Jankovic J, Roos RA. Chorea associated with Huntington’s disease: to treat or not to treat? Mov Disord. 2014;29(11):1414–8. https://doi.org/10.1002/mds.25996.
Austedo® (deutetrabenazine) tablets [prescribing information]. Parsippany: Teva Neuroscience, Inc; 2023.
Huntington Study Group KINECT-HD Collaborators, Furr-Stimming E, Claassen DO, Kayson E, Goldstein J, Mehanna R, Zhang H, et al. Safety and efficacy of valbenazine for the treatment of chorea associated with Huntington’s disease (KINECT-HD): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2023;22(6):494–504. https://doi.org/10.1016/S1474-4422(23)00127-8.
Xenazine® (tetrabenazine) tablets [prescribing information]. Deerfield: Neopharm Ltd.; 2023.
Gibson JS, Claassen DO. State-of-the-art pharmacological approaches to reduce chorea in Huntington’s disease. Expert Opin Pharmacother. 2021;22(8):1015. https://doi.org/10.1080/14656566.2021.1876666.
Schneider F, Stamler D, Bradbury MJ, Loupe PS, Gordon MF, Rabinovich-Guilatt L. The effect of potent CYP2D6 inhibition on the pharmacokinetics and safety of deutetrabenazine in healthy volunteers. Eur J Clin Pharmacol. 2022;78(1):11–8. https://doi.org/10.1007/s00228-021-03202-0.
Huntington Study Group, Frank S, Testa CM, Stamler D, Kayson E, Davis C, Edmondson MC, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40–50. https://doi.org/10.1001/jama.2016.8655.
Huntington Study Group/ARC-HD Investigators and Coordinators, Frank S, Testa C, Edmondson MC, Goldstein J, Kayson E, Leavitt BR, et al. The safety of deutetrabenazine for chorea in Huntington disease: an open-label extension study. CNS Drugs. 2022;36(11):1207–16. https://doi.org/10.1007/s40263-022-00956-8.
Huntington Study Group/Alternatives for Reducing Chorea in Huntington Disease Investigators, Frank S, Stamler D, Kayson E, Claassen DO, Colcher A, Davis C, et al. Safety of converting from tetrabenazine to deutetrabenazine for the treatment of chorea. JAMA Neurol. 2017;74(8):977–82. https://doi.org/10.1001/jamaneurol.2017.1352.
Liu GF, Wang J, Liu K, Snavely DB. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med. 2006;25(8):1275–86. https://doi.org/10.1002/sim.2335.
Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–42. https://doi.org/10.1002/mds.870110204.
Hauser RA, Barkay H, Fernandez HH, Factor SA, Jimenez-Shahed J, Gross N, et al. Long-term deutetrabenazine treatment for tardive dyskinesia is associated with sustained benefits and safety: a 3-year, open-label extension study. Front Neurol. 2022;13: 773999. https://doi.org/10.3389/fneur.2022.773999.
Tyson RJ, Park CC, Powell JR, Patterson JH, Weiner D, Watkins PB, et al. Precision dosing priority criteria: drug, disease, and patient population variables. Front Pharmacol. 2020;11:420. https://doi.org/10.3389/fphar.2020.00420.
Claassen DO, Ayyagari R, Goldschmidt D, Zhou M, Leo S, Ribalov R. Defining utility values for chorea health states in patients with Huntington’s disease. Adv Ther. 2022;39(4):1784–93. https://doi.org/10.1007/s12325-022-02046-z.
Claassen DO, DeCourcy J, Mellor J, Johnston C, Iyer RG. Impact of chorea on self-care activity, employment, and health-care resource use in patients with Huntington’s disease. J Health Econ Outcomes Res. 2021;8(1):99–105. https://doi.org/10.36469/001c.24620.
Acknowledgments
The authors would like to thank Juha-Matti Savola, MD, PhD (of Teva Pharmaceuticals at the time of this research), and Mary C. Edmondson, MD (of the Huntington Study Group) for their contributions to the study. Medical writing support for the development of the outline and subsequent drafts of this manuscript, under the direction of the authors, was provided by Anneke Brand, PhD, and Jennifer C. Jaworski, MS, BCMAS, CMPP, and editing support by Kelsey Gribbon, MS, and Beverly Stanley, ELS, all of Ashfield MedComms, an Inizio company, and were funded by Teva Branded Pharmaceutical Products R&D, Inc.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was supported by Teva Branded Pharmaceutical Products R&D, Inc.
Conflict of Interest
Samuel Frank and Claudia M. Testa served as co-principal investigators of the First-HD study. Samuel Frank was compensated for his professional role as the chair of the Data and Safety Monitoring Board for the KINECT-HD study. Jody Goldstein, Elise Kayson, Christine O’Neill, and Jacquelyn Whaley report no conflicts of interest. Blair R. Leavitt is an employee of the Department of Medical Genetics and Division of Neurology, Department of Medicine, and a senior scientist at the Centre for Molecular Medicine and Therapeutics, The University of British Columbia, and BC Children’s Hospital. In the last 5 years, he has held or applied for research grants in the area of neurodegenerative disease from the Canadian Institutes of Health Research, The Huntington Society of Canada, Weston Brain Foundation, Brain Canada, uniQure, Triplet Therapeutics, and the Nanomedicines Innovations Network. He has served on advisory boards for sRNAlytics, the Huntington’s Disease Society of America, and the Huntington Society of Canada. In addition, he has acted as a paid consultant for Roche, uniQure, Novartis, PTC Therapeutics, Triplet Therapeutics, Genentech, Takeda, and Ionis. He is also a shareholder, co-founder, and CEO of Incisive Genetics Inc. David Oakes has received research support from Auspex (for the First-HD and related studies), Vaccinex, Prana Pharmaceuticals, Biogen, and the National Institutes of Health, and received honoraria from Raptor Pharmaceuticals and Voyager Inc. Nicholas Gross, Nayla Chaijale, Steve Barash, and Mark Forrest Gordon are employees and shareholders of Teva Pharmaceuticals.
Availability of Data and Material
Consistent with the policies of the Huntington Study Group, the corresponding author is confirmed as the guarantor of the work, and all authors had full access to the data. All authors have the right to publish any and all data separate and apart from any sponsor, and we take full responsibility for the data, the analysis and interpretation, and the conduct of the research.
Ethics Approval
This article is based on previously conducted studies and does not contain any new studies with human participants. All involved study centers of the original trial (NCT01897896) received approval by local ethics committees and informed consent was received for all participants.
Consent to Participate
Consent was obtained during the original clinical study and no new consent forms were required/collected for this analysis.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
S.F., N.C., and M.F.G. contributed to the drafting/revision of the manuscript for content, including medical writing for content, and had a major role in the conceptualization, data curation, methodology, data analysis, supervision, validation, and reviewing. C.T., J.G., E.K., and J.W. contributed to the drafting/revision of the manuscript for content, including medical writing for content, and had a major role in the acquisition of data and the analysis or interpretation of data. B.R.L. and C.O. had a major role in the acquisition of data. D.O., N.G., and S.B. contributed to the drafting/revision of the manuscript for content, including medical writing for content, and the analysis or interpretation of data. All authors reviewed and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Frank, S., Testa, C.M., Goldstein, J. et al. Safety and Efficacy of Deutetrabenazine at High versus Lower Daily Dosages in the ARC-HD Study to Treat Chorea in Huntington Disease. CNS Drugs 39, 185–195 (2025). https://doi.org/10.1007/s40263-024-01139-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01139-3