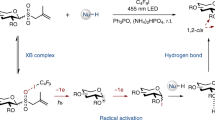
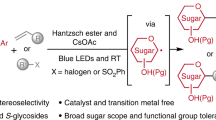
Abstract
Carbohydrate chemistry has benefited a lot from the intrinsic reactivity of sulfoxide since it was introduced in glycosylation reactions by Kahne in 1989. Since then, extensive studies have been explored by employing sulfoxide as glycosyl donors and activation reagents in construction of glycosidic bonds. As glycosyl donors, the sulfinyl groups could locate either directly or remotely at anomeric position. This chapter focuses on the establishment and development of sulfoxides as glycosyl donors in glycosylation reactions, with an emphasis on their applications and postulated mechanisms.











































Similar content being viewed by others
Abbreviations
- ADMB:
-
4-allyl-1,2-dimethoxybenzene
- CB:
-
Carboxybenzyl
- CIPs:
-
Contact ion pairs
- DDQ:
-
2,3-dicyano-5,6-dichlorobenzoquinone
- DIPEA:
-
N,N-diisopropylethylamine
- DMAP:
-
4-dimethylaminopyridine
- DMDO:
-
Dimethyldioxirane
- DTBMP:
-
2,6-di-tert-butyl-4-methylpyridine
- E:
-
Electrophile
- Fmoc:
-
9-Fluorenylmethyl
- IAD:
-
Intramolecular aglycon delivery
- LAH:
-
Lithium aluminum hydride
- mCPBA:
-
3-chloroperbenzoic acid
- MOM:
-
Methoxymethyl
- Nap:
-
Naphthyl
- NMP:
-
N-methyl-2-pyrrolidone
- Nu:
-
Nucleophile
- Piv:
-
Pivaloyl
- PMB:
-
p-methoxybenzyl
- PSB:
-
2-[(propan-2-yl)sulfinyl]benzyl
- PTB:
-
2-[(propan-2-yl)thio]benzyl
- SSIPs:
-
Solven-separated ion pairs
- TBDPS:
-
tert-Butyldiphenylsilyl
- TBS:
-
t-butyldimethylsilyl
- TCP:
-
N-tetrachlorophthalimido
- Tf2O:
-
Trifluoromethanesulfonic anhydride
- TFAP:
-
3-trifluoroacetamidopropyl
- TfOH:
-
Trifluoromethanesulfonic acid
- TMP:
-
1,3,5-trimethoxybenzene
- TMSE:
-
2-(trimethylsilyl)ethyl
- TMSOTf:
-
Trimethylsilyl trifluoromethanesulfonate
- TTBP:
-
2,4,5-tri-tert-butylpyrimidine
- β-hFSH:
-
β-domain of human follicle-stimulating hormone
References
Willer R (2000) Sulfoxides, in Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons, Hoboken
Pummerer R (1909) Über phenyl-sulfoxyessigsäure. Ber Dtsch Chem Ges 42:2282–2291
Smith LHS, Coote SC, Sneddon HF, Procter DJ (2010) Beyond the Pummerer reaction: recent developments in thionium ion chemistry. Angew Chem Int Ed 49:5832–5844
Akai S, Kita Y (2006) Recent advances in Pummerer reactions. In: Schaumann E (ed) Sulfur-mediated rearrangements I. Top curr chem, vol 274. Springer, Berlin
Feldman KS (2006) Modern Pummerer-type reactions. Tetrahedron 62:5003–5034
Bur SK, Padwa A (2004) The Pummerer reaction: methodology and strategy for the synthesis of heterocyclic compounds. Chem Rev 104:2401–2432
Pfitzner KE, Moffatt JG (1963) A new and selective oxidation of alcohols. J Am Chem Soc 85:3027–3028
Omura K, Swern D (1978) Oxidation of alcohols by “activated” dimethyl sulfoxide. A preparative, steric and mechanistic study. Tetrahedron 34:1651–1660
Evans DA, Andrews GC (1974) Allylic sulfoxides. Useful intermediates in organic synthesis. Acc Chem Res 7:147–155
Carreno MC (1995) Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem Rev 95:1717–1760
Kahne D, Walker S, Cheng Y, Van Engen D (1989) Glycosylation of unreactive substrates. J Am Chem Soc 111:6881–6882
Aversa MC, Barattucci A, Bonaccorsi P (2008) Glycosulfoxides in carbohydrate chemistry. Tetrahedron 64:7659–7683
Fascione MA, Brabham R, Turnbull WB (2016) Mechanistic investigations into the application of sulfoxides in carbohydrate synthesis. Chem Eur J 22:3916–3928
Crich D, Bowers AA (2008) Sulfoxides, sulfimides and sulfones. In: Demchenko AV (ed) Handbook of chemical glycosylation. Wiley-VCH Verlag Gmbh & Co. KGaA, Weinheim, pp 303–329
Micheel F, Schmitz H (1939) Das verhalten von sulfoxyden gegenüber sulfit. Ber Dtsch Chem Ges 72:992–994
Khiar N, Alonso I, Rodriguez N, Fernandez-Mayoralas A, Jimenez-Barbero J, Nieto O, Cano F, Foces-Foces C, Martin-Lomas M (1997) Chemical and enzymatic diastereoselective cleavage of β-d-galactopyranosylsulfoxides. Tetrahedron Lett 38:8267–8270
Ferrières V, Joutel J, Boulch R, Roussel M, Toupet LC, Plusquellec D (2000) Sulfur atom configuration of sulfinyl galactofuranosides determines different reactivities in glycosylation reactions. Tetrahedron Lett 41:5515–5519
Karthaus O, Shoda S-I, Kobayashi S (1994) Diastereoselective cleavage of β-glucosylsulfoxides by β-glucosidase. Tetrahedron Asymmetry 5:2213–2216
Moya-Lopez JF, Elhalem E, Recio R, Alvarez E, Fernandez I, Khiar N (2015) Studies on the diastereoselective oxidation of 1-thio-β-d-glucopyranosides: synthesis of the usually less favoured RS sulfoxide as a single diastereoisomer. Org Biomol Chem 13:1904–1914
Raghavan S, Kahne D (1993) A one-step synthesis of the ciclamycin trisaccharide. J Am Chem Soc 115:1580–1581
Gildersleeve J, Smith A, Sakurai K, Raghavan S, Kahne D (1999) Scavenging byproducts in the sulfoxide glycosylation reaction: application to the synthesis of ciclamycin 0. J Am Chem Soc 121:6176–6182
Crich D, Sun S (1997) Are glycosyl triflates intermediates in the sulfoxide glycosylation method? A chemical and 1H, 13C, and 19F NMR spectroscopic investigation. J Am Chem Soc 119:11217–11223
Crich D, Sun S (1998) Direct formation of β-mannopyranosides and other hindered glycosides from thioglycosides. J Am Chem Soc 120:435–436
Martichonok V, Whitesides GM (1996) A practical method for the synthesis of sialyl α-glycosides. J Am Chem Soc 118:8187–8191
Martichonok V, Whitesides GM (1997) Studies on α-sialylation using sialyl donors with an auxiliary 3-thiophenyl group. Carbohy Res 302:123–129
Lian G, Zhang X, Yu B (2015) Thioglycosides in carbohydrate research. Carbohydr Res 403:13–22
Shiao TC, Roy R (2010) “Active-latent” thioglycosyl donors and acceptors in oligosaccharide syntheses. In: Fraser-Reid B, Cristóbal LJ (eds) Reactivity tuning in oligosaccharide assembly. Top curr chem, 301st edn. Springer, Berlin, pp 69–108
Gildersleeve J, Pascal RA, Kahne D (1998) Sulfenate intermediates in the sulfoxide glycosylation reaction. J Am Chem Soc 120:5961–5969
Sliedregt LAJM, van der Marel GA, van Boom JH (1994) Trimethylsilyl triflate mediated chemoselective condensation of arylsulfenyl glycosides. Tetrahedron Lett 35:4015–4018
Alonso I, Khiar N, Martín-Lomas M (1996) A new promoter system for the sulfoxide glycosylation reaction. Tetrahedron Lett 37:1477–1480
Marsh SJ, Kartha KPR, Firld RA (2003) Observation on iodine-promoted & β-mannosylation. Syn Lett 2003:1376–1378
Wipf P, Reeves JT (2001) Glycosylation via Cp2ZrCl2/AgClO4-mediated activation of anomeric sulfoxides. J Org Chem 66:7910–7914
Nagai H, Matsumura S, Toshima K (2000) A novel promoter, heteropoly acid, mediated chemo- and stereoselective sulfoxide glycosidation reactions. Tetrahedron Lett 41:10233–10237
Nagai H, Kawahara K, Matsumura S, Toshima K (2001) Novel stereocontrolled α- and β-glycosidations of mannopyranosyl sulfoxides using environmentally benign heterogeneous solid acids. Tetrahedron Lett 42:4159–4162
Palanivel A, Chennaiah A, Dubbu S, Mallick A, Vankar YD (2017) AuCl3–AgOTf promoted O-glycosylation using anomeric sulfoxides as glycosyl donors at room temperature. Carbohydr Res 437:43–49
Stork G, Kim G (1992) Stereocontrolled synthesis of disaccharides via the temporary silicon connection. J Am Chem Soc 114:1087–1088
Stork G, La Clair JJ (1996) Stereoselective synthesis of β-mannopyranosides via the temporary silicon connection method. J Am Chem Soc 118:247–248
Chung SK, Park KH (2001) A novel approach to the stereoselective synthesis of β-d-mannopyranosides. Tetrahedron Lett 42:4005–4007
Gu ZY, Zhang JX, Xing GW (2012) N-Acetyl-5-N,4-O-oxazolidinone-protected sialyl sulfoxide: an α-selective sialyl donor with Tf2O/(Tol)2SO in dichloromethane. Chem Asian J 7:1524–1528
Gadikota RR, Callam CS, Lowary TL (2001) Stereocontrolled synthesis of 2,3-anhydro-β-d-lyxofuranosyl glycosides. Org Lett 3:607–610
Gadikota RR, Callam CS, Wagner T, Del Fraino B, Lowary TL (2003) 2,3-Anhydro sugars in glycoside bond synthesis. Highly stereoselective syntheses of oligosaccharides containing α- and β-arabinofuranosyl linkages. J Am Chem Soc 125:4155–4165
Callam CS, Gadikota RR, Krein DM, Lowary TL (2003) 2,3-Anhydrosugars in glycoside bond synthesis. NMR and computational investigations into the mechanism of glycosylations with 2,3-anhydrofuranosyl glycosyl sulfoxides. J Am Chem Soc 125:13112–13119
Bai Y, Lowary TL (2006) 2,3-Anhydrosugars in glycoside bond synthesis. Application to α-d-galactofuranosides. J Org Chem 71:9658–9671
Amaya T, Takahashi D, Tanaka H, Takahashi T (2003) Synthesis of 2,3,6-trideoxysugar-containing disaccharides by cyclization and glycosidation through the sequential activation of sulfoxide and methylsulfanyl groups in a one-pot procedure. Angew Chem Ed 42:1833–1836
Merrifield RB (1963) Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc 85:2149–2154
Yan L, Taylor CM, Goodnow R, Kahne D (1994) Glycosylation on the Merrifield resin using anomeric sulfoxides. J Am Chem Soc 116:6953–6954
Liang R, Yan L, Loebach J, Ge M, Uozumi Y, Sekanina K, Horan N, Gildersleeve J, Thompson C, Smith A, Biswas K, Still WC, Kahne D (1996) Parallel Synthesis and screening of a solid phase carbohydrate library. Science 274:1520–1522
Silva DJ, Wang H, Allanson NM, Jain RK, Sofia MJ (1999) Stereospecific solution- and solid-phase glycosylations. Synthesis of β-linked saccharides and construction of disaccharide libraries using phenylsulfenyl 2-deoxy-2-trifluoroacetamido glycopyranosides as glycosyl donors. J Org Chem 64:5926–5929
Ikemoto N, Schreiber SL (1992) Total synthesis of (−)-hikizimycin employing the strategy of two-directional chain synthesis. J Am Chem Soc 114:2524–2536
Ge M, Thompson C, Kahne D (1998) Reconstruction of vancomycin by chemical glycosylation of the pseudoaglycon. J Am Chem Soc 120:11014–11015
Kim SH, Augeri D, Yang D, Kahne D (1994) Concise synthesis of the calicheamicin oligosaccharide using the sulfoxide glycosylation method. J Am Chem Soc 116:1766–1775
Yan L, Kahne D (1996) Generalizing glycosylation: synthesis of the blood group antigens Lea, Leb, and Lex using a standard set of reaction conditions. J Am Chem Soc 118:9239–9248
Yeung BKS, Hill DC, Janicka M, Petillo PA (2000) Synthesis of two hyaluronan trisaccharides. Org Lett 2:1279–1282
Nicolaou KC, Li Y, Fylaktakidou KC, Mitchell HJ, Sugita K (2001) Total synthesis of apoptolidin: part 2. Coupling of key building blocks and completion of the synthesis. Angew Chem Ed 40:3854–3857
Hederos M, Konradsson P (2005) Synthesis of the core tetrasaccharide of Trypanosoma cruzi glycoinositolphospholipids: Manp(α1 → 6)-Manp(α1 → 4)-6-(2-aminoethylphosphonic acid)-GlcNp(α1 → 6)-myo-Ins-1-PO4. J Org Chem 70:7196–7207
Taylor JG, Li X, Oberthür M, Zhu W, Kahne D (2006) The total synthesis of moenomycin A. J Am Chem Soc 128:15084–15085
Zhang Y, Fechter EJ, Wang TSA, Barrett D, Walker S, Kahne DE (2007) Synthesis of heptaprenyl-lipid IV to analyze peptidoglycan glycosyltransferases. J Am Chem Soc 129:3080–3081
Nguyen MH, Imanishi M, Kurogi T, Smith AB (2016) Total synthesis of (−)-mandelalide A exploiting anion relay chemistry (ARC): identification of a type II ARC/CuCN cross-coupling protocol. J Am Chem Soc 138:3675–3678
Nigudkar SS, Demchenko AV (2015) Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem Sci 6:2687–2704
Zhu X, Schmidt RR (2009) New principles for glycoside-bond formation. Angew Chem Int Ed 48:1900–1934
Ishiwata A, Lee YJ, Ito Y (2010) Recent advances in stereoselective glycosylation through intramolecular aglycon delivery. Org Biomol Chem 8:3596–3608
Ishiwata A, Ito Y (2017) Intramolecular aglycon delivery toward 1,2-cis selective glycosylation. In: Bennett CS (ed) Selective glycosylations: synthetic methods and catalysts, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, pp 79–96
Jung KH, Müller M, Schmidt RR (2000) Intramolecular O-glycoside bond formation. Chem Rev 100:4423–4442
Pistorio SG, Yasomanee JP, Demchenko AV (2014) Hydrogen-bond-mediated aglycone delivery: focus on β-mannosylation. Org Lett 16:716–719
Liu QW, Bin HC, Yang JS (2013) β-Arabinofuranosylation using 5-O-(2-quinolinecarbonyl) substituted ethyl thioglycoside donors. Org Lett 15:3974–3977
Zhu Y, Yu B (2015) Highly stereoselective β-mannopyranosylation via the 1-α-glycosyloxy-isochromenylium-4-gold(I) intermediates. Chem Eur J 21:8771–8780
Sun P, Wang P, Zhang Y, Zhang X, Wang C, Liu S, Lu J, Li M (2015) Construction of β-mannosidic bonds via gold(I)-catalyzed glycosylations with mannopyranosyl ortho-hexynylbenzoates and its application in synthesis of acremomannolipin A. J Org Chem 80:4164–4175
Elferink H, Mensink RA, White PB, Boltje TJ (2016) Angew Chem Int Ed 55:11217–11220
Takahashi D, Tanaka M, Nishi N, Toshima K (2017) Novel 1,2-cis-stereoselective glycosylations utilizing organoboron reagents and their application to natural products and complex oligosaccharide synthesis. Carbohydr Res 452:64–77
Crich D, Sun S (1996) Formation of β-mannopyranosides of primary alcohols using the sulfoxide method. J Org Chem 61:4506–4507
Huang X, Huang L, Wang H, Ye XS (2004) Iterative one-pot synthesis of oligosaccharides. Angew Chem Int Ed 43:5221–5224
Wu Y, Xiong DC, Chen SC, Wang YS, Ye XS (2017) Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat Commun 8:14851
Crich D, Sun S (1997) Direct synthesis of β-mannopyranosides by the sulfoxide method. J Org Chem 62:1198–1199
Crich D, Li H (2000) Direct stereoselective synthesis of β-thiomannosides. J Org Chem 65:801–805
Crich D, Dudkin V (2000) Efficient, diastereoselective chemical synthesis of a β-mannopyranosyl phosphoisoprenoid. Org Lett 2:3941–3943
Crich D, Cai W (1999) Chemistry of 4,6-O-benzylidene-d-glycopyranosyl triflates: contrasting behavior between the gluco and manno series. J Org Chem 64:4926–4930
Kim KS, Kim JH, Lee YJ, Lee YJ, Park J (2001) 2-(Hydroxycarbonyl)benzyl glycosides: a novel type of glycosyl donors for highly efficient β-mannopyranosylation and oligosaccharide synthesis by latent-active glycosylation. J Am Chem Soc 123:8477–8481
Codée JDC, Kröck L, Castagner B, Seeberger PH (2008) Automated solid-phase synthesis of protected oligosaccharides containing β-mannosidic linkages. Chem Eur J 14:3987–3994
Tsuda T, Arihara R, Sato S, Koshiba M, Nakamura S, Hashimoto S (2005) Direct and stereoselective synthesis of β-d-mannosides using 4,6-O-benzylidene-protected mannosyl diethyl phosphite as a donor. Tetrahedron 61:10719–10733
Baek JY, Choi TJ, Jeon HB, Kim KS (2006) A highly reactive and stereoselective β-mannopyranosylation system: mannosyl 4-pentenoate/PhSeOTf. Angew Chem Int Ed 45:7436–7440
Tanaka SI, Takashina M, Tokimoto H, Fujimoto Y, Tanaka K, Fukase K (2005) Highly β-selective mannosylation towards Manβ1-4GlcNAc synthesis: tMSB(C6H5)4 as Lewis acid/cation trap catalyst. Synlett 2005:2325–2328
Heuckendorff M, Bols PS, Barry CB, Frihed TG, Pedersen CM, Bols M (2015) β-Mannosylation with 4,6-benzylidene protected mannosyl donors without preactivation. Chem Commun 51:13283–13285
Crich D, Dai Z (1998) Direct synthesis of β-d-Xyl-(1 → 2)-β-d-Man-(1 → 4)-α-d-Glc-OME: a trisaccharide component of the Hyriopsis schlegelii glycosphingolipid. Tetrahedron Lett 39:1681–1684
Crich D, Li H, Yao Q, Wink DJ, Sommer RD, Rheingold AL (2001) Direct synthesis of β-mannans. A hexameric [→ 3)-β-d-man-(1 → 4)-β-d-man-(1]3 subunit of the antigenic polysaccharides from Leptospira biflexa and the octameric (1 → 2)-linked β-d-mannan of the Candida albicans phospholipomannan. X-ray crystal structure of a protected tetramer. J Am Chem Soc 123:5826–5828
Karelin AA, Tsvetkov YE, Paulovičová E, Paulovičová L, Nifantiev NE (2016) A blockwise approach to the synthesis of (1 → 2)-linked oligosacchrides corresponding to fragments of the acid-stable β-mannan from the Candida albicans cell wall. Eur J Org Chem 2016:1173–1181
Nagorny P, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ (2009) Toward fully synthetic homogeneous β-human follicle-stimulating hormone (β-hFSH) with a biantennary N-linked dodecasaccharide. Synthesis of β-hFSH with chitobiose units at the natural linkage sites. J Am Chem Soc 131:5792–5799
Kim JH, Yang H, Park J, Boons GJ (2005) A general strategy for stereoselective glycosylations. J Am Chem Soc 127:12090–12097
Boltje TJ, Kim JH, Park J, Boons GJ (2011) Stereoelectronic effects determine oxacarbenium vs β-sulfonium ion mediated glycosylations. Org Lett 13:284–287
Fang T, Gu Y, Huang W, Boons GJ (2016) Mechanism of glycosylation of anomeric sulfonium ions. J Am Chem Soc 138:3002–3011
Fascione MA, Adshead SJ, Stalford SA, Kilner CA, Leach AG, Turnbull WB (2009) Stereoselective glycosylation using oxathiane glycosyl donors. Chem Commun 2009:5841–5843
Fascione MA, Kilner CA, Leach AG, Turnbull WB (2012) Do glycosyl sulfonium ions engage in neighbouring-group participation? A study of oxathiane glycosyl donors and the basis for their stereoselectivity. Chem Eur J 18:321–333
Fascione MA, Turnbull WB (2010) Benzyne arylation of oxathiane glycosyl donors. Beilstein J Org Chem 6:19
Fascione MA, Webb NJ, Kilner CA, Warriner SL, Turnbull WB (2012) Stereoselective glycosylations using oxathiane spiroketal glycosyl donors. Carbohydr Res 348:6–13
Fang T, Mo KF, Boons GJ (2012) Stereoselective assembly of complex oligosaccharides using anomeric sulfonium ions as glycosyl donors. J Am Chem Soc 134:7545–7552
Huang W, Gao Q, Boons GJ (2015) Assembly of a complex branched oligosaccharide by combining fluorous-supported synthesis and stereoselective glycosylations using anomeric sulfonium ions. Chem Eur J 21:12920–12926
Crich D (2010) Mechanism of a chemical glycosylation reaction. Acc Chem Res 43:1144–1153
Bohé L, Crich D (2017) Glycosylation with glycosyl sulfonates. In: Bennett CS (ed) Selective Glycosylations: Synthetic Methods and Catalysts. Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, pp 115–133
Martin A, Arda A, Désiré J, Martin-Mingot A, Probst N, Sinaÿ P, Jiménez-Barbero J, Thibaudeau S, Blériot Y (2015) Catching elusive glycosyl cations in a condensed phase with HF/SbF5 superacid. Nature Chem 8:186–191
Bohé L, Crich D (2015) A propos of glycosyl cations and the mechanism of chemical glycosylation; the current state of the art. Carbohydr Res 403:48–59
Walvoort MTC, Dinkelaar J, van den Bos LJ, Lodder G, Overkleeft HS, Codée JDC, van der Marel GA (2010) The impact of oxacarbenium ion conformers on the stereochemical outcome of glycosylations. Carbohydr Res 345:1252–1263
Smith DM, Woerpel KA (2006) Electrostatic interactions in cations and their importance in biology and chemistry. Org Biomol Chem 4:1195–1201
Cumpstey I (2012) On a so-called “kinetic anomeric effect” in chemical glycosylation. Organic & Org Biomol Chem 10:2503–2508
Kim KS, Fulse DB, Baek JY, Lee BY, Jeon HB (2008) Stereoselective direct glycosylation with anomeric hydroxy sugars by activation with phthalic anhydride and trifluoromethanesulfonic anhydride involving glycosyl phthalate intermediates. J Am Chem Soc 130:8537–8547
Walvoort MTC, van den Elst H, Plante OJ, Kröck L, Seeberger PH, Overkleeft HS, van der Marel GA, Codée JDC (2012) Automated solid-phase synthesis of β-mannuronic acid alginates. Angew Chem Int Ed 51:4393–4396
Rencurosi A, Lay L, Russo G, Caneva E, Poletti L (2006) NMR evidence for the participation of triflated ionic liquids in glycosylation reaction mechanisms. Carbohydr Res 341:903–908
Zeng Y, Wang Z, Whitfield D, Huang X (2008) Installation of electron-donating protective groups, a strategy for glycosylating unreactive thioglycosyl acceptors using the preactivation-based glycosylation method. J Org Chem 73:7952–7962
Frihed TG, Bols M, Pedersen CM (2015) Mechanisms of glycosylation reactions studied by low-temperature nuclear magnetic resonance. Chem Rev 115:4963–5013
Crich D, Chandrasekera NS (2004) Mechanism of 4,6-O-benzylidene-directed β-mannosylation as determined by α-deuterium kinetic isotope effects. Angew Chem Int Ed 43:5386–5389
Huang M, Garrett GE, Birlirakis N, Bohé L, Pratt DA, Crich D (2012) Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects. Nature Chem 4:663–667
Huang M, Furukawa T, Retailleau P, Crich D, Bohé L (2016) Further studies on cation clock reactions in glycosylation: observation of a configuration specific intramolecular sulfenyl transfer and isolation and characterization of a tricyclic acetal. Carbohydr Res 427:21–28
Huang M, Retailleau P, Bohé L, Crich D (2012) Cation clock permits distinction between the mechanisms of α- and β-O- and β-C-glycosylation in the mannopyranose series: evidence for the existence of a mannopyranosyl oxocarbenium ion. J Am Chem Soc 134:14746–14749
Adero PO, Furukawa T, Huang M, Mukherjee D, Retailleau P, Bohé L, Crich D (2015) Cation clock reactions for the determination of relative reaction kinetics in glycosylation reactions: applications to gluco- and mannopyranosyl sulfoxide and trichloroacetimidate type donors. J Am Chem Soc 137:10336–10345
Shu P, Xiao X, Zhao Y, Xu Y, Yao W, Tao J, Wang H, Yao G, Lu Z, Zeng J, Wan Q (2015) Interrupted Pummerer reaction in latent-active glycosylation: glycosyl donors with a recyclable and regenerative leaving group. Angew Chem Int Ed 54:14432–14436
Xiao X, Zhao Y, Shu P, Zhao X, Liu Y, Sun J, Zhang Q, Zeng J, Wan Q (2016) Remote activation of disarmed thioglycosides in latent-active glycosylation via interrupted Pummerer reaction. J Am Chem Soc 138:13402–13407
Shu P, Yao W, Xiao X, Sun J, Zhao X, Zhao Y, Xu Y, Tao J, Yao G, Zeng J, Wan Q (2016) Glycosylation via remote activation of anomeric leaving groups: development of 2-(2-propylsulfinyl)benzyl glycosides as novel glycosyl donors. Org Chem Front 3:177–183
Meng L, Zeng J, Wan Q (2017) Interrupted Pummerer reaction in lacent/active glycosylation. Synlett 2017. https://doi.org/10.1055/s-0036-1588582
Bates DK, Winters RT, Picard JA (1992) Intramolecular capture of Pummerer rearrangement intermediates. Interrupted Pummerer rearrangement: capture of tricoordinate sulfur species generated under Pummerer rearrangement conditions. J Org Chem 57:3094–3097
Bates DK, Xia M (1998) A sulfoxide-based ring annelation approach to fused, many-membered ring N,S-heterocycles. J Org Chem 63:9190–9196
Bates DK, Sell BA, Picard JA (1987) An interrupted Pummerer reaction induced by Vilsmeier reagent (POCl3/DMF). Tetrahedron Lett 28:3535–3538
Zeng J, Sun G, Yao W, Zhu Y, Wang R, Cai L, Liu K, Zhang Q, Liu XW, Wan Q (2017) 3-Aminodeoxypyranoses in glycosylation: diversity-oriented synthesis and assembly in oligosaccharides. Angew Chem Int Ed 56:5227–5231
Xu Y, Zhang Q, Xiao Y, Wu P, Chen W, Song Z, Xiao X, Meng L, Zeng J, Wan Q (2017) Practical synthesis of latent disarmed S-2-(2-propylthio)benzyl glycosides for interrupted Pummerer reaction mediated glycosylation. Tetrahedron Lett 58:2381–2384
Chen W, Zeng J, Wang H, Xiao X, Meng L, Wan Q (2017) Tracking the leaving group in the remote activation of O-2-[(propan-2-yl)sulfinyl]benzyl (OPSB) glycoside. Carbohydr Res 452:1–5
Kristensen SK, Salamone S, Rasmussen MR, Marqvorsen MHS, Jensen HH (2016) Glycosyl ortho-methoxybenzoates: catalytically activated glycosyl donors with an easily removable and recyclable leaving group. Eur J Org Chem 2016:5365–5376
Nigudkar SS, Stine KJ, Demchenko AV (2014) Regenerative glycosylation under nucleophilic catalysis. J Am Chem Soc 136:921–923
Garcia BA, Poole JL, Gin DY (1997) Direct glycosylations with 1-hydroxy glycosyl donors using trifluoromethanesulfonic anhydride and diphenyl sulfoxide. J Am Chem Soc 119:7597–7598
Codée JDC, Hossain LH, Seeberger PH (2005) Efficient installation of β-mannosides using a dehydrative coupling strategy. Org Lett 7:3251–3254
Boebel TA, Gin DY (2005) Probing the mechanism of sulfoxide-catalyzed hemiacetal activation in dehydrative glycosylation. J Org Chem 70:5818–5826
Di Bussolo V, Kim YJ, Gin DY (1998) Direct oxidative glycosylations with glycal gonors. J Am Chem Soc 120:13515–13516
Honda E, Gin DY (2002) C2-Hydroxyglycosylation with glycal gonors. probing the mechanism of sulfonium-mediated oxygen transfer to glycal enol ethers. J Am Chem Soc 124:7343–7352
Crich D (2002) Chemistry of glycosyl triflates: synthesis of & β-mannopyranosides. J Carbohydr Chem 21:667–690
Acknowledgements
We thank the National Natural Science Foundation of China (21672077, 21772050, 21472054, 21702068), the State Key Laboratory of Bioorganic and Natural Products Chemistry (SKLBNPC13425), Natural Science Funds of Hubei Province for Distinguished Young Scholars (2015CFA035), Wuhan Creative Talent Development Fund, “Thousand Talents Program” Young Investigator Award, and Huazhong University of Science and Technology for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Sulfur Chemistry”; edited by Xuefeng Jiang.
Rights and permissions
About this article
Cite this article
Zeng, J., Liu, Y., Chen, W. et al. Glycosyl Sulfoxides in Glycosylation Reactions. Top Curr Chem (Z) 376, 27 (2018). https://doi.org/10.1007/s41061-018-0205-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-018-0205-4