Abstract
Studying groundwater renewal time is a valuable tool to deepen the comprehension of sustainable groundwater resources specific to arid regions. Tritium is a radioactive isotope of hydrogen often used as an environmental tracer to study groundwater renewal time. The present work reports groundwater renewal times by the environmental tracer tritium to understand sustainable water resources in arid regions. First, groundwater samples were collected from wells in northeastern arid regions of Saudi Arabia. Then, an electrolysis process was employed to significantly increase the tritium level from twenty-five to thirty times the original concentration. Subsequently, the enriched water was analyzed using a liquid scintillation counter under optimized measurement conditions to determine the tritium concentration precisely. Two internationally recognized tritium laboratories conducted independent assessments to validate the estimated tritium levels. The verified tritium concentration was then used to estimate the groundwater renewal time using the Morgenstern and Pimenta curves. The results suggest that most of the monitored wells in the surveyed areas are more than a century old. Conversely, a few monitoring wells exhibit renewal times of several hundred years and may be considered nonrenewable water sources. These studies help to understand the geochemical characteristics of arid regions to ensure the sustainable management and protection of groundwater resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
In arid regions, extreme climatic conditions and limited water resources pose unique challenges for scientists and environmentalists [1]. Groundwater, hidden beneath the Earth's surface, is often a vital water source. Studying environmental tracers and groundwater dating has become a powerful tool for understanding the geochemical traits that define arid landscapes. Age dating is a vital aspect of sustainable water management, as it involves determining the time that has passed since a water parcel entered a saturated zone [2, 3]. The rate of recharge and the accessibility of water in an aquifer can provide information on sustainable groundwater resources [4]. In addition, dating can assist in recognizing the time and source of contamination, which is crucial for assessing groundwater pollution. If an aquifer contains recently accumulated precipitation, it suggests rapid replenishment and heightened susceptibility to surface contamination due to improper waste disposal or inadequate agricultural practices [5, 6].
The atmosphere naturally generates numerous isotopes due to solar radiation and cosmic rays. When it rains, these isotopes combine with the water and can infiltrate the aquifer's saturated zone during water recharge. The water is examined for age, dating to the amounts of isotopes or natural tracers in the groundwater [7, 8]. Therefore, the existence of isotopes in the water is beneficial in calculating the groundwater renewal time [9,10,11]. Tritium has a known half-life, making it advantageous for dating groundwater. The concentration of tritium in water decreases predictably with time, allowing for the renewal time of groundwater to be calculated based on its initial concentration. Measuring of tritium concentration in environmental samples can offer valuable insights into the timing and speed of groundwater recharge and aid in evaluating the long-term viability of groundwater resources. Hence, the tritium concentration in groundwater relates to atmospheric tritium levels when the water encounters the atmosphere. Consequently, detecting any measurable tritium concentration in groundwater can yield valuable information, including the renewal time within the aquifer [12].
Many studies of groundwater dating have been conducted in different parts of Saudi Arabia to ascertain the groundwater renewal time. Groundwater from the Wajid aquifer in Wadi Al-Dawasir, southern Saudi Arabia, was investigated using isotopic methods [13]. Okofo et al. employed various environmental tracers to investigate groundwater renewal time and recharge mechanisms in the northeastern region of Ghana. They found that the groundwater has a wide range of ages, from less than a year to over 50 years [14]. Schlosser et al. estimated groundwater ages in a shallow urban aquifer using environmental tracers [15]. Based on the tracer measurements at each monitoring well, it was found to be over 50 years old. A report in an area very close to the present site measured tritium concentrations in wells and springs and found a mixture of sub-modern and modern water. The report concluded that the groundwater was unsuitable for drinking but could be used for household [16]. No relevant study has been reported in the northeastern part of Saudi Arabia. Hence, it is essential to assess the groundwater renewal time to understand the geochemical characteristics in these areas.
In previous work, we reported an optimized method designed to reduce the detection limit in low level tritium measurement by liquid scintillation counter [17,18,19]. We have estimated and validated renewal time for limited groundwater wells. In the present study, we use tritium as an environmental tracer to estimate groundwater renewal times, specifically in arid regions highly sensitive to water sustainability issues. The research involves an innovative electrolysis process to significantly increase tritium concentration, allowing for precise analysis using a liquid scintillation counter. The study also emphasizes independent validation of results by two international tritium laboratories, further ensuring data accuracy. Additionally, it employs the Morgenstern and Pimenta curves to estimate groundwater age, revealing critical insights into the long-term sustainability of water resources in these regions, including identifying some nonrenewable sources. Our other objective is to distinguish old groundwater resources from young resources by environmental tritium through quality management to eradicate possible contamination. The results can be used to screen for planned, sustainable, and quality water management, referring to public health and safety for drinking, domestic, and irrigation uses.
2 Materials and methods
2.1 Study areas and hydrogeology
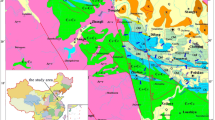
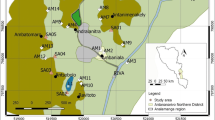
The current research focuses on three primary cities in northeastern Saudi Arabia: Hafr Al Batin, Thybiyah, and Qaisumah. The study area is between the longitude of 28° 26ʹ 3ʺ N and latitude of 45° 57ʹ 49ʺ E (Fig. 1). Hafr Al Batin, also known as Al Batin Valley, derives its name from its location within the arid valley of Wadi Al Batin. This valley is a segment of the larger Wadi al Rummah, the most extensive and longest dry river in the Arabian Peninsula, spanning approximately 2000 km in length [20,21,22]. The primary segment of Wadi Al Rummah traverses the Al Qassim oasis, one of the key agricultural regions in Saudi Arabia [22]. The climate in this area is characterized by extremely dry and hot summer days, with temperatures ranging from 40 to 50 °C. On the other hand, during winter nights, the temperatures drop to −2 to 8 °C. Rainfall is infrequent in the winter season, and no rainfall is recorded during the summer months.
The Eastern Province's territory has water quality well-suited for various uses. According to Vincent [23] and Alsharhan [24], the primary aquifers can be categorized based on their primary and secondary origins. The primary origin aquifers include the Quaternary sands of the Wadi system, quartz sandstones, conglomerates with primary porosity, and calcarenite, coquinite, and oolitic limestone with secondary porosity [23, 25]. In Hafr Al Batin, a Quaternary sand aquifer is present, and deep wells in this area provide relatively good water compared to shallow wells.
2.2 Groundwater collection and electrolytic enrichment
Different groundwater samples were collected from a total of forty-five distinct sites, which were believed to contain extremely low levels of tritium. These samples were directly taken from monitoring wells, handled with care, and stored in glass bottles immediately after collection. To ensure greater precision, measurements were typically carried out within a short time of sample collection unless otherwise mentioned. Additionally, a blank sample consisting of virtually no tritium (referred to as "dead water") was employed as a baseline for comparison.
The electrolysis enrichment process involves measuring low levels of tritium in water samples, which typically cannot be detected using a conventional liquid scintillation counter (LSC). The tritium concentration is enriched by electrolysis per instructions from the International Atomic Energy Association (IAEA) to enable detection. Initially, the groundwater undergoes primary distillation to remove impurities and contaminants. The distillation process eliminates dissolved ions and solids commonly found in groundwater and confirms their absence through testing with a YSI 9500 photometer and a Horiba digital meter. Electrolytic enrichment is performed using batches of electrolytic cells, each with a maximum capacity of 800 ml water. These cells are equipped with stainless steel anodes and mild steel cathodes. The 500–800 ml of water enrichment takes one to two weeks, with up to nine cells running simultaneously. The resulting water sample volume is collected after enrichment and an enrichment factor of approximately twenty-five to thirty times with an estimated error of ± 0.5 is calculated. The next step involves neutralizing the alkalinity of the solution, which is caused by electrolytes used in the electrolytic process. Neutralization was achieved by adding an acidic substance that reacted with the alkaline components, forming a more neutral product by introducing carbon dioxide into the alkaline water. A Horiba LAQUAtwin digital pH meter was used to monitor the water's alkalinity. Finally, the solution underwent final distillation by heating it to its boiling point, with the vapor being collected and condensed. The purified water was tested for contaminants (Cl−, SO4−, Ca−, CO3−, Mg++, Na+, K+, and dissolved solids) using a YSI 9500 photometer and a Horiba digital meter. Details of the electrolysis enrichment process, including enrichment time, factors, and data validation, are outlined in a previous study [19].
2.3 Sample preparation and tritium measurement
For liquid scintillation counting (LSC), a 10 ml enriched water sample was mixed with an equal volume (10 ml) of Hidex scintillation cocktail (Aqualight Plus, London, UK). This mixture converts energy released during tritium decay into light flashes. The samples were carefully stored in a cool, dark place and left for at least 4 h before measurement to avoid luminescence effects. Light flashes from tritium decay are counted using a liquid scintillation counter (Hidex 300 SL) based on the triple-to-double coincidence ratio (TDCR) principle, as shown in Figure S1 in the supporting document. Each sample was measured five times for 200 min. Also periodic calibration and background radiation was monitored to maintain consistency. A mathematical formulation was applied to calculate the efficiency and tritium concentration. Decay correction was considered if samples were left for an extended period, utilizing the radioactive decay equation and tritium's half-life as described earlier [19]. Additionally, groundwater samples were send to commercial commercial tritium laboratories, IsodetectUmwelmonitoring GmbH (Germany), and the University of Miami (USA) to ensure data accuracy and consistency. Both laboratories employ standardized equipment and procedures, conducting multiple analyses to validate results. A rigorous methodology was applied to ensure the reliability of the obtained data.
2.4 Mathematical formula for the scintillation counter’s efficiency and tritium concentration measurement
Efficiency measurement for the scintillation counter was conducted using a standard tritium source from Hidex, UK, and the assessment was conducted under identical conditions as those applied to the unknown groundwater samples. The tritium measurement efficiency, εH-3, was obtained from the measured net count rate of the standard sample, Rn (s−1); the known activity concentration, cn (Bq/L); and the sample volume, mM as
Flashes were quantified using a liquid scintillation counter (Hidex 300 SL) and recorded in counts per minute (CPM). Each sample underwent five measurements over 200 min, and the average of these five runs was calculated. A standard tritium source and a dead water sample containing zero tritium (provided by Hidex) were also tested for comparison under similar experimental conditions.
After the enrichment step, the tritium activity concentration c at the time of sampling was calculated using the following equation:
For both standard and background samples, where the enrichment step is no longer needed, Eq. (1) can be rewritten as
where.
cactivity concentration of tritium in Bq.m−3;
fAcorrection factor for the decay;
λdecay constant of tritium in s−1;
tAtime between sampling and beginning of the measurement in s;
Rnnet count rate of the counting source in s−1;
with Rn = Rg—Ro.
εdetection efficiency in Bq−1.s−1;
ρthe density of the groundwater in kg.m−3;
mnmass of the solution remaining in the electrolysis cell after electrolysis, in g;
mVmass of the solution filled into the electrolysis cell before electrolysis, in g;
mMmass of distillate in the scintillation vial, in g;
ϕprocedural calibration factor Bq.s.m−3;
The total gross count rate Rg was calculated from the individual gross count rate Rg,j using the following equation:
where.
Rg,jgross count rate of the single measurement j of the counting source, in s−1;
tm,jduration of the single measurement j, in s;
ttotsum of the durations of a single measurement in s.
The details of this mathematical formulation with applications can be obtained in the previous article [26].
3 Results and discussion
3.1 Tritium concentration in standard sample and background calibration
Accurate measurement of tritium concentration using a scintillation counter requires calibration. This calibration process involves efficiency calculations to ensure that the counter accurately detects and quantifies the radioactive emissions from tritium. To do so, the Liquid Scintillation Counter (LSC) performance is periodically monitored to maintain the accuracy of tritium concentration measurements. This monitoring is typically done using standard samples with known concentrations of radioactive isotopes. The calibration process for the scintillation counter involves using certified sources of carbon-14 (14C) and tritium (3H), as well as activity-free water (which may contain negligible levels of radioactive isotopes). These sources provide reference points for calibrating the counter's response to different levels of radioactivity. The results of the calibration process, as illustrated in Figure S2 (supporting document), show that the activity-free water consistently produces readings with minimal experimental error over an eight-year period. This consistency indicates that the radiation level in the activity-free water and the surrounding environment remains stable over time. Carbon-14, one of the isotopes used in the calibration process, has a longer half-life of approximately 5715 ± 40 years [27]. This longer half-life makes carbon-14 well-suited for long-term calibration standards, ensuring the stability and reliability of the counter over extended periods. In contrast to carbon-14, the count of the standard tritium (3H) source exhibits an exponential decrease over the eight-year period since the initialization of the device, as shown in Figure S2b. This decrease indicates the radioactive decay of tritium over time. By analyzing the exponential decay curve of tritium counts, a rough estimation suggests a half-life of approximately 11.75 years for tritium. This estimation closely aligns with the half-life of tritium reported in the literature, which is approximately 12.3 years [28]. The stable background count and consistent count for carbon-14 over time indicate the excellent performance of the liquid scintillation device in maintaining calibration stability. Additionally, the negligible effect of background count can be disregarded if the measured tritium value is approximately 1000 times higher than the background count.
3.2 Tritium concentration in the monitored wells
Before any measurement, the scintillation device was checked for efficiency and accurate results. The liquid scintillation counter (LSC) was calibrated periodically using certified activity-free water, carbon-14 (14C), and tritium (3H) sources. Over a few years, activity-free water consistently showed minimal experimental error, indicating a low level of radiation in the solution and its environment (data not shown here). The 14C source remained stable due to its longer half-life [27]. The details of the calibration process and efficiency were described previously [26, 29, 30]. These results affirm that TDCR values can effectively measure tritium activity concentration across multiple repetitions.
The estimated tritium concentrations for the groundwater samples using a liquid scintillation counter are presented in Fig. 2.
Figure 2 presents the measured tritium concentrations for 45 groundwater samples collected from the monitoring wells. The observed concentrations varied between 0.1 and 0.8 TU, with an average of 0.36 TU. Various national and international entities have a pivotal role in establishing comprehensive guidelines and safety thresholds for tritium levels in groundwater, ensuring the protection of public health and environmental well-being on a global scale. These guidelines serve as essential benchmarks for assessing and managing tritium contamination risks. The United States Environmental Protection Agency (USEPA) has mentioned a threshold of 11.1 Bq/L (~ 93 TU) as the permissible limit for tritium concentration in groundwater [31]. This limit reflects the agency's commitment to safeguarding groundwater quality and safe drinking water sources. The UNSCEAR published a safe limit of 40 Bq/L (~ 338 TU) [32], while the European Commission (EC) and World Health Organization (WHO) have identified an action limit of 100 Bq/L (~ 847 TU) [33, 34]. Remarkably, all the groundwater samples subjected to the present study unequivocally exhibited tritium levels well below these internationally recognized limits, reaffirming the region's commitment to ensuring the safety of its groundwater resources. Moreover, approximately 90% of the examined samples exhibited tritium concentrations that were markedly lower than those reported (1.0 to 80 TU) in previous studies documented in other regions in Saudi Arabia [34,35,36].
Figure 2 shows that shallow wells located in the northernmost region, specifically within the lower valley, were found to have notably higher tritium concentrations. In contrast, wells positioned in hillside areas demonstrated comparatively lower levels of tritium. This disparity in tritium concentration across these regions suggests a potential correlation with the geological composition of the underlying rocks and sediments through which the water traverses. The unique characteristics of the geological formations in these areas may significantly influence the transport and retention of tritium within the groundwater, resulting in the observed variations in concentration levels. Further investigation into the specific geological properties and hydrological dynamics of these regions could provide valuable insights into understanding the distribution patterns of tritium in groundwater systems.
3.3 Data validation for tritium activity
The tritium concentration data were validated by comparison with known values of spiked samples. Spiked samples were laboratory-prepared samples that contain a known concentration of tritium. They were used to calibrate the analytical methods and verify the accuracy of the obtained data. By comparing the obtained data with the spiked samples, we found minor errors in the analytical methods. The details of the data validation using spiked samples have been published previously [19].
Another data validation process involves comparing the acquired data to ensure its accuracy and reliability. This validation was performed by sending several water samples to two reputable international tritium laboratories: Isodetect Umwelmonitoring GmbH in Germany and the University of Miami in the United States. The tritium activity obtained from these laboratories is tabulated in Table 1. In two cases, negative values were found due to experimental error or variations in measurement. When measuring tritium levels, the count rate of the sample is compared to the count rate of a tritium-free sample (background or blank sample). The net tritium count rate is then calculated as the difference between these counts. Theoretically, when measuring samples with no tritium, the net results should be symmetrical around zero tritium units (TU). For certain applications, especially those where the absolute presence of tritium is crucial, a value of 0 TU should be used instead of negative values. However, in other cases where statistical analysis is the primary concern, negative values may be retained to maintain the integrity of the dataset. But, these negative values are reported to ensure unbiased statistical treatment of the data sets. By comparing the obtained data from three laboratories, the data are identical with a small error. The data collected independently from three separate laboratories exhibit complete consistency, providing strong evidence that the results obtained from all three laboratories are identical. This remarkable agreement in the collected data underscores the reliability and robustness of the experimental findings, as it reaffirms the accuracy and precision of the measurements conducted across these diverse research environments. Such uniformity in results not only lends credibility to the findings but also enhances the overall confidence in the validity of the scientific observations made in this study, reinforcing the significance of the research outcomes.
3.4 Groundwater renewal time
The estimated tritium activity of the monitoring wells was used to estimate the groundwater renewal time. The Morgenstern and Pimenta [37, 38] plots are useful tools in environmental science, particularly for estimating groundwater renewal times and ages. The plot is used to estimate groundwater age based on tritium concentrations. The Morgenstern and Pimenta plot visually represent the relationship between groundwater renewal time and tritium concentration. The plot typically shows tritium concentration on one axis and renewal time on the other, where data points often form a straight line if the system behaves predictably. The plot provides valuable insights into how long water has been in an aquifer, offering important information for water resource management. This method assumes that the aquifer behaves as a linear system and that the hydraulic head and time relationship follows a power-law relationship. It's important to note that while the Morgenstern and Pimenta plots can provide useful groundwater renewal time estimation, their accuracy depends on the validity of these assumptions and the quality of the data used. The assumptions that the aquifer behaves as a linear system and the quality of the data, including accurate tritium measurements, play a crucial role in the reliability of the results. Consequently, any deviations from these assumptions or data inconsistencies may affect the precision of the groundwater age estimation. Such a plot of the renewal time against the tritium concentration is shown in Fig. 3 (solid black line). The tritium activity data from three different laboratories are embedded in the plot. The plot shows that the groundwater containing the lowest tritium activities has a longer renovation time; conversely, the higher value of tritium activities represents a shorter renovation time. However, the data from our laboratory (solid red circle) show a wide variation in groundwater renewal time, ranging from 80 to 600 years, indicating significant mixing between different water sources [39]. According to the results, only a few samples were one hundred years old, while the remaining wells had ages exceeding one hundred years, even several hundred years old. It is important to note that tritium has a relatively short half-life. Depending on the initial concentration and decay rate, it can be used for groundwater dating from several decades to a couple hundred years. However, tritium concentrations diminish significantly beyond this temporal window, rendering accurate measurement challenging and, eventually, impractical. Consequently, tritium becomes unsuitable for estimating groundwater renewal time for older water sources. In the context of the present study, where, in some cases, tritium concentrations fall below 0.1 TU, the estimated renewal time is not reliable and does not have precision and significance [34]. This suggests that groundwater sources with estimated renewal times exceeding 200 years (as indicated by the oval shape with an arrow) need further analysis utilizing alternative isotopic tracers with longer half-lives. Examples of such isotopes include carbon-14 and noble gases like helium-4. These isotopes can provide insights into groundwater age dating over longer time scales, ranging from hundreds to thousands of years. These isotopes undergo radioactive decay at rates conducive to dating ancient groundwater sources with greater accuracy. We are currently employing carbon-14 as a tracer with the same scintillation counter, but the result shows a few decades as an error. However, the report will be published elsewhere. Notably, a study conducted in the alluvial aquifer of the Rio Grande in the United States revealed diverse groundwater ages [39, 40]. The complex hydrological processes controlling water movement in the aquifer were considered responsible.
The renewal time as a function of tritium activity (TU) was analyzed at various locations: our laboratory in Saudi Arabia (indicated by a red circle), the Isodetect lab in Germany (marked with a green circle), and the University of Miami lab in the United States (represented by a blue circle). The inset image shows an expanded area marked by a rectangular broken line. The solid and broken lines are the well-known "Morgenstern and Pimenta" curves for ages [37, 38, 41]
In contrast, tritium activity in groundwater wells over extended periods reveals deep aquifer waters to be as old as 200 years in the upper Jequitibá River Basin, Brazil [38]. Conversely, on shorter timescales, tritium's radioactive decay tracks the movement of groundwater over recent periods, such as shallow aquifer waters in Brazil, which are less than 37 years old, and the Rock Island Aquifer of Tobago, with ages ranging between 18 and over 60 years. Studies in the Ganges–Brahmaputra-Meghna delta, Bangladesh, show groundwater ages ranging from 7 to 28 years across wells, reflecting the complex dynamics of aquifer systems [42, 43]. Further investigation of isotopic ratios and water chemistry is necessary to understand groundwater intermingling and age-dating accuracy. Overall, our analysis suggests that groundwater in the surveyed region is predominantly old, with ages exceeding several hundred years.
The comparison with the water renewal time from the monitoring wells shows that most groundwater in the surveyed area is between 50 and 200 years old (Fig. 4). In contrast, only a few wells (sites 5, 11, 12, 19, and 26) show old water with a significant error that can be treated as dead water, but the reliability of the data is very poor. The differences in the groundwater ages across various locations of the research areas can vary significantly for several reasons. One of the reason might be the geological and hydrogeological characteristics of the research areas. Other reasons might be the rate at which water infiltrates the ground and the porosity and permeability of the subsurface materials. The porosity and permeability of subsurface materials significantly influence groundwater flow and age. Porosity refers to the volume of pore spaces within rocks or sediments, while permeability measures the ease with which water can move through these spaces. High porosity and permeability allow for faster groundwater movement, potentially leading to younger groundwater.
In contrast, low porosity and permeability can impede flow, leading to the accumulation of older groundwater. The presence of confining layers, also known as aquitards or aquicludes, can create isolated pockets of groundwater with distinct ages. These confining layers restrict the vertical movement of water and can lead to the development of artesian aquifers. Water trapped between confining layers can be quite old since it may have been isolated from the main aquifer for a long time.
In hydrogeology and groundwater studies, the GIS-based contour of the age distribution is a graphical representation that illustrates how the age of groundwater varies across a specific geographical area or within an aquifer. The GIS-based analysis provides valuable insights into the spatial and temporal distribution of groundwater ages, helping hydrogeologists and researchers understand groundwater flow patterns and assess groundwater resources' overall health and sustainability. Figure 5 shows the groundwater renewal time and describes the proportion of different ages within the aquifer. Areas with closely spaced contour lines indicate rapid groundwater flow, where water is relatively young and moves quickly through the aquifer. Widely spaced contour lines suggest slower groundwater movement, where water has resided in the aquifer for longer periods and may be older. Isolated patches of distinct age contours may indicate the presence of localized flow systems or geological features affecting groundwater renewal time. The size and shape of an aquifer can influence the renewal time of groundwater. Larger aquifers often have longer flow pathways, leading to older groundwater in their deeper portions.
On the other hand, smaller aquifers may have shorter flow pathways and potentially younger groundwater. The results imply that relatively older water is in the hillside areas of most of the northeastern side of the study area. Geological structures, such as faults and fractures, can act as preferential pathways for groundwater flow. Water can move more quickly through these conduits, potentially leading to variations in groundwater renewal time along these pathways. However, regions with less fractured geology may exhibit slower and more uniform groundwater flow and renewal time distribution. Compared with renewal time data from other countries, these findings suggest that most groundwater in the surveyed area is several hundred years old.
4 Conclusion
Groundwater renewal time was estimated using environmental tritium isotopes measured using a liquid scintillation counter by assessing the levels of tritium in the water. Since tritium levels in the water sample were initially low, the electrolysis process enriched the tritium level ten to thirty times. Shallow wells in the northernmost region, particularly in the lower valley, showed relatively elevated tritium concentrations. However, wells located in hillside areas exhibited lower concentrations.The groundwater renewal time varies significantly across different locations and even within the same aquifer due to differences in water flow, recharge rates, and the types of rocks and sediments the water passes through. Tritium concentrations were validated by two international tritium laboratories, confirming the accuracy of the estimated values with a significant error. The results indicate that most groundwater in the surveyed wells is over 100 years old, suggesting limited recharge. The presence of "dead water" in some wells emphasizes the need for sustainable groundwater management in these regions. This data is valuable for governmental agencies aiming to manage water resources effectively.
Data availability
The author declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request. Source data are provided in this paper.
References
Wang Z, Hu L, Pan S. Groundwater in arid regions of China. Ann Arid Zone. 2009;48:329–40.
Post VEA, Vandenbohede A, Werner AD, Maimun, Teubner MD. Groundwater ages in coastal aquifers. Adv Water Res. 2013;57:1–11. https://doi.org/10.1016/j.advwatres.2013.03.011.
Grünenbaum N, Greskowiak J, Sültenfuß J, Massmann G. Groundwater flow and residence times below a meso-tidal high-energy beach: a model-based analyses of salinity patterns and 3H–3He groundwater ages. J Hydrol. 2020;587: 124948. https://doi.org/10.1016/j.jhydrol.2020.124948.
Redda MA, Kebede S, Birhanu B, Hussien B. Estimating groundwater recharge rates in the Upper Awash Basin, Ethiopia under different combinations of model complexity and objective functions. Hydrol Res. 2024;55(3):263–79.
Bethke C, Johnson T. Groundwater age and groundwater age dating. Annu Rev Earth Planet Sci. 2008;36:121–52. https://doi.org/10.1146/annurev.earth.36.031207.124210.
Sánchez-Úbeda JP, López-Chicano M, Calvache ML, Purtschert R, Engesgaard P, Martín-Montañés C, et al. Groundwater age dating in Motril-Salobreña coastal aquifer with environmental tracers (δ18O/δ2H, 3H/3He, 4He, 85Kr, and 39Ar). In: Calvache ML, Duque C, Pulido-Velazquez D, editors., et al., Groundwater and Global Change in the Western Mediterranean Area. Cham: Springer International Publishing; 2018. p. 287–95.
Bovolo CI, Parkin G, Sophocleous M. Groundwater resources, climate and vulnerability. Environ Res Lett. 2009;4(3): 035001.
Li J, Zhang Y-K, Zhou Z, Guo Y, Zhao J, Liang X. Using multiple isotopes to determine groundwater source, age, and renewal rate in the Beishan preselected area for geological disposal of high-level radioactive waste in China. J Hydrol. 2024;629: 130592.
Howcroft W, Cartwright I, Fifield LK, Cendón DI. Differences in groundwater and chloride residence times in saline groundwater: the Barwon River Catchment of Southeast Australia. Chem Geol. 2017;451:154–68. https://doi.org/10.1016/j.chemgeo.2017.01.015.
Cook PG, Robinson NI. Estimating groundwater recharge in fractured rock from environmental 3H and 36Cl, Clare Valley South Australia. Water Res Res. 2002;38(8):11–21. https://doi.org/10.1029/2001WR000772.
Gomes AR, Abrantes J, Libânio A, Madruga MJ, Reis M. Determination of tritium in water using electrolytic enrichment: methodology improvements. J Radioanal Nucl Chem. 2017;314(2):669–74. https://doi.org/10.1007/s10967-017-5456-y.
Muhammad SB, Nasiru R. Procedure for radio dating of water using nuclear hydrological isotopes techniques. IOSR J Appl Phys. 2013. https://doi.org/10.9790/4861-06214953.
Benaafi M, Al-Shaibani A. Hydrochemical and Isotopic Investigation of the Groundwater from Wajid Aquifer in Wadi Al-Dawasir, Southern Saudi Arabia. Water. 2021;13(13):1855.
Okofo Boansi L, Adonadaga M-G, Martienssen M. Groundwater age dating using multi-environmental tracers (SF6, CFC-11, CFC-12, δ18O, and δD) to investigate groundwater residence times and recharge processes in Northeastern Ghana. J Hydrol. 2022;610: 127821. https://doi.org/10.1016/j.jhydrol.2022.127821.
Schlosser P, Stute M, Dörr H, Sonntag C, Münnich KO. Tritium/3He dating of shallow groundwater. Earth Planet Sci Lett. 1988;89(3):353–62. https://doi.org/10.1016/0012-821X(88)90122-7.
Al-Shamma’a A, Ghafel K, Al S, Al M. Estimating of groundwater age and quality of Al-Shanafiya Area Southwest Iraq. Iraqi J Sci. 2014;55:1061–10701061.
Mamun A, Alazmi AS. Risk assessment of radon exposure by ingestion and inhalation of groundwater within different age groups. Groundwater Monitor Remed. 2023;43(1):69–77. https://doi.org/10.1111/gwmr.12558.
Mamun A, Alazmi AS. Advancement of a liquid scintillation counter and semiconductor alpha spectroscopy detector to estimate the radon concentration in groundwater. Water. 2022;14(23):3849.
Mamun A. Estimation of tritium concentration in the rain- and groundwater in the dry river of Hafr Al Batin. Saudi Arabia Limnological Review. 2023;23(2):93–107.
El-Taher A, Al-Turki A. Radon activity measurements in irrigation water from Qassim Province by RAD7. J Environ Biol. 2016;37:1299–302.
El-Taher AM, Abojassim AA, Najam LA, Mraity HAAB. Assessment of annual effective dose for different age groups based on radon concentrations in the groundwater of Qassim, Saudi Arabia. Iranian J Med Phys. 2020;17(1):15–20. https://doi.org/10.22038/ijmp.2019.37379.1476.
Aly AA, Al-Omran AM, Alharby MM. The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin. Saudi Arabia Arabian J Geosci. 2015;8(6):4177–90. https://doi.org/10.1007/s12517-014-1463-2.
Vincent P. Saudi Arabia: an environmental overview. 1st. Edition. CRC Press: London; 2008.
Alsharhan AS, Rizk ZA, Nairn AEM, Bakhit DW, Alhajari SA. Hydrogeology of an arid region: the Arabian Gulf and adjoining areas. Amsterdam: Elsevier Science; 2001.
Alsharhan AS, Rizk ZA, Nairn AEM, Bakhit DW, Alhajari SA. Preface hydrogeology of an arid region: the Arabian Gulf and adjoining areas. Amsterdam: Elsevier Science; 2001.
Mamun A. An advanced optimization method to minimize the detection limit of liquid scintillation counter to measure low-level tritium activity in groundwater. Radiation. 2023;3(3):138–52.
Choppin GR, Liljenzin J-O, Rydberg JAN. CHAPTER 5—radionuclides in nature. In: Choppin GR, Liljenzin J-O, Rydberg JAN, editors. Radiochemistry and Nuclear Chemistry. 3rd ed. Woburn: Butterworth-Heinemann; 2002. p. 94–122.
Lucas LL, Unterweger MP. Comprehensive review and critical evaluation of the half-life of tritium. J Res Nat Inst Stand Technol. 2000;105(4):541–9. https://doi.org/10.6028/jres.105.043.
Mamun A. Enrichment of low-level tritium in groundwater via an electrolysis process for liquid scintillation counting applications. Geosciences. 2023;13(10):290.
Arun B, Viswanathan S, Venkatesan S, Jose MT, Balasubramaniam V. Study of triple to double coincidence method for tritium measurements. Radiochemistry. 2021;63:221–6. https://doi.org/10.1134/S1066362221020120.
USEPA. National primary drinking water regulations radionuclides; final rule. Fed Reg. 2000;65(236):76708.
UNSCEAR. The general assembly with scientific annex. United Nations; 2000.
EC. Radon in workplaces: implementing the requirements in Council Directive. Publications Office; 2019.
WHO. Guidelines for drinking-water quality. Geneva: World Health Organization; 2011.
Al-Jaseem QK, Almasoud FI, Ababneh AM, Al-Hobaib AS. Radiological assessment of water treatment processes in a water treatment plant in Saudi Arabia: Water and sludge radium content, radon air concentrations and dose rates. Sci Total Environ. 2016;563–564:1030–6. https://doi.org/10.1016/j.scitotenv.2016.04.049.
Aljaloud KB, ElBatouti M. Statistical analysis of 222Rn concentration in Zamzam and other water sources in the Kingdom of Saudi Arabia. Heliyon. 2021;7(2): e06057. https://doi.org/10.1016/j.heliyon.2021.e06057.
Morgenstern U, Taylor CB. Ultra low-level tritium measurement using electrolytic enrichment and LSC. Isot Environ Health Stud. 2009;45(2):96–117. https://doi.org/10.1080/10256010902931194.
Pimenta R, Rocha Z, Viana J, Gardini G, Duarte M, Moreira R. Use Of Environmental Tritium In Groundwater Dating In The Upper jequitibá River Basin, Municipality Of Sete Lagoas, Minas Gerais, Brazil. 2017.
Plummer LN, Glynn PD. Radiocarbon dating in groundwater systems. Chapter 4. vol;Other: ISBN 978–92–0–137210–9; TRN: XA13M0262055166. IAEA: IAEA; Vienna (International Atomic Energy Agency (IAEA)); International Atomic Energy Agency, Division of Physical and Chemical Sciences, Vienna (Austria); 2013.
Plassin S, Koch J, Paladino S, Friedman JR, Spencer K, Vaché KB. A socio-environmental geodatabase for integrative research in the transboundary Rio Grande/Río Bravo basin. Scientific Data. 2020;7(1):80. https://doi.org/10.1038/s41597-020-0410-1.
Jurgens BC, Böhlke JK, Eberts SM. TracerLPM (Version 1): An Excel® workbook for interpreting groundwater age distributions from environmental tracer data. Techniques and Methods. Reston, VA2012.
Hoque MA, Burgess WG. 14C dating of deep groundwater in the Bengal Aquifer System, Bangladesh: Implications for aquifer anisotropy, recharge sources and sustainability. J Hydrol. 2012;444–445:209–20. https://doi.org/10.1016/j.jhydrol.2012.04.022.
Sadeak S, Al Amin M, Chowdhury T, Mia MB, Alam MJ, Ahmed KM, et al. Comparison of the groundwater recharge estimations of the highly exploited aquifers in Bangladesh and their sustainability. Groundw Sustain Dev. 2023;20: 100896. https://doi.org/10.1016/j.gsd.2022.100896.
Acknowledgements
The author would like to thank the Deanship of Scientific Research at the University of Hafr Al Batin for generously providing the necessary experimental facilities. Additionally, this research has been made possible through financial support from the Institutional Funding Project (IFP-A-202-1-3) granted by the Ministry of Education in Saudi Arabia. The author is also grateful for the prompt assistance and data validation provided by IsodetectUmwelmonitoring GmbH in Germany and the University of Miami in the United States.
Author information
Authors and Affiliations
Contributions
Al Mamun wrote the main manuscript text and prepared figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mamun, A. Groundwater renewal time by environmental tritium isotopes as a tracer for sustainable water resource management. Discov Water 4, 84 (2024). https://doi.org/10.1007/s43832-024-00135-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00135-4
Keywords
Profiles
- Al Mamun View author profile